High Serum Adrenomedullin and Mid-Regional Pro-Atrial Natriuretic Peptide Concentrations in Early Pregnancy Predict the Development of Gestational Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Measurements
2.3. Statistical Analysis
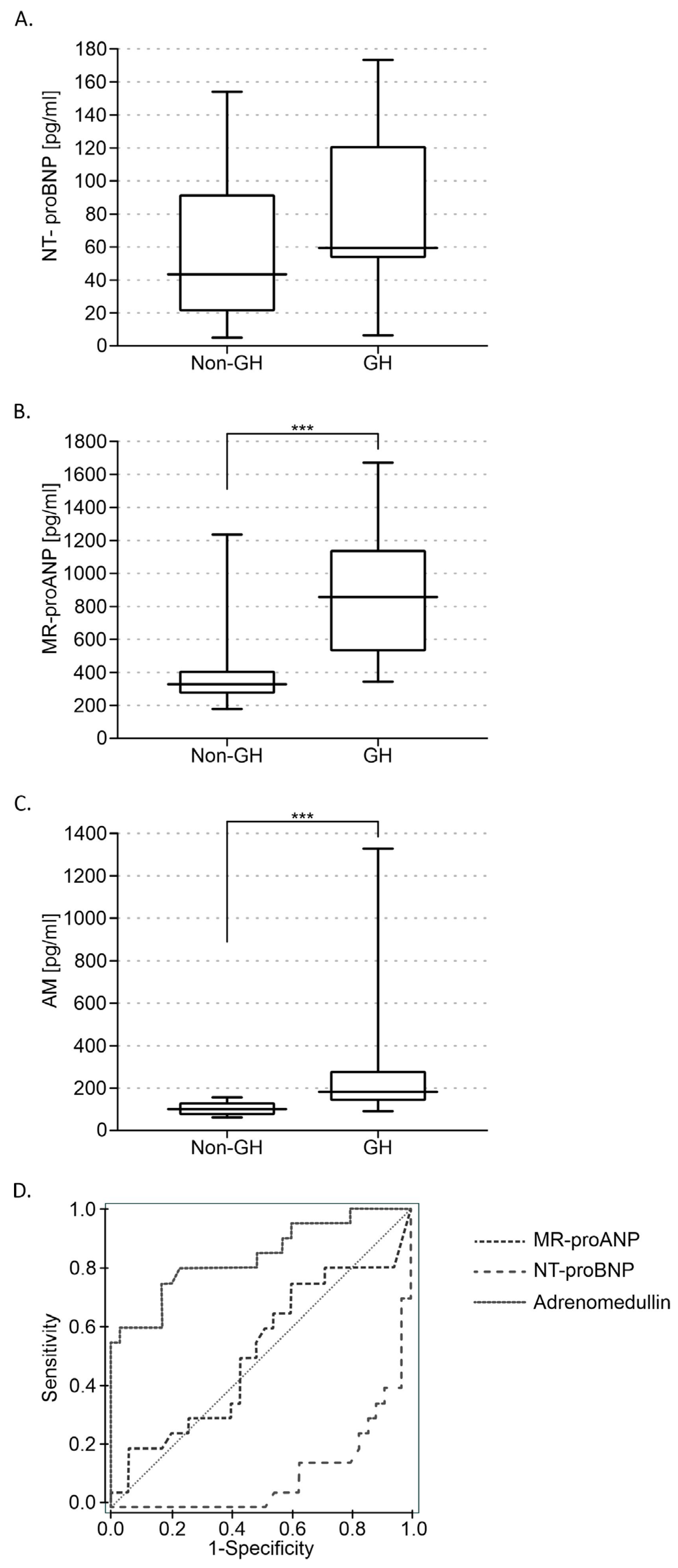
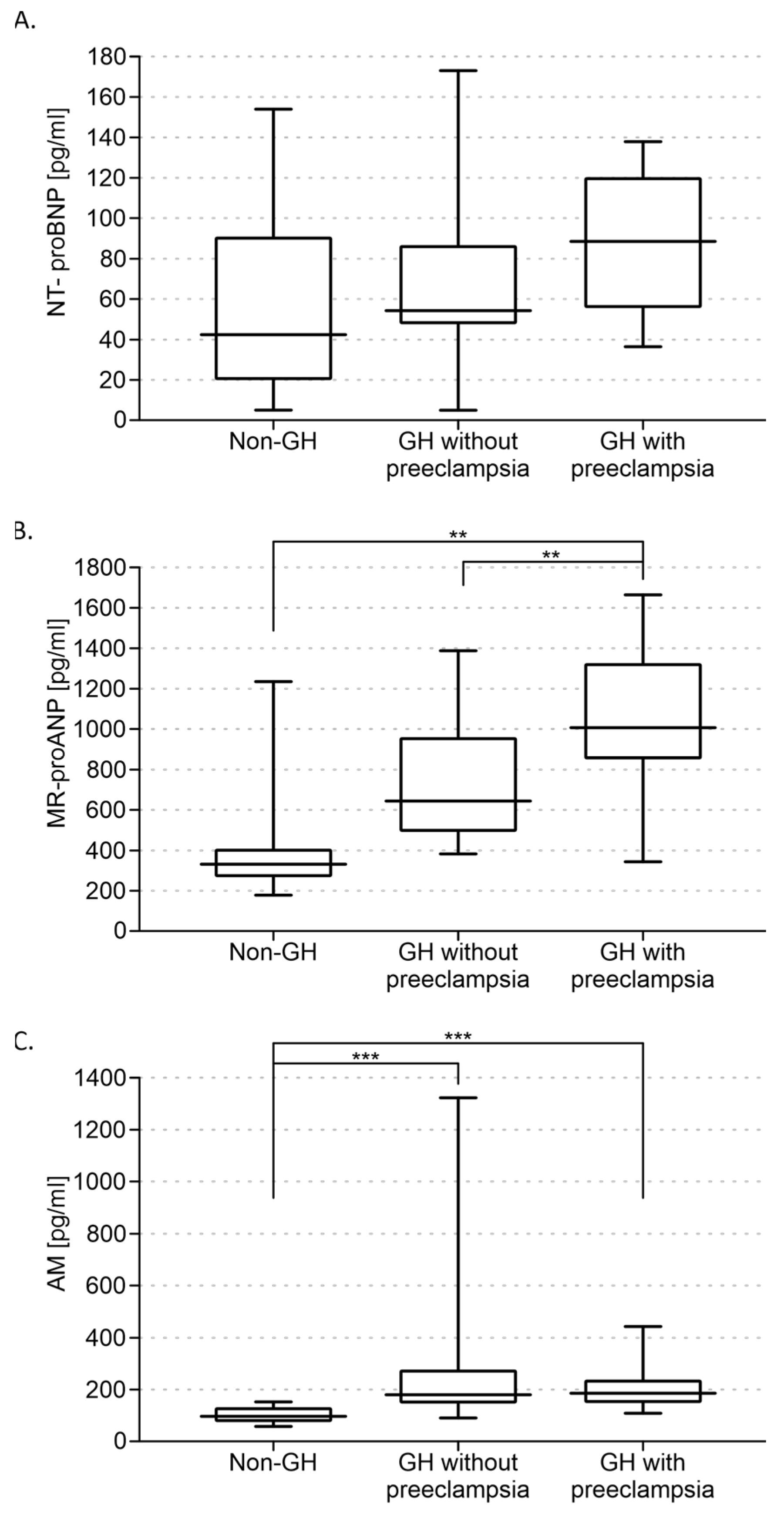
3. Results
3.1. Characteristic of the Study Group
3.2. Biochemical Measurements Results
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchbinder, A.; Sibai, B.M.; Caritis, S.; MacPherson, C.; Hauth, J.; Lindheimer, M.D.; Klebanoff, M.; VanDorsten, P.; Landon, M.; Paul, R.; et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am. J. Obstet. Gynecol. 2002, 186, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hauth, J.C.; Ewell, M.G.; Levine, R.J.; Esterlitz, J.R.; Sibai, B.; Curet, L.B.; Catalano, P.M.; Morris, C.D.; Calcium for Preeclampsia Prevention Study Group. Pregnancy outcomes in healthy nulliparas who developed hypertension. Obstet. Gynecol. 2000, 95, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Yoder, S.R.; Thornburg, L.L.; Bisognano, J.D. Hypertension in Pregnancy and Women of Childbearing Age. Am. J. Med. 2009, 122, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Gillon, T.E.; Pels, A.; von Dadelszen, P.; MacDonell, K.; Magee, L.A. Hypertensive disorders of pregnancy: A systematic review of international clinical practice guidelines. PLoS ONE 2014, 9, e113715. [Google Scholar] [CrossRef]
- Brown, M.; Magee, L.; Kenny, L.; Karumanchi, S.A.; Mccarthy, F.; Saito, S.; Hall, D.R.; Warren, C.; Adoyi, G.; Ishaku, S. The Hypertensive Disorders of Pregnancy: Isshp Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Deaton, C.; Simpson, I.; Aboyans, V.; Agewall, S.; Barbato, E.; Calda, P.; Coca, A.; Coman, I.; De Backer, J.; Delgado, V.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Gondek, A.; Jagodzińska, A.; Pietrzak, B.; Mamcarz, A.; Cudnoch-Jędrzejewska, A. Relevance of the Assessment of Natriuretic Peptide Plasma Concentrations in Hypertensive Pregnant Women. Biomarkers 2020, 25, 449–457. [Google Scholar] [CrossRef]
