Circulating MicroRNAs as Biomarkers for the Early Diagnosis of Lung Cancer and Its Differentiation from Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Subjects
2.3. Plasma Preparation and RNA Isolation
2.4. Obtaining cDNA and Quantitative PCR
2.5. Study Design
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Compared Groups
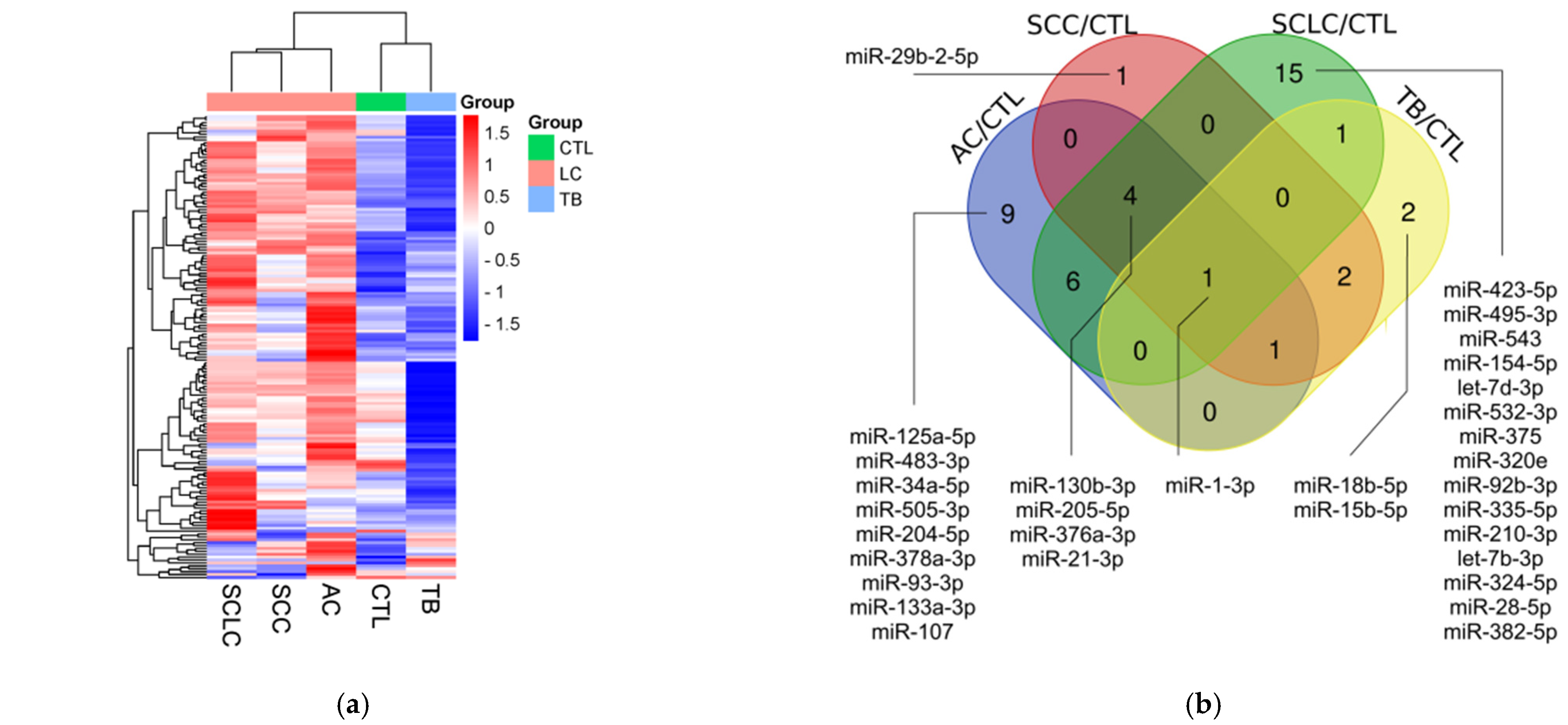
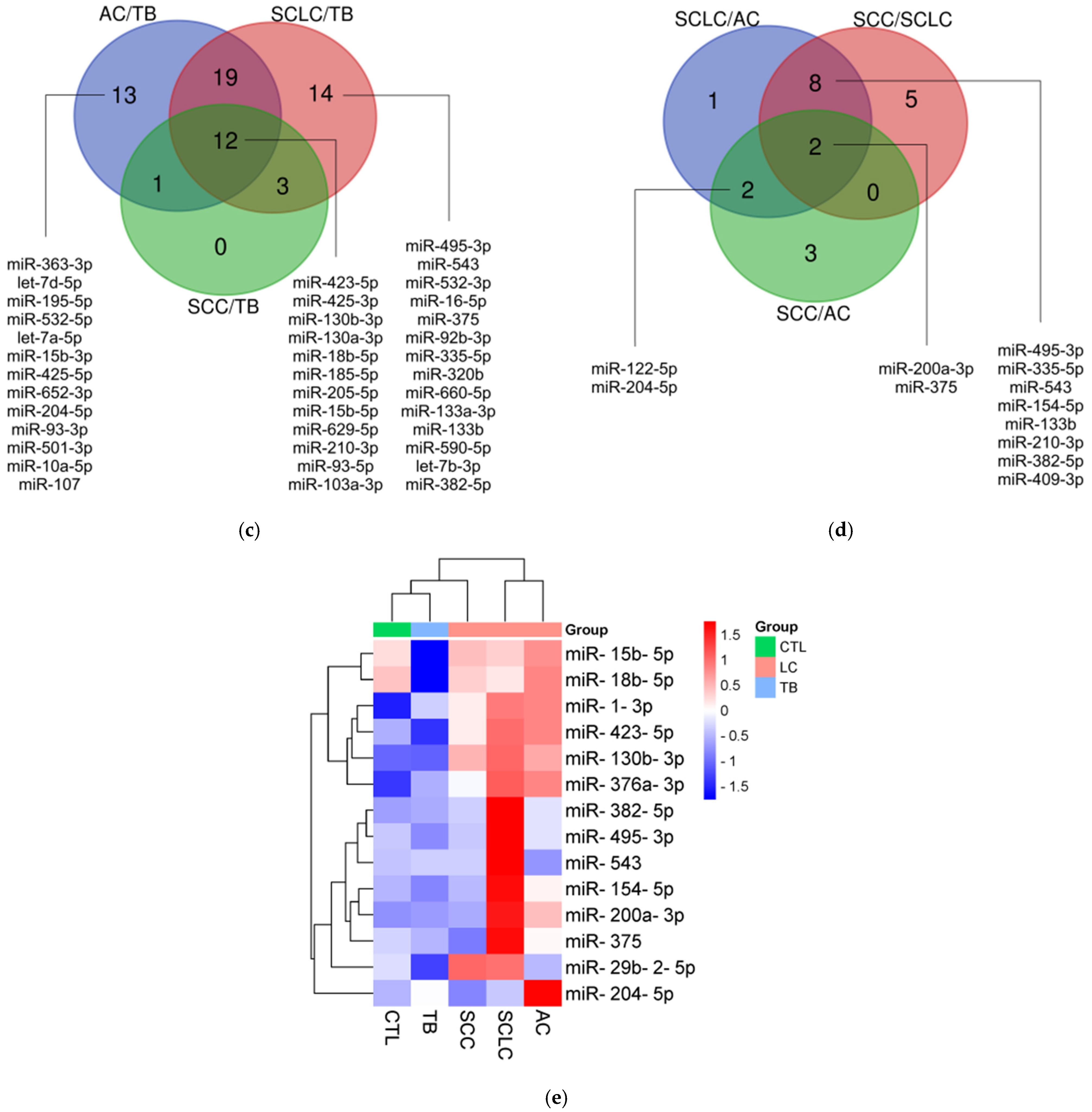
3.2. miRNA Profiling
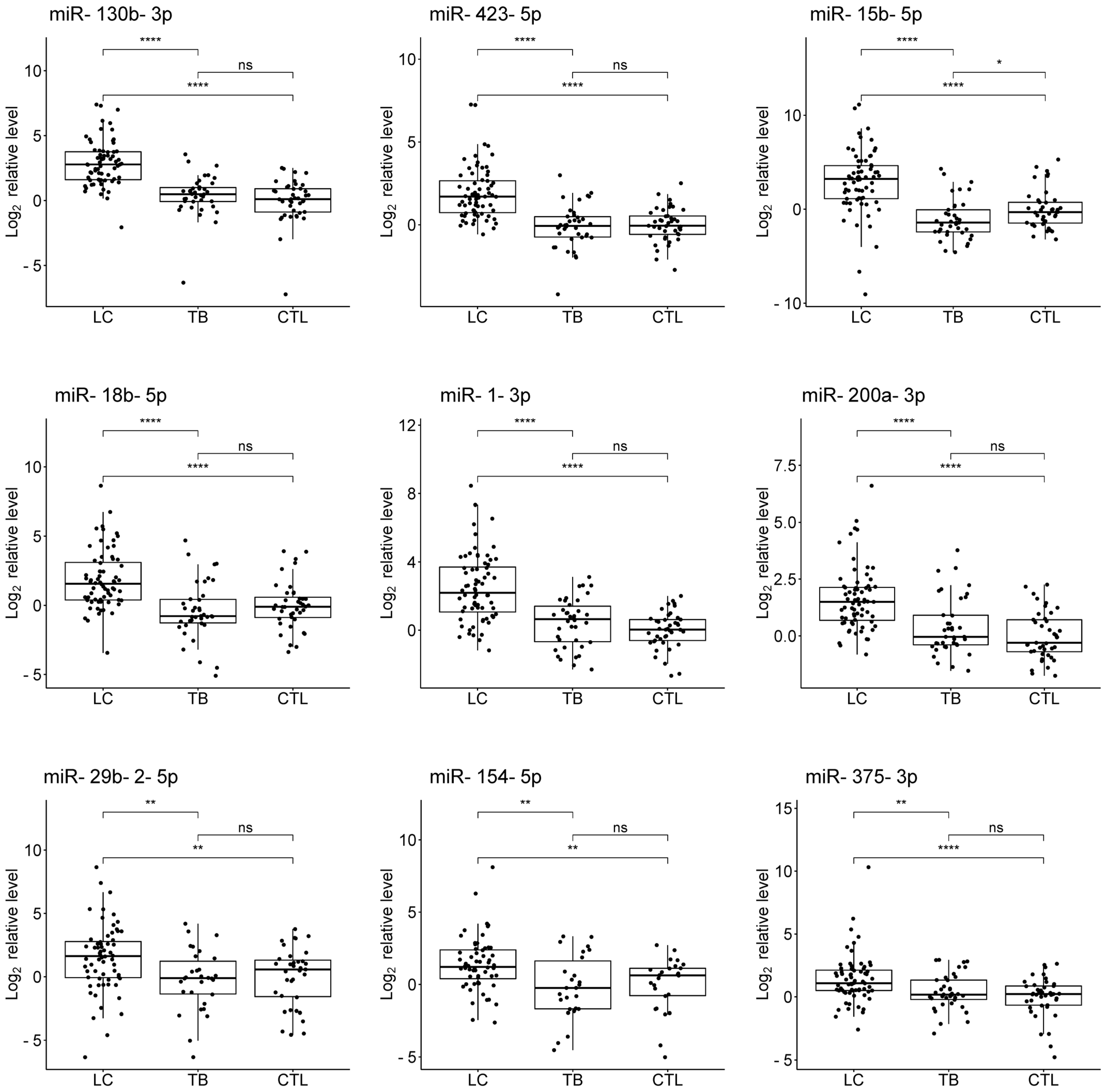
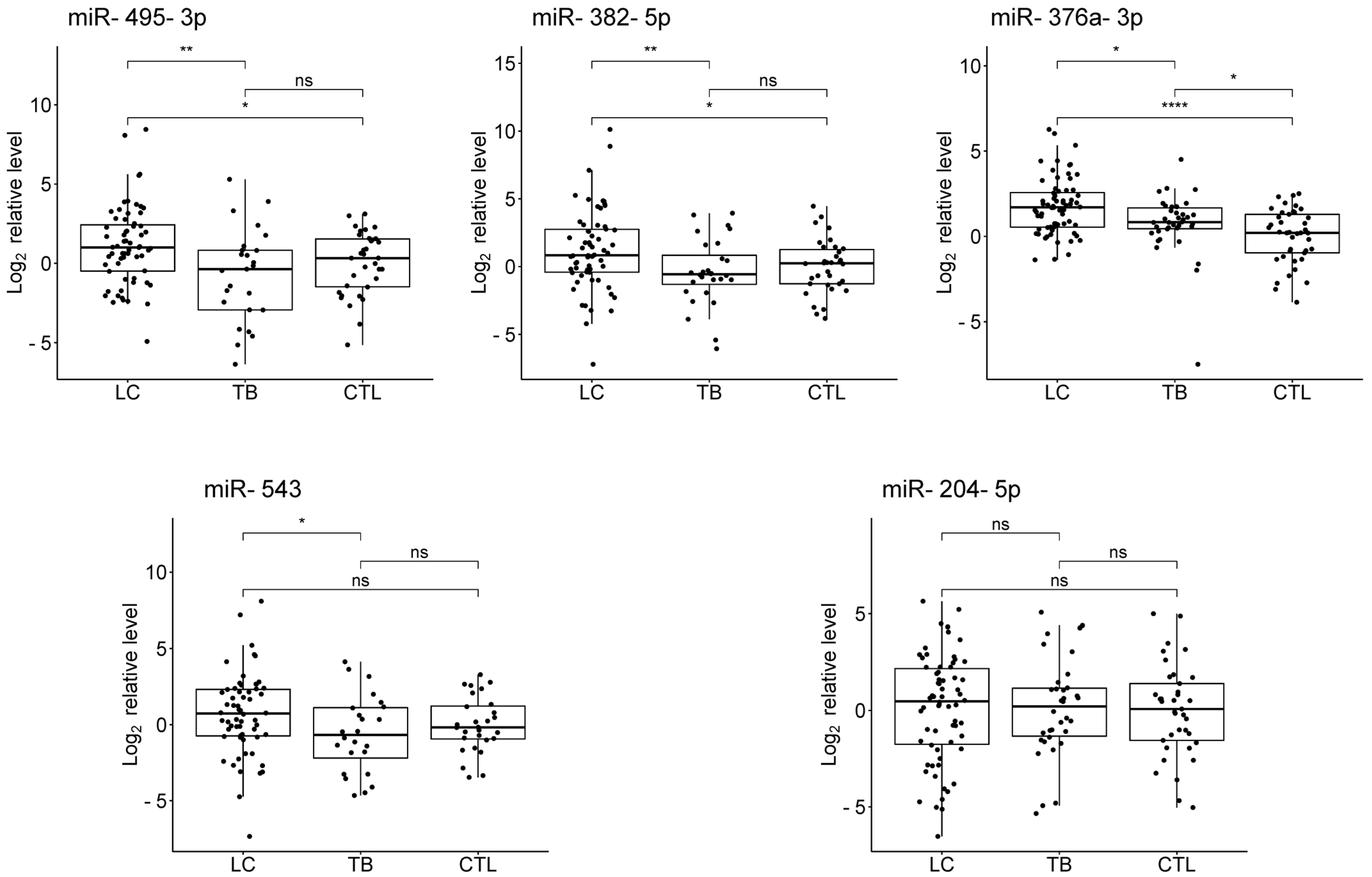
3.3. Validation
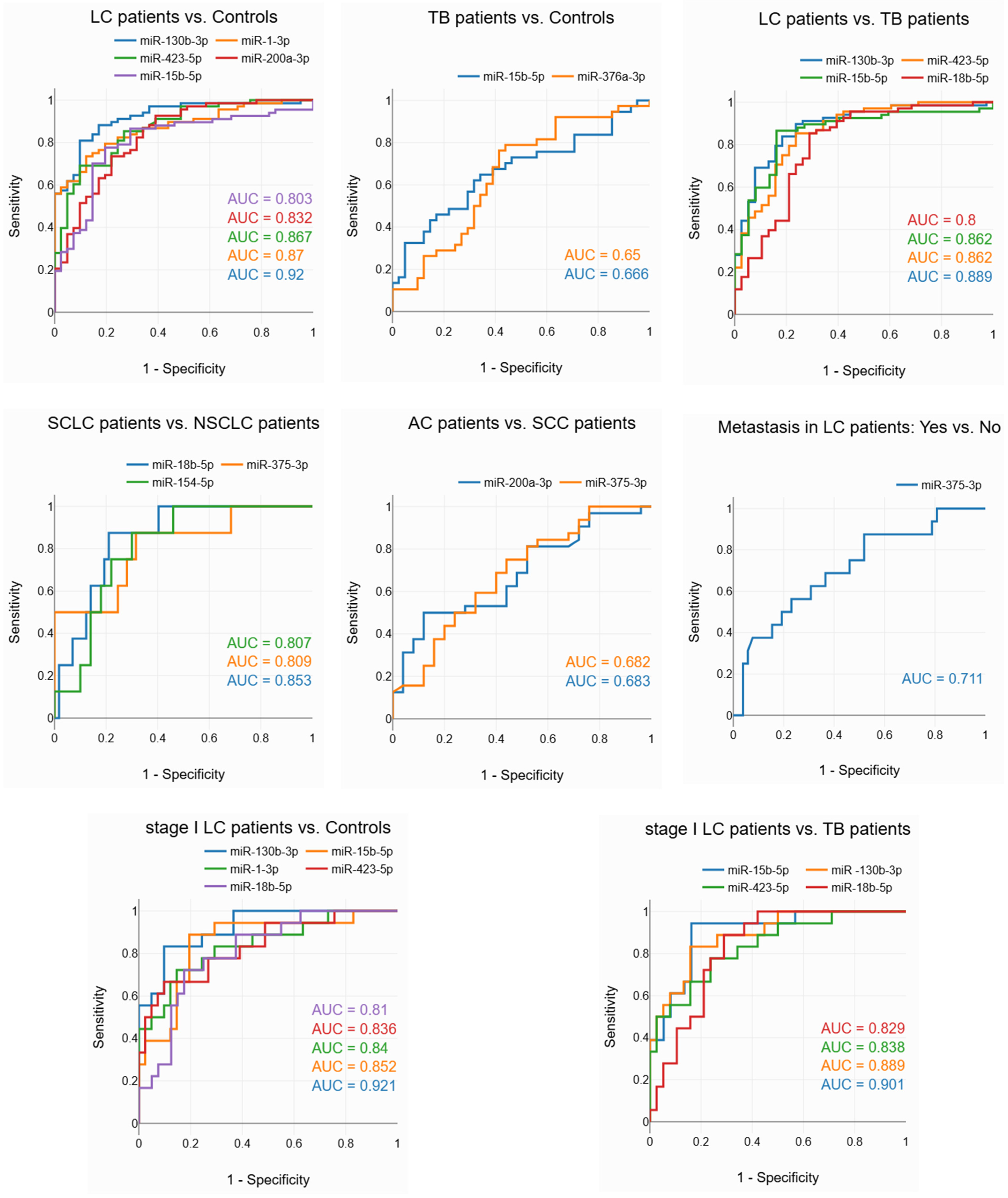
3.4. Evaluation of the Diagnostic Potential of miRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023, 1st ed.; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Pai, M.; Nicol, M.P.; Boehme, C.C. Tuberculosis Diagnostics: State of the Art and Future Directions. Microbiol. Spectr. 2016, 4, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.M.; Finn, S. The Liquid Biopsy for Lung Cancer: State of the Art, Limitations and Future Developments. Cancers 2021, 13, 3923. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, C.; He, Y.; Zhang, Q. Cancer or Tuberculosis: A Comprehensive Review of the Clinical and Imaging Features in Diagnosis of the Confusing Mass. Front. Oncol. 2021, 11, 644150. [Google Scholar] [CrossRef] [PubMed]
- Preda, M.; Tănase, B.C.; Zob, D.L.; Gheorghe, A.S.; Lungulescu, C.V.; Dumitrescu, E.A.; Stănculeanu, D.L.; Manolescu, L.S.C.; Popescu, O.; Ibraim, E.; et al. The Bidirectional Relationship between Pulmonary Tuberculosis and Lung Cancer. Int. J. Environ. Res. Public Health 2023, 20, 1282. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.-X.; Fang, G.; Huang, Y.; Hu, C.-M.; Chen, W. Misdiagnosis of a Multi-Organ Involvement Hematogenous Disseminated Tuberculosis as Metastasis: A Case Report and Literature Review. Infect. Dis. Poverty 2020, 9, 66. [Google Scholar] [CrossRef]
- Varol, Y.; Varol, U.; Unlu, M.; Kayaalp, I.; Ayranci, A.; Dereli, M.S.; Guclu, S.Z. Primary Lung Cancer Coexisting with Active Pulmonary Tuberculosis. Int. J. Tuberc. Lung Dis. 2014, 18, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Moya, J.M.; Vilella, F.; Simón, C. MicroRNA: Key Gene Expression Regulators. Fertil. Steril. 2014, 101, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Mott, J. Overview of MicroRNA Biology. Semin. Liver Dis. 2015, 35, 003–011. