Mixed Pancreatobiliary Ductal Adenocarcinoma and Squamous Cell Carcinoma Arising from an Ectopic Pancreas in a Gastric Duplication Cyst—A Rare Double Diagnosis
Abstract
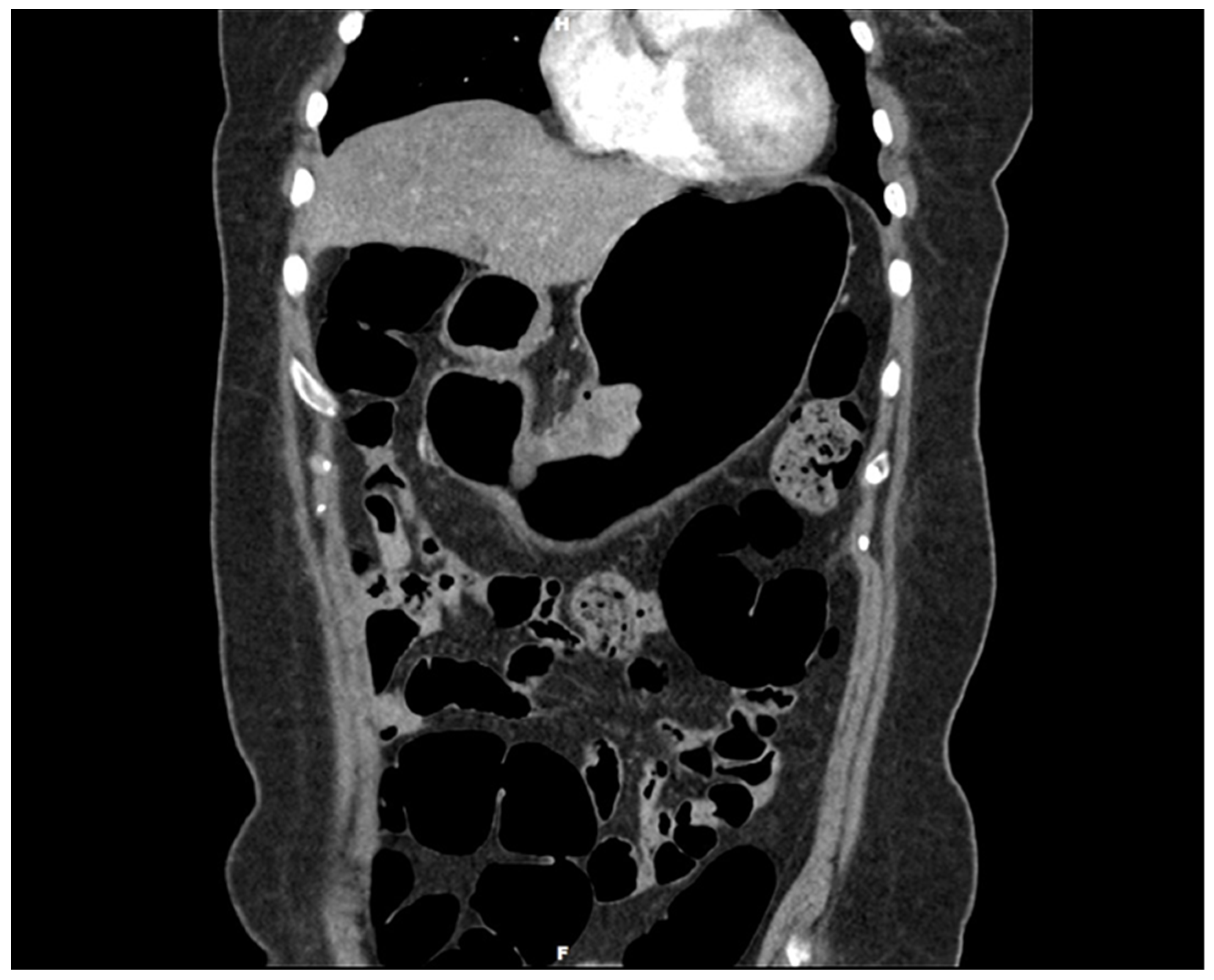
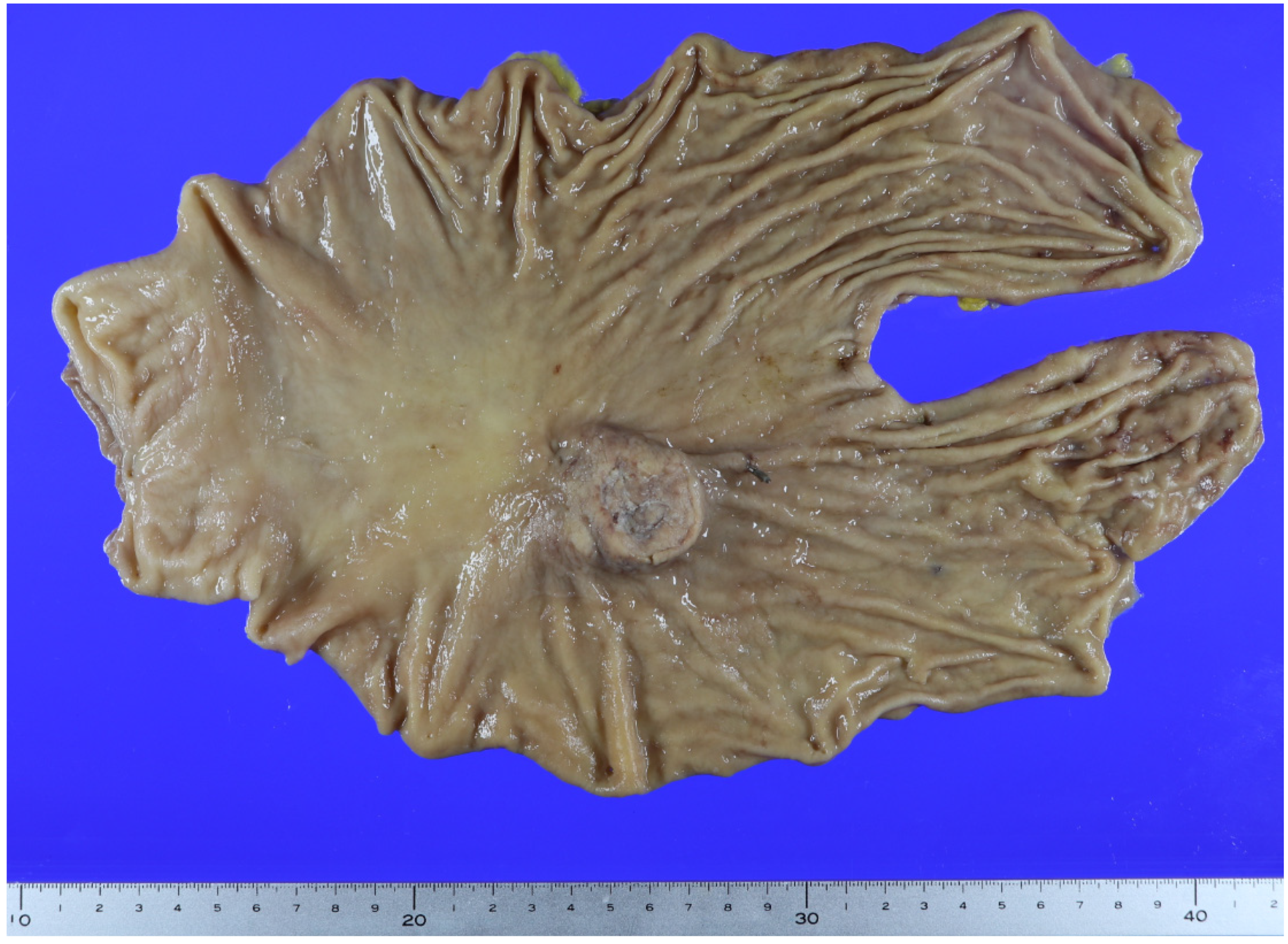


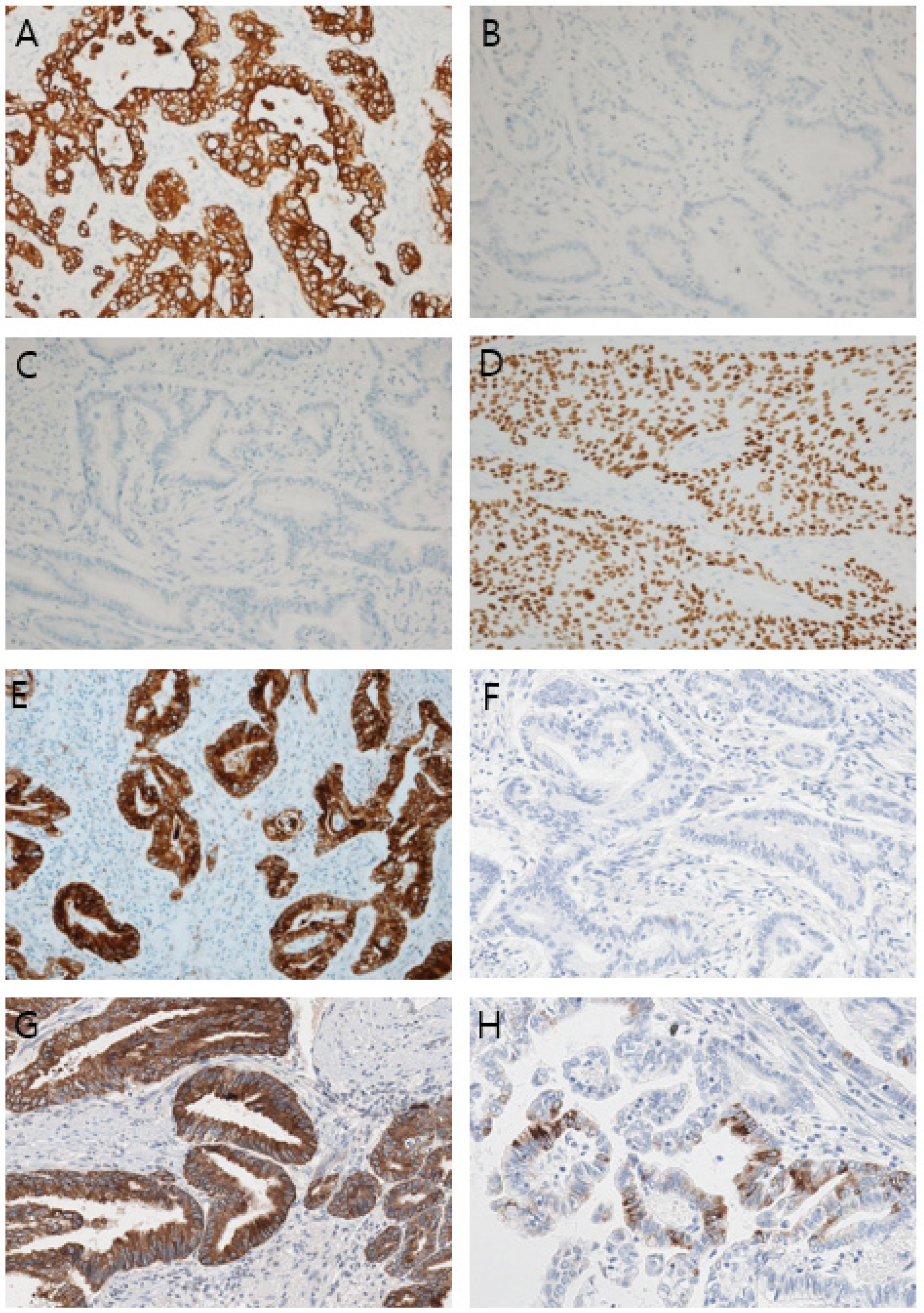
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García Nebreda, M.; Paseiro Crespo, G.; Álvaro Cifuentes, E.; Marqués Medina, E.; Burdaspal Moratilla, A. Gastric duplication cyst with respiratory epithelium: An uncommon injury that has a difficult differential diagnosis. Cir. Esp. (Engl. Ed.) 2019, 97, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, S.; Monma, H.; Sakamoto, Y.; Watanabe, T.; Fujimoto, M. Adenocarcinoma arising from a gastric duplication cyst with lymph node metastasis. Cureus 2020, 12, e12320. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.S.; Nam, E.S.; Shin, H.S. Foregut duplication cyst of the stomach. Pathol. Int. 2000, 50, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Rajdeo, H.; Bhuta, K.; Savino, J.A. Gastric duplication cyst: Two case reports and review of the literature. Case Rep. Surg. 2013, 2013, 605059. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.A.M.; Al Saeed, M.; Ameer Alshaikh, S.; Nabar, U.J. Adenocarcinoma arising from a gastric duplication cyst: A case report and literature review. Int. Med. Case Rep. J. 2017, 10, 367–372. [Google Scholar] [CrossRef]