- Albrecht, E.D.; Pepe, G.J. Regulation of Uterine Spiral Artery Remodeling: A Review. Reprod. Sci. 2020, 27, 1932–1942. [Google Scholar] [CrossRef]
- Reslan, O.M.; Khalil, R.A. Molecular and Vascular Targets in the Pathogenesis and Management of the Hypertension Associated with Preeclampsia. Cardiovasc. Hematol. Agents Med. Chem. 2010, 8, 204–226. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Latest advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Yanagawa, B.; Nagaya, N. Adrenomedullin: Molecular mechanisms and its role in cardiac disease. Amino Acids 2007, 32, 157–164. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Fukai, N.; Sato, R.; Sugiyama, T.; Ozawa, N.; Shichiri, M.; Hirata, Y. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology 2004, 145, 3331–3337. [Google Scholar] [CrossRef]
- Saito, T.; Itoh, H.; Chun, T.H.; Fukunaga, Y.; Yamashita, J.; Doi, K.; Tanaka, T.; Inoue, M.; Masatsugu, K.; Sawada, N.; et al. Coordinate regulation of endothelin and adrenomedullin secretion by oxidative stress in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1364–H1371. [Google Scholar] [CrossRef]
- Er, H.; Doğanay, S.; Özerol, E.; Yürekli, M. Adrenomedullin and leptin levels in diabetic retinopathy and retinal diseases. Ophthalmologica 2005, 219, 107–111. [Google Scholar] [CrossRef]
- Yallampalli, C.; Chauhan, M.; Endsley, J.; Sathishkumar, K. Calcitonin gene related family peptides: Importance in normal placental and fetal development. Adv. Exp. Med. Biol. 2014, 814, 229–240. [Google Scholar] [CrossRef]
- Marinoni, E.; Scavo, D.; Letizia, C.; Cosmi, E.V. Adrenomedullin in pregnancy. Lancet 1997, 349, 328. [Google Scholar] [CrossRef]
- Di Iorio, R.; Marinoni, E.; Letizia, C.; Alo, P.; Villaccio, B.; Cosmi, E.V. Adrenomedullin, a New Vasoactive Peptide, Is Increased in Preeclampsia. Hypertension 1998, 32, 758–763. [Google Scholar] [CrossRef]