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Y.; Fu, B.; Guo, D.; Zaky, M.Y.; Lin, X.; Wu, H. MicroRNAs as Immune Regulators and Biomarkers in Tuberculosis. Front. Immunol. 2022, 13, 1027472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Fan, S.; Li, Y.; Wei, L.; Yang, X.; Jiang, T.; Chen, Z.; Wang, C.; Liu, J.; et al. Screening and Identification of Six Serum microRNAs as Novel Potential Combination Biomarkers for Pulmonary Tuberculosis Diagnosis. PLoS ONE 2013, 8, e81076. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, X.; Li, C.; Yang, T.; Wang, J.; Pan, L.; Jia, H.; Li, Z.; Sun, Q.; Yue, L.; et al. Small RNA Profiles of Serum Exosomes Derived From Individuals With Latent and Active Tuberculosis. Front. Microbiol. 2019, 10, 1174. [Google Scholar] [CrossRef]
- Ndzi, E.N.; Nkenfou, C.N.; Mekue, L.M.; Zentilin, L.; Tamgue, O.; Pefura, E.W.Y.; Kuiaté, J.-R.; Giacca, M.; Ndjolo, A. MicroRNA Hsa-miR-29a-3p Is a Plasma Biomarker for the Differential Diagnosis and Monitoring of Tuberculosis. Tuberculosis 2019, 114, 69–76. [Google Scholar] [CrossRef]
- Tu, H.; Yang, S.; Jiang, T.; Wei, L.; Shi, L.; Liu, C.; Wang, C.; Huang, H.; Hu, Y.; Chen, Z.; et al. Elevated Pulmonary Tuberculosis Biomarker miR-423-5p Plays Critical Role in the Occurrence of Active TB by Inhibiting Autophagosome-Lysosome Fusion. Emerg. Microbes Infect. 2019, 8, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Nishimura, T.; Nishio, K.; Kohsaka, A.; Tamizu, E.; Nakano, Y.; Kagyo, J.; Nakajima, Y.; Arai, R.; Hasegawa, H.; et al. Potential Biomarker Enhancing the Activity of Tuberculosis, Hsa-miR-346. Tuberculosis 2021, 129, 102101. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Liao, P. Diagnostic Performance of microRNA-29a in Active Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Clinics 2023, 78, 100290. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.-M.; Wei, L.-L.; Shi, L.-Y.; Pan, Z.-F.; Mao, L.-G.; Wan, X.-C.; Ping, Z.-P.; Jiang, T.-T.; Chen, Z.-L.; et al. A Group of Novel Serum Diagnostic Biomarkers for Multidrug-Resistant Tuberculosis by iTRAQ-2D LC-MS/MS and Solexa Sequencing. Int. J. Biol. Sci. 2016, 12, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.; Urhekar, A.; Modi, D. Levels of microRNA miR-16 and miR-155 Are Altered in Serum of Patients with Tuberculosis and Associate with Responses to Therapy. Tuberculosis 2017, 102, 24–30. [Google Scholar] [CrossRef]