- Chan, B.P.H.; Hyrcza, M.; Ramsay, J.; Tse, F. Adenocarcinoma Arising from a Gastric Duplication Cyst. ACG Case Rep. J. 2018, 5, e42. [Google Scholar] [CrossRef]
- Kaneko, T.; Ohara, M.; Okamura, K.; Fujiwara-Kuroda, A.; Miyasaka, D.; Yamabuki, T.; Takahashi, R.; Komuro, K.; Suzuoki, M.; Iwashiro, N.; et al. Adenocarcinoma arising from an ectopic pancreas in the duodenum: A case report. Surg. Case Rep. 2019, 5, 126–128. [Google Scholar] [CrossRef]
- Kang, H.J.; Jang, S.J.; Park, Y.S. Adenocarcinoma arising in gastric duplication cyst. Korean J. Pathol. 2014, 48, 159–161. [Google Scholar] [CrossRef]
- Losefsky, Q.P.; Cho, E.; Jeyarajah, D.R. Adenocarcinoma arising in a gastric duplication cyst. J. Gastrointest. Surg. 2022, 26, 1336–1337. [Google Scholar] [CrossRef]
- Barussaud, M.L.; Meurette, G.; Cassagnau, E.; Dupas, B.; Le Borgne, J. Mixed adenocarcinoma and squamous cell carcinoma arising in a gastric duplication cyst. Gastroenterol. Clin. Biol. 2008, 32, 188–191. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, G.H. Collision tumor arising from a gastric duplication cyst. Gastrointest. Endosc. 2017, 86, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; You, L.; Dai, M.; Zhao, Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J. Cell. Mol. Med. 2020, 24, 10279–10289. [Google Scholar] [CrossRef] [PubMed]
