The Evaluation of Radiolabeled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Initial Staging in Intermediate-Risk Prostate Cancer Patients: A Retrospective Multicenter Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. PSMA PET/CT Protocol
2.3. Image Analysis
2.4. Multiparametric MRI Imaging
2.5. Change in Management
2.6. Statistical Analysis
3. Results
3.1. MpMRI and PSMA PET/CT
3.2. PSMA PET/CT and Change Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mottet, N.; Bellmunt, J.; Briers, E.; Bolla, M.; Bourke, L.; Cornford, P.; De Santis, M.; Henry, A.; Joniau, S.; Lam, T.; et al. Members of the EAU—ESTRO—ESUR—SIOG Prostate Cancer Guidelines Panel. In EAU—ESTRO—ESUR—SIOG Guidelines on Prostate Cancer. Edn. Presented at the EAU Annual Congress Milan; EAU Guidelines Office: Arnhem, The Netherlands, 2021; ISBN 978-94-92671-13-4. [Google Scholar]
- Zelic, R.; Garmo, H.; Zugna, D.; Stattin, P.; Richiardi, L.; Akre, O.; Pettersson, A. Predicting Prostate Cancer Death with Different Pretreatment Risk Stratification Tools: A Head-to-head Comparison in a Nationwide Cohort Study. Eur. Urol. 2020, 77, 180. [Google Scholar] [CrossRef] [PubMed]
- Dess, R.T.; Suresh, K.; Zelefsky, M.J.; Freedland, S.J.; Mahal, B.A.; Cooperberg, M.R.; Davis, B.J.; Horwitz, E.M.; Terris, M.K.; Amling, C.L.; et al. Development and Validation of a Clinical Prognostic Stage Group System for Nonmetastatic Prostate Cancer Using Disease-Specific Mortality Results From the International Staging Collaboration for Cancer of the Prostate. JAMA Oncol. 2020, 6, 1912. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A., Jr.; Scardino, P.T.; Resnick, M.I.; Hernandez, A.D.; Rose, S.C.; Marlene, J. Transrectal ultrasound versus digital rectal examination for the staging of carcinoma of the prostate: Results of a prospective, multi-institutional trial. J. Urol. 1997, 157, 902. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, M.; Hamoen, E.H.J.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. 2Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Thoeny, H.C.; Urs, E.S. Current Status of Lymph Node Imaging in Bladder and Prostate Cancer. Urology 2016, 96, 1–7. [Google Scholar] [CrossRef]
- Hovels, A.M.; Heesakkers, R.A.M.; Adang, E.M.; Jager, G.J.; Strum, S.; Hoogeveen, Y.L.; Severens, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kairemo, K. Meta-analysis of (11)C-choline and (18)F-choline PET/CT for management of patients with prostate cancer. Nucl. Med. Commun. 2014, 35, 221. [Google Scholar] [CrossRef]
- Van den Bergh, L.; Evelyne, L.; Karin, H.; Christophe, M.D.; Raymond, O.; Sofie, S.; Tom, B.; Filip, A.; Felix, M.M.; Kris, B.; et al. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol. Oncol. 2015, 33, e23-31. [Google Scholar] [CrossRef]
- Heck, M.M.; Souvatzoglou, M.; Retz, M.; Nawroth, R.; Kübler, H.; Maurer, T.; Thalgott, M.; Gramer, B.M.; Weirich, G.; Rondak, I.-C.; et al. Prospective comparison of computed tomography, diffusion-weighted magnetic resonance imaging and [11C]choline positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 694. [Google Scholar] [CrossRef]
- Abrams-Pompe, R.S.; Fanti, S.; Schoots, I.G.; Moore, C.M.; Turkbey, B.; Vickers, A.J.; Walz, J.; Steuber, T.; James, A. The Role of Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Primary Staging of Newly Diagnosed Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. Oncol. 2021, 4, 370–395. [Google Scholar] [CrossRef]