- Barry, S.E.; Ellis, M.; Yang, Y.; Guan, G.; Wang, X.; Britton, W.J.; Saunders, B.M. Identification of a Plasma microRNA Profile in Untreated Pulmonary Tuberculosis Patients That Is Modulated by Anti-Mycobacterial Therapy. J. Infect. 2018, 77, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B. MicroRNA (miRNA) in Cancer. Cancer Cell Int. 2015, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H. Clinical Relevance of Circulating, Cell-Free and Exosomal microRNAs in Plasma and Serum of Breast Cancer Patients. Oncol. Res. Treat. 2017, 40, 423–429. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of Exosomal miRNA on Cancer Biology and Clinical Applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Truini, A.; Nakata, A.; Lin, J.; Reddy, R.M.; Chang, A.C.; Ramnath, N.; Gotoh, N.; Beer, D.G.; Chen, G. A Novel Serum 4-microRNA Signature for Lung Cancer Detection. Sci. Rep. 2015, 5, 12464. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Krawczyk, P.; Kowalski, D.M.; Kuźnar-Kamińska, B.; Winiarczyk, K.; Olszyna-Serementa, M.; Batura-Gabryel, H.; Milanowski, J. Application of Plasma Circulating microRNA-448, 506, 4316, and 4478 Analysis for Non-Invasive Diagnosis of Lung Cancer. Tumor Biol. 2016, 37, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Karimipoor, M.; Irani, S.; Kiani, A.; Zeinali, S.; Tafsiri, E.; Sheikhy, K. Potential Circulating miRNA Signature for Early Detection of NSCLC. Cancer Genet. 2017, 216–217, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, F.; Shen, T.; Luo, Q.; Ding, Z.; Qian, L.; Huang, J. Plasma miR-145, miR-20a, miR-21 and miR-223 as Novel Biomarkers for Screening Early-Stage Non-Small Cell Lung Cancer. Oncol. Lett. 2017, 13, 669–676. [Google Scholar] [CrossRef]
- Shao, C.; Yang, F.; Qin, Z.; Jing, X.; Shu, Y.; Shen, H. The Value of miR-155 as a Biomarker for the Diagnosis and Prognosis of Lung Cancer: A Systematic Review with Meta-Analysis. BMC Cancer 2019, 19, 1103. [Google Scholar] [CrossRef]
- Tao, S.; Ju, X.; Zhou, H.; Zeng, Q. Circulating microRNA-145 as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer: A Systemic Review and Meta-Analysis. Int. J. Biol. Markers 2020, 35, 51–60. [Google Scholar] [CrossRef]