- Puligandla, P.S.; Nguyen, L.T.; St-Vil, D.; Flageole, H.; Bensoussan, A.L.; Nguyen, V.H.; Laberge, J.M. Gastrointestinal duplications. J. Pediatr. Surg. 2003, 38, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, K.; Nakayama, H.; Kagawa, T.; Ichikawa, T.; Yasui, W. Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: A case report with literature review. J. Clin. Pathol. 2004, 57, 428–431. [Google Scholar] [CrossRef]
- Van Pham, N.; Van Mai, D.; Duong, P.D.T.; Lam, H.H.; Ly, H.H.V.; Van Nguyen, L. Duplication cyst in adult cases: A journey from diagnosis to treatment. J. Surg. Case Rep. 2024, 2024, rjae460. [Google Scholar] [CrossRef]
- Ahmed, M.A.H.; Liyanaarachchi, K.S.; Preston, S.R.; Hewish, M.; Bagwan, I.N. Sarcomatoid carcinoma arising in a gastric duplication cyst. ACG Case Rep. J. 2021, 8, e00584. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Machicado, J.; Davogustto, G. Gastrointestinal stromal tumor arising from a gastric duplication cyst. ACG Case Rep. J. 2016, 3, 175–177. [Google Scholar] [CrossRef]
- Horne, G.; Ming-Lum, C.; Kirkpatrick, A.W.; Parker, R.L. High-grade neuroendocrine carcinoma arising in a gastric duplication cyst: A case report with literature review. Int. J. Surg. Pathol. 2007, 15, 187–191. [Google Scholar] [CrossRef]