- Briganti, A.; Passoni, N.; Ferrari, M.; Capitanio, U.; Suardi, N.; Gallina, A.; Da Pozzo, L.F.; Picchio, M.; Di Girolamo, V.; Salonia, A.; et al. When to perform bone scan in patients with newly diagnosed prostate cancer: External validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur. Urol. 2010, 57, 551. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, M.J.; Chang, J.I.; Stricker, P.D.; van Leeuwen, P.J.; Nguyen, Q.A.; Ho, B.; Delprado, W.; Lee, J.; Thompson, J.E.; Cusick, T.; et al. Diagnostic accuracy of 68 Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediategrade intra-prostatic prostate cancer using whole-mount pathology: Impact of the addition of 68Ga-PSMA PET to mpMRI. BJU Int. 2019, 124, 42–49. [Google Scholar] [PubMed]
- Hagens, M.J.; Luining, W.I.; Jager, A.; Donswijk, M.L.; Cheung, Z.; Wondergem, M.; Oprea-Lager, D.E.; Vis, A.N.; van Leeuwen, P.J.; van der Poel, H.G. The Diagnostic Value of PSMA PET/CT in Men with Newly Diagnosed Unfavorable Intermediate-Risk Prostate Cancer. J. Nucl. Med. 2023, 64, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Luining, W.I.; Jager, A.; Donswijk, M.L.; Cheung, Z.; Wondergem, M.; Oprea-Lager, D.E.; Vis, A.N.; van Leeuwen, P.J.; van der Poel, A.G. The PRIMARY Score: Using Intraprostatic 68Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J. Nucl. Med. 2022, 63, 1644–1650. [Google Scholar]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen-Targeted PET Imaging: PSMA-RADS Version 1.0. J. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J. Urol. 2018, 199, 990–997. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, V.A.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Dekalo, S.; Kuten, J.; Campbell, J.; Mintz, I.; Bar-Yosef, Y.; Keizman, D.; Sarid, D.; Even-Sapir, E.; Yossepowitch, O.; Mano, R. 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography for patients with favorable intermediate-risk prostate cancer. Can. Urol. Assoc. J. 2022, 16, E381–E385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chikatamarla, V.A.; Okano, S.; Jenvey, P.; Ansaldo, A.; Roberts, M.J.; Ramsay, S.C.; Thomas, P.A.; David, A.P. Risk of metastatic disease using [18F]PSMA-1007 PET/CT for primary prostate cancer staging. EJNMMI Res. 2021, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Arnfield, E.G.; Thomas, P.A.; Roberts, M.J.; Pelecanos, A.M.; Ramsay, S.C.; Lin, C.Y.; Latter, M.J.; Garcia, P.L.; Pattison, D.A. Clinical insignificance of [18F]PSMA-1007 avid non-specific bone Lesions: A Retrosp. Evaluation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4495–4507. [Google Scholar] [CrossRef]
- Grunig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Joachim, M. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging. 2021, 48, 4483–4494. [Google Scholar] [CrossRef]
- Hagens, M.J.; Oprea-Lager, D.E.; Vis, A.N.; Wondergem, M.; Donswijk, M.L.; Meijer, D.; Emmett, L.; van Leeuwen, P.J.; van der Poel, H.G. Reproducibility of PSMA PET/CT imaging for primary staging of treatment-naïve prostate cancer patients depends on the applied radiotracer: A retrospective study. J. Nucl. Med. 2022, 63, 1531–1536. [Google Scholar] [CrossRef]
- Presented at the EAU Annual Congress Paris; Edn; EAU Guidelines: Arnhem, The Netherlands, 2024; ISBN 978-94-92671-23-3.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Prostate Cancer; Version 4.2024—17 May 2024; NCCN Guidelines for Patients®: Philadelphia, PA, USA, 2024; Available online: https://www.nccn.org/patients (accessed on 15 July 2024).