- Shen, X.; Li, L.; Zhang, L.; Liu, W.; Wu, Y.; Ma, R. Diagnostic and Prognostic Value of microRNA-486 in Patients with Lung Cancer: A Systematic Review and Meta-Analysis. Int. J. Biol. Markers 2022, 37, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Liu, C.; Zhang, X.; Wu, Y.; Diao, M.; Tan, S.; Huang, S.; Cheng, Y.; You, T. MicroRNA-21 as a Diagnostic and Prognostic Biomarker of Lung Cancer: A Systematic Review and Meta-Analysis. Biosci. Rep. 2022, 42, BSR20211653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Yang, J.; Wei, Y.; Peng, J.; Fu, C.; Huang, M.; Wang, R.; Wang, P.; Sun, G.; et al. MiR-205-5p Promotes Lung Cancer Progression and Is Valuable for the Diagnosis of Lung Cancer. Thorac. Cancer 2022, 13, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Peng, M.; Gong, J.; Li, C.; Cheng, H.; Li, Y.; Tang, Y. Circulating Exosomal microRNA-4497 as a Potential Biomarker for Metastasis and Prognosis in Non-Small-Cell Lung Cancer. Exp. Biol. Med. 2023, 248, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Dotto, G.-P. Racial Differences in Cancer Susceptibility and Survival: More Than the Color of the Skin? Trends Cancer 2017, 3, 181–197. [Google Scholar] [CrossRef]
- Tuasha, N.; Petros, B. Heterogeneity of Tumors in Breast Cancer: Implications and Prospects for Prognosis and Therapeutics. Scientifica 2020, 2020, 4736091. [Google Scholar] [CrossRef] [PubMed]
- Nassar, F.J.; Talhouk, R.; Zgheib, N.K.; Tfayli, A.; El Sabban, M.; El Saghir, N.S.; Boulos, F.; Jabbour, M.N.; Chalala, C.; Boustany, R.-M.; et al. microRNA Expression in Ethnic Specific Early Stage Breast Cancer: An Integration and Comparative Analysis. Sci. Rep. 2017, 7, 16829. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guan, Z.; Cuk, K.; Zhang, Y.; Brenner, H. Circulating MicroRNA Biomarkers for Lung Cancer Detection in East Asian Populations. Cancers 2019, 11, 415. [Google Scholar] [CrossRef]
- Königshoff, M.; Kramer, M.; Balsara, N.; Wilhelm, J.; Amarie, O.V.; Jahn, A.; Rose, F.; Fink, L.; Seeger, W.; Schaefer, L.; et al. WNT1-Inducible Signaling Protein–1 Mediates Pulmonary Fibrosis in Mice and Is Upregulated in Humans with Idiopathic Pulmonary Fibrosis. J. Clin. Investig. 2009, 119, 772–787. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Bioinformatics & Evolutionary Genomics: Calculate and Draw Custom Venn Diagrams. Available online: http://bioinformatics.psb.ugent.be/webtools/Venn (accessed on 29 October 2024).
- Jamovi. Available online: https://www.jamovi.org/ (accessed on 29 October 2024).