- Rolo, A.; Oliveira, R.C.; Lima, B.; Barbosa, A.; Faustino, I. Pancreatobiliary adenocarcinoma in a gastric duplication cyst: A doubly rare diagnosis. Cureus 2021, 13, e16025. [Google Scholar] [CrossRef]
- Passos, I.D.; Chatzoulis, G.; Milias, K.; Tzoi, E.; Christoforakis, C.; Spyridopoulos, P. Gastric duplication cyst (gdc) associated with ectopic pancreas: Case report and review of the literature. Int. J. Surg. Case Rep. 2017, 31, 109–113. [Google Scholar] [CrossRef]
- Cazacu, I.M.; Luzuriaga Chavez, A.A.; Nogueras Gonzalez, G.M.; Saftoiu, A.; Bhutani, M.S. Malignant transformation of ectopic pancreas. Dig. Dis. Sci. 2019, 64, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Jäger, D.; Büchler, M.W.; Strobel, O.; Hackert, T.; Palmer, D.H.; Neoptolemos, J.P. Chemotherapy for pancreatic cancer. Presse Med. 2019, 48, e159–e174. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 2017, CD004064. [Google Scholar] [CrossRef]
- Wld, A.T.; Dholakia, A.S.; Fan, K.Y.; Kumar, R.; Moningi, S.; Rosati, L.M.; Laheru, D.A.; Zheng, L.; De Jesus-Acosta, A.; Ellsworth, S.G.; et al. Efficacy of platinum chemotherapy agents in the adjuvant setting for adenosquamous carcinoma of the pancreas. J. Gastrointest. Oncol. 2015, 6, 115–125. [Google Scholar] [CrossRef]