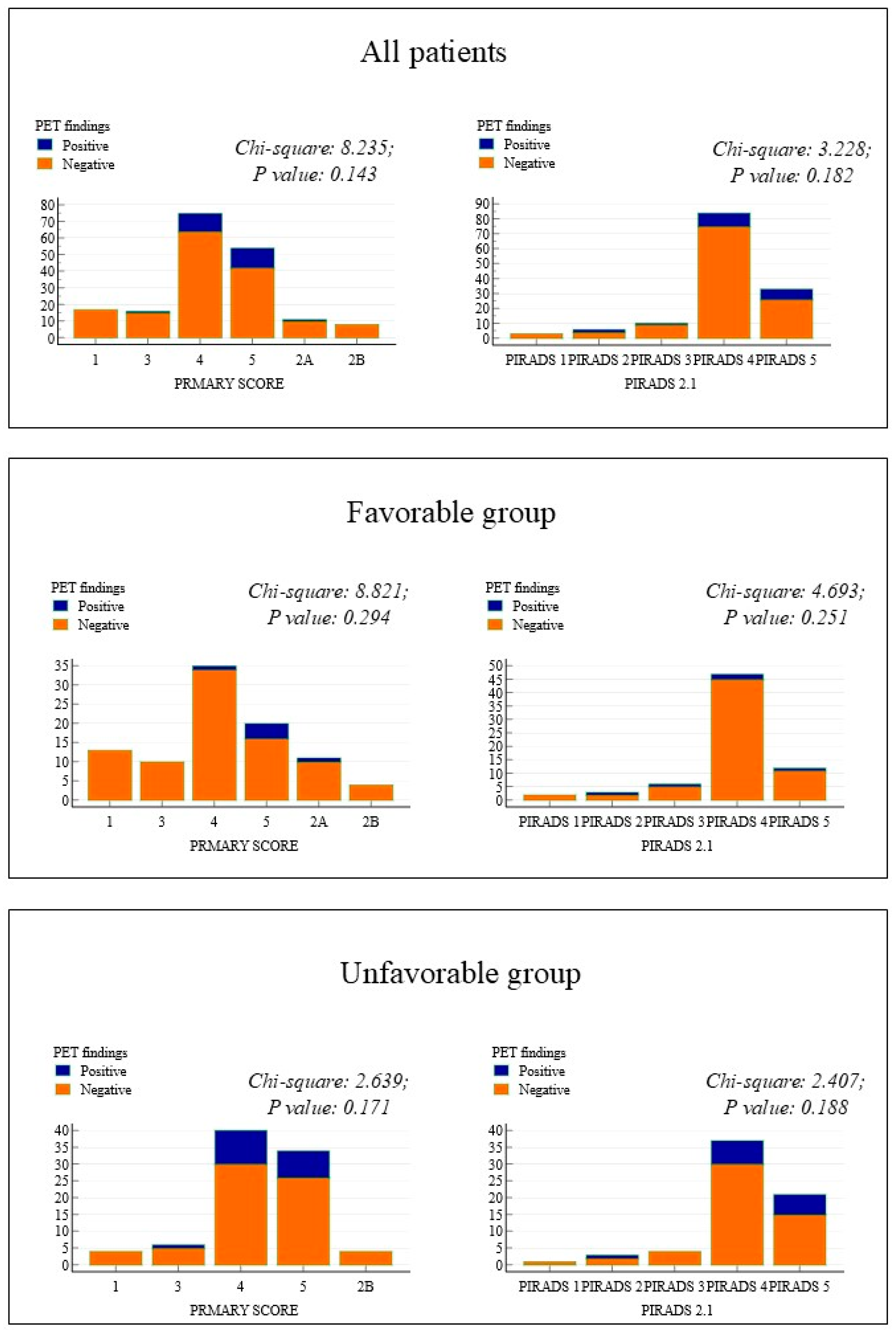

| Variables | Risk Category | ||
|---|---|---|---|
| Favorable | Unfavorable | ||
| N of patients | 181 | 93 | 88 |
| Median (range) age in years | 70 (48–84) | 69 (52–84) * | 72 (48–83) * |
| Initial PSA | |||
| Median (range), ng/mL | 6.6 (1.17–20) | 6.1 (1.9–12.9) * | 7.2 (1.2–20) * |
| ISUP (biopsy), n (%) | |||
| Favorable (2) | 93 (51.4%) | - | |
| Unfavorable (3) | 88 (48.6%) | - | |
| Type of treatment | |||
| ADT + new-generation HT | 1 (0.5%) | 0 * | 1 (1.1%) * |
| Surgery | 108 (59.7%) | 66 (71%) * | 42 (47.7%) * |
| HIFU | 6 (3.3%) | 5 (5.4%) * | 1 (1.1%) * |
| ADT | 6 (3.3%) | 0 * | 6 (6.8%) * |
| RT | 35 (19.3%) | 17 (18.3%) * | 18 (20.6%) * |
| RT + ADT | 24 (13.4%) | 4 (4.3%) * | 20 (22.7%) * |
| TURP + ADT | 1 (0.5%) | 1 (1.1%) * | 0 * |
| TP | TN | FP | FN | Sens (95%CI) | Spec (95%CI) | PPV (95%CI) | NPV (95%CI) | Acc (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|
| N *–All | 13 | 166 | 2 | 0 | 100 | 99 (97–100) | 87 (68–100) | 100 | 99 (97–100) |
| N–Fav | 1 | 91 | 1 | 0 | 100 | 99 (97–100) | 50 (1–100) | 100 | 99 (97–100) |
| N–Unf | 11 | 76 | 1 | 0 | 100 | 99 (96–100) | 91 (75–100) | 100 | 99 (97–100) |
| M *–All | 9 | 164 | 8 | 0 | 100 | 95 (92–98) | 53 (20–86) | 100 | 96 (92–98) |
| M–Fav | 2 | 88 | 3 | 0 | 100 | 97 (93–100) | 40 (27–100) | 100 | 97 (93–100) |
| M–Unf | 7 | 76 | 5 | 0 | 100 | 93 (88–99) | 58 (22–94) | 100 | 94 (89–99) |
| Variables | Risk Category | |||||
|---|---|---|---|---|---|---|
| Favorable | Unfavorable | |||||
| Negative PET/CT | Positive PET/CT | p Value | Negative PET | Positive PET | p Value | |
| N of patients | 87 | 6 | 69 | 19 | ||
| Median (range) age in years | 69 (52–84) | 74 (58–78) | 0.219 | 71 (48–83) | 73 (58–83) | 0.239 |
| Initial PSA Median (range), ng/mL | 6.2 (1.9–12.9) | 5.1 (4.2–10) | 0.578 | 7 (1.2–20) | 8 (2.9–20) | 0.156 |
| N patients available for MRI | 65 | 5 | 52 | 14 | ||
| MRI | ||||||
| Monofocal | 49 (75%) | 4 (80%) | 0.153 | 37 (71%) | 10 (71%) | 0.976 |