- FDR Online Calculator. Available online: https://www.sdmproject.com/utilities/?show=FDR (accessed on 29 October 2024).
- Center for Diagnostics & Telemedicine: Web Tool for ROC Analysis. Available online: https://telemedai.ru/en/nauka/razrabotki/veb-instrument-dlya-roc-analiza (accessed on 25 November 2024).
- Statistics Kingdom: ROC Calculator. Available online: https://www.statskingdom.com/roc-calculator.html (accessed on 29 October 2024).
- Muller, M.P.; Tomlinson, G.; Marrie, T.J.; Tang, P.; McGeer, A.; Low, D.E.; Detsky, A.S.; Gold, W.L. Can Routine Laboratory Tests Discriminate between Severe Acute Respiratory Syndrome and Other Causes of Community-Acquired Pneumonia? Clin. Infect. Dis. 2005, 40, 1079–1086. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Li, Z.; Zhu, Y.; Liu, F.; Cai, J. CircDENND2D Inhibits PD-L1-Mediated Non-Small Cell Lung Cancer Metastasis and Immune Escape by Regulating miR-130b-3p/STK11 Axis. Biochem. Genet. 2023, 61, 2691–2709. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yan, J.; Song, T.; Zhong, C.; Kuang, J.; Mo, Y.; Tan, J.; Li, D.; Sui, Z.; Cai, K.; et al. microRNA-130b-3p Contained in MSC-Derived EVs Promotes Lung Cancer Progression by Regulating the FOXO3/NFE2L2/TXNRD1 Axis. Mol. Ther. Oncolytics 2021, 20, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, C.; Yang, Z.; Liu, W.; Yuan, Y.; Li, K.; Zhang, Y.; Wang, Y.; Shi, Y.; Qiu, Y.; et al. Dysregulated Sp1/miR-130b-3p/HOXA5 Axis Contributes to Tumor Angiogenesis and Progression of Hepatocellular Carcinoma. Theranostics 2020, 10, 5209–5224. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Y.; Chen, Y.; Guo, Y.; Li, Q.; Wei, X. Exosomal miR-130b-3p Promotes Progression and Tubular Formation Through Targeting PTEN in Oral Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 616306. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, P.; Li, D.; Wang, Z.; Ding, Z.; Zhou, M.; Chen, X.; Miao, M.; Ding, J.; Lin, W.; et al. STAT3/miR-130b-3p/MBNL1 Feedback Loop Regulated by mTORC1 Signaling Promotes Angiogenesis and Tumor Growth. J. Exp. Clin. Cancer Res. 2022, 41, 297. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xie, F.; Wei, H.; Cui, D. Identification of Key Circulating Exosomal microRNAs in Gastric Cancer. Front. Oncol. 2021, 11, 693360. [Google Scholar] [CrossRef]