- Li, H.S.; Liu, X.; Zhang, M.Y.; Cheng, K.; Chen, Y.; Zhou, Y.W.; Liu, J.Y. Clinicopathologic characteristics, survival, and treatments for gastric adenosquamous carcinoma: A population-based study. Curr. Oncol. 2020, 27, e527–e536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Yang, J.; Song, D.; An, H.; Kim, D. Mixed Pancreatobiliary Ductal Adenocarcinoma and Squamous Cell Carcinoma Arising from an Ectopic Pancreas in a Gastric Duplication Cyst—A Rare Double Diagnosis. Diagnostics 2024, 14, 2727. https://doi.org/10.3390/diagnostics14232727
Kim M, Yang J, Song D, An H, Kim D. Mixed Pancreatobiliary Ductal Adenocarcinoma and Squamous Cell Carcinoma Arising from an Ectopic Pancreas in a Gastric Duplication Cyst—A Rare Double Diagnosis. Diagnostics. 2024; 14(23):2727. https://doi.org/10.3390/diagnostics14232727
Chicago/Turabian StyleKim, Minhye, Jungwook Yang, Daehyun Song, Hyojung An, and Dongchul Kim. 2024. "Mixed Pancreatobiliary Ductal Adenocarcinoma and Squamous Cell Carcinoma Arising from an Ectopic Pancreas in a Gastric Duplication Cyst—A Rare Double Diagnosis" Diagnostics 14, no. 23: 2727. https://doi.org/10.3390/diagnostics14232727
APA StyleKim, M., Yang, J., Song, D., An, H., & Kim, D. (2024). Mixed Pancreatobiliary Ductal Adenocarcinoma and Squamous Cell Carcinoma Arising from an Ectopic Pancreas in a Gastric Duplication Cyst—A Rare Double Diagnosis. Diagnostics, 14(23), 2727. https://doi.org/10.3390/diagnostics14232727