| Plurifocal | 11 (17%) | 0 | 12 (23%) | 3 (21%) | ||
| Missing | 5 (8%) | 1 (20%) | 3 (6%) | 1 (7%) | ||
| MRI–PIRADS 2.1 | ||||||
| 1 | 2 (3%) | 0 | 1 (2%) | 0 | ||
| 2 | 2 (3%) | 1 (20%) | 0.251 | 2 (4%) | 1 (7%) | 0.661 |
| 3 | 5 (8%) | 1 (20%) | 4 (8%) | 0 | ||
| 4 | 45 (69%) | 2 (40%) | 30 (58%) | 7 (50%) | ||
| 5 | 11 (17%) | 1 (20%) | 15 (28%) | 6 (43%) | ||
| MRI EPE | ||||||
| No | 61 (94%) | 3 (60%) | <0.005 | 46 (88%) | 10 (72%) | |
| Yes | 1 (2%) | 2 (40%) | 4 (8%) | 3 (21%) | 0.308 | |
| Doubtful | 3 (4%) | 0 | 2 (4%) | 1 (7%) | ||
| MRI SVI | ||||||
| No | 64 (98%) | 5 (100%) | 0.033 | 49 (94%) | 12 (86%) | 0.288 |
| Yes | 1 (2%) | 0 | 3 (6%) | 2 (14%) | ||
| Patient n# | Risk | Initial Treatment | Post-PET Treatment | Change in Management |
|---|---|---|---|---|
| 1 | Favorable | Surgery | Systemic therapy | Major |
| 2 | Favorable | Radiotherapy | RT planning | Minor |
| 3 | Unfavorable | Radiotherapy | RT planning | Minor |
| 4 | Unfavorable | Radiotherapy | Systemic therapy | Major |
| 5 | Unfavorable | Radiotherapy | Systemic therapy | Major |
| 6 | Favorable | Radiotherapy | RT planning | Minor |
| 7 | Unfavorable | Radiotherapy | Systemic therapy | Major |
| 8 | Favorable | Surgery | Tailoring of LND template | Minor |
| 9 | Favorable | Surgery | Tailoring of LND template | Minor |
| 10 | Favorable | Surgery | Tailoring of LND template | Minor |
| 11 | Unfavorable | Radiotherapy | Systemic therapy | Major |
| 12 | Unfavorable | Radiotherapy | RT planning | Minor |
| 13 | Favorable | Surgery | Tailoring of LND template | Minor |
| 14 | Favorable | Radiotherapy | RT planning | Minor |
| 15 | Favorable | Surgery | Tailoring of LND template | Minor |
| 16 | Unfavorable | Radiotherapy | RT planning and systemic treatment | Major |
| 17 | Unfavorable | Surgery | Tailoring of LND template | Minor |
| 18 | Favorable | Radiotherapy | RT planning | Minor |
| 19 | Unfavorable | Radiotherapy | Systemic therapy | Major |
| 20 | Unfavorable | Radiotherapy | Systemic therapy | Major |