- Song, D.; Zhang, Q.; Zhang, H.; Zhan, L.; Sun, X. MiR-130b-3p Promotes Colorectal Cancer Progression by Targeting CHD9. Cell Cycle 2022, 21, 585–601. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Peng, X.; Liu, K.; Zhang, C.; Chen, X.; Han, Y.; Lai, Y. Evaluation of miR-130 Family Members as Circulating Biomarkers for the Diagnosis of Bladder Cancer. J. Clin. Lab. Anal. 2020, 34, e23517. [Google Scholar] [CrossRef]
- Liang, H.; Lu, Y.; Huang, X.; Ye, T. LncRNA BRCAT54 Is Downregulated and Inhibits Cancer Cell Proliferation by Downregulating miR-130b-3p through Methylation in Prostate Cancer. J. Biochem. Mol. Toxicol. 2024, 38, e23552. [Google Scholar] [CrossRef]
- Lv, M.; Li, X.; Zheng, C.; Tian, W.; Yang, H.; Yin, Z.; Zhou, B. Exosomal miR-130b-3p Suppresses Metastasis of Non-Small Cell Lung Cancer Cells by Targeting DEPDC1 via TGF-β Signaling Pathway. Int. J. Biol. Macromol. 2024, 275, 133594. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, G. MiR-423-5p Aggravates Lung Adenocarcinoma via Targeting CADM1. Thorac. Cancer 2021, 12, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, J.; Dang, Y.; Xu, S. LncRNA PGM5-AS1 Inhibits Non-Small Cell Lung Cancer Progression by Targeting miRNA-423-5p/SLIT2 Axis. Cancer Cell Int. 2024, 24, 216. [Google Scholar] [CrossRef]
- Sun, G.; Ding, X.; Bi, N.; Wu, L.; Wang, J.; Zhang, W.; Dong, X.; Lv, N.; Song, Y.; Zhan, Q.; et al. MiR-423-5p in Brain Metastasis: Potential Role in Diagnostics and Molecular Biology. Cell Death Dis. 2018, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cai, W.; Zhou, T.; Men, B.; Chen, S.; Tu, D.; Guo, W.; Wang, J.; Zhao, F.; Wang, Y. CEACAM1 Increased the Lymphangiogenesis through miR-423-5p and NF- kB in Non-Small Cell Lung Cancer. Biochem. Biophys. Rep. 2024, 40, 101833. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B.; Jia, Y.; Shi, H.; Wang, H.; Guo, Q.; Li, H. LncRNA LOXL1-AS1 Regulates the Tumorigenesis and Development of Lung Adenocarcinoma through Sponging miR-423-5p and Targeting MYBL2. Cancer Med. 2020, 9, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Yun, S.; Shin, S.-H.; Youn, D.H.; Son, G.-H.; Lee, J.J.; Hong, J.Y. Tuberculous Pleural Effusion-Derived Exosomal miR-130b-3p and miR-423-5p Promote the Proliferation of Lung Cancer Cells via Cyclin D1. Int. J. Mol. Sci. 2024, 25, 10119. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, A.R.; Bjaanæs, M.; LeBlanc, M.; Holm, A.M.; Bolstad, N.; Rubio, L.; Peñalver, J.C.; Cervera, J.; Mojarrieta, J.C.; López-Guerrero, J.A.; et al. A unique set of 6 circulating microRNAs for early detection of non-small cell lung cancer. Oncotarget 2016, 7, 37250–37259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, K.; Zha, Y.; Chen, D.; He, J.; Ma, H.; Liu, X.; Le, H.; Zhang, Y. Diagnostic Value of Serum miR-182, miR-183, miR-210, and miR-126 Levels in Patients with Early-Stage Non-Small Cell Lung Cancer. PLoS ONE 2016, 11, e0153046. [Google Scholar] [CrossRef]
- Kim, D.J.; Linnstaedt, S.; Palma, J.; Park, J.C.; Ntrivalas, E.; Kwak-Kim, J.Y.; Gilman-Sachs, A.; Beaman, K.; Hastings, M.L.; Martin, J.N.; et al. Plasma components affect accuracy of circulating cancer-related microRNA quantitation. J. Mol. Diagn. 2012, 14, 71–80. [Google Scholar] [CrossRef]
| Characteristics | LC Patients | TB Patients | Healthy Controls | |||
|---|---|---|---|---|---|---|
| Profiling | Validation | Profiling | Validation | Profiling | Validation | |
| Total | 29 | 68 | 10 | 38 | 10 | 41 |
| Male/female | 25/4 | 47/21 | 8/2 | 17/21 | 8/2 | 30/11 |
| Kazakhs/other ethnic groups * | 25/4 | 56/12 | 8/2 | 34/4 | 10/0 | 39/2 |
| Non-smokers/smokers | 9/20 | 36/32 | 6/4 | 34/4 | 5/5 | 27/14 |
| DS-TB/DR-TB | - | - | 9/1 | 28/10 | - | - |
| AC 1/SCC 2/SCLC 3/SA 4/AtC 5 | 10/10/9/0/0 | 32/25/8/2/1 | - | - | - | - |
| Tumour stage (I/II/III/IV) | 1/1/18/9 | 18/8/25/17 | - | - | - | - |
| Age, years (mean ± SD) | 62.0 ± 8.1 | 59. 7 ± 11.5 | 47.5 ± 13.3 | 36.5 ± 17.3 | 49.2 ± 6.5 | 58.5 ± 8.1 |
| miRNA | LC Patients vs. TB Patients | LC Patients vs. Controls | TB Patients vs. Controls | |||
|---|---|---|---|---|---|---|
| Log2FC (95% CI) | p-Value | Log2FC (95% CI) | p-Value | Log2FC (95% CI) | p-Value | |
| miR-130b-3p | 2.30 (1.72; 2.86) | 3.8 × 10−11 * | 2.66 (2.09; 3.25) | 2.6 × 10−13 * | 0.34 (−0.19; 0.88) | 0.188 |
| miR-423-5p | 1.80 (1.30; 2.35) | 7.4 × 10−10 * | 1.69 (1.23; 2.20) | 1.5 × 10−10 * | −0.10 (−0.57; 0.38) | 0.700 |
| miR-15b-5p | 4.31 (3.22; 5.26) | 1.2 × 10−9 * | 3.20 (2.19; 4.07) | 1.3 × 10−7 * | −1.13 (−1.95; −0.29) | 0.012 * |
| miR-18b-5p | 2.05 (1.37; 2.83) | 3.5 × 10−7 * | 1.67 (1.00; 2.40) | 2.2 × 10−6 * | −0.48 (−1.11; 0.28) | 0.193 |
| miR-1-3p | 1.89 (1.19; 2.63) | 7.4 × 10−7 * | 2.29 (1.64; 2.95) | 1.1 × 10−10 * | 0.46 (−0.18; 1.04) | 0.118 |
| miR-200a-3p | 1.24 (0.73; 1.71) | 8.1 × 10−6 * | 1.54 (1.09; 1.99) | 7.1 × 10−9 * | 0.31 (−0.14; 0.80) | 0.167 |
| miR-29b-2-5p | 1.74 (0.57; 2.69) | 0.0028 * | 1.33 (0.37; 2.36) | 0.0099 * | −0.46 (−1.40; 0.95) | 0.517 |
| miR-154-5p | 1.56 (0.60; 2.44) | 0.0029 * | 1.06 (0.34; 1.88) | 0.0045 * | −0.32 (−1.52; 0.99) | 0.605 |
| miR-375-3p | 0.87 (0.33; 1.48) | 0.0031 * | 1.10 (0.53; 1.67) | 5.7 × 10−5 * | 0.16 (−0.32; 0.85) | 0.555 |
| miR-495-3p | 1.77 (0.46; 3.07) | 0.0081 * | 0.90 (0.00; 1.89) | 0.049 | −0.78 (−2.17; 0.56) | 0.255 |
| miR-382-5p | 1.47 (0.37; 2.64) | 0.0084 * | 1.12 (0.11; 2.10) | 0.032 | −0.57 (−1.41; 0.74) | 0.523 |
| miR-376a-3p | 0.76 (0.17; 1.33) | 0.010 * | 1.57 (0.95; 2.26) | 3.1 × 10−6 * | 0.69 (0.14; 1.46) | 0.021 * |
| miR-543 | 1.30 (0.14; 2.59) | 0.036 | 0.69 (−0.28; 1.73) | 0.166 | −0.66 (−1.92; 0.70) | 0.348 |
| miR-204-5p | 0.15 (−1.05; 1.29) | 0.791 | 0.24 (−0.85; 1.31) | 0.658 | 0.10 (−1.07; 1.20) | 0.836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashirbekov, Y.; Khamitova, N.; Satken, K.; Abaildayev, A.; Pinskiy, I.; Yeleussizov, A.; Yegenova, L.; Kairanbayeva, A.; Kadirshe, D.; Utegenova, G.; et al. Circulating MicroRNAs as Biomarkers for the Early Diagnosis of Lung Cancer and Its Differentiation from Tuberculosis. Diagnostics 2024, 14, 2684. https://doi.org/10.3390/diagnostics14232684
Ashirbekov Y, Khamitova N, Satken K, Abaildayev A, Pinskiy I, Yeleussizov A, Yegenova L, Kairanbayeva A, Kadirshe D, Utegenova G, et al. Circulating MicroRNAs as Biomarkers for the Early Diagnosis of Lung Cancer and Its Differentiation from Tuberculosis. Diagnostics. 2024; 14(23):2684. https://doi.org/10.3390/diagnostics14232684
Chicago/Turabian StyleAshirbekov, Yeldar, Nazgul Khamitova, Kantemir Satken, Arman Abaildayev, Ilya Pinskiy, Askar Yeleussizov, Laura Yegenova, Anargul Kairanbayeva, Danara Kadirshe, Gulzhakhan Utegenova, and et al. 2024. "Circulating MicroRNAs as Biomarkers for the Early Diagnosis of Lung Cancer and Its Differentiation from Tuberculosis" Diagnostics 14, no. 23: 2684. https://doi.org/10.3390/diagnostics14232684
APA StyleAshirbekov, Y., Khamitova, N., Satken, K., Abaildayev, A., Pinskiy, I., Yeleussizov, A., Yegenova, L., Kairanbayeva, A., Kadirshe, D., Utegenova, G., Jainakbayev, N., & Sharipov, K. (2024). Circulating MicroRNAs as Biomarkers for the Early Diagnosis of Lung Cancer and Its Differentiation from Tuberculosis. Diagnostics, 14(23), 2684. https://doi.org/10.3390/diagnostics14232684