| 21 | Unfavorable | Radiotherapy | RT planning | Minor |
| 22 | Favorable | Surgery | Systemic therapy | Major |
| 23 | Unfavorable | Radiotherapy | RT planning | Minor |
| 24 | Unfavorable | Surgery | Systemic therapy | Major |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelista, L.; Guglielmo, P.; Giacoppo, G.; Setti, L.; Aricò, D.; Muraglia, L.; Marzo, K.; Buffi, N.; Fasulo, V.; Rodari, M.; et al. The Evaluation of Radiolabeled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Initial Staging in Intermediate-Risk Prostate Cancer Patients: A Retrospective Multicenter Analysis. Diagnostics 2024, 14, 2751. https://doi.org/10.3390/diagnostics14232751
Evangelista L, Guglielmo P, Giacoppo G, Setti L, Aricò D, Muraglia L, Marzo K, Buffi N, Fasulo V, Rodari M, et al. The Evaluation of Radiolabeled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Initial Staging in Intermediate-Risk Prostate Cancer Patients: A Retrospective Multicenter Analysis. Diagnostics. 2024; 14(23):2751. https://doi.org/10.3390/diagnostics14232751
Chicago/Turabian StyleEvangelista, Laura, Priscilla Guglielmo, Giulia Giacoppo, Lucia Setti, Demetrio Aricò, Lorenzo Muraglia, Katia Marzo, Nicolò Buffi, Vittorio Fasulo, Marcello Rodari, and et al. 2024. "The Evaluation of Radiolabeled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Initial Staging in Intermediate-Risk Prostate Cancer Patients: A Retrospective Multicenter Analysis" Diagnostics 14, no. 23: 2751. https://doi.org/10.3390/diagnostics14232751
APA StyleEvangelista, L., Guglielmo, P., Giacoppo, G., Setti, L., Aricò, D., Muraglia, L., Marzo, K., Buffi, N., Fasulo, V., Rodari, M., Jandric, J., Salvaggio, A., Bonacina, M., Lazzeri, M., & Lughezzani, G. (2024). The Evaluation of Radiolabeled Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Initial Staging in Intermediate-Risk Prostate Cancer Patients: A Retrospective Multicenter Analysis. Diagnostics, 14(23), 2751. https://doi.org/10.3390/diagnostics14232751