- Marinoni, E.; Di Iorio, R.; Letizia, C.; Villaccio, B.; Scucchi, L.; Cosmi, E.V. Immunoreactive adrenomedullin in human fetoplacental tissues. Am. J. Obstet. Gynecol. 1998, 179 Pt 1, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Betancourt, A.; Balakrishnan, M.; Mishra, A.; Espinosa, J.; Shamshirsaz, A.A.; Fox, K.; Belfort, M.; Yallampalli, C. Calcitonin Gene Related Peptide, Adrenomedullin, and Adrenomedullin 2 Function in Uterine Artery During Human Pregnancy. Endocrinology 2022, 163, bqab204. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Ross, G.R.; Yallampalli, U.; Yallampalli, C. Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: Influence of pregnancy. Endocrinology 2007, 148, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Yallampalli, U.; Reed, L.; Yallampalli, C. Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol. Reprod. 2006, 75, 940–947. [Google Scholar] [CrossRef]
- Gutkowska, J. ANP. In Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: San Diego, CA, USA, 2013; pp. 1011–1018. [Google Scholar]
- Kinnunen, P.; Vuolteenaho, O.; Ruskoaho, H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: Effect of stretching. Endocrinology 1993, 132, 1961–1970. [Google Scholar] [CrossRef]
- Richards, A.M.; Doughty, R.; Nicholls, M.G.; Macmahon, S.; Ikram, H.; Sharpe, N.; Espiner, E.A.; Frampton, C.; Yandle, T.G. Neurohumoral prediction of benefit from carvedilol in ischemic left ventricular dysfunction. Circulation 1999, 99, 786–792. [Google Scholar] [CrossRef]
- Afshani, N.; Moustaqim-Barrette, A.; Biccard, B.M.; Rodseth, R.N.; Dyer, R.A. Utility of B-Type Natriuretic Peptides in Preeclampsia: A Systematic Review. Int. J. Obstet. Anesth. 2013, 22, 96–103. [Google Scholar] [CrossRef]
- Resnik, J.L.; Hong, C.; Resnik, R.; Kazanegra, R.; Beede, J.; Bhalla, V.; Maisel, A. Evaluation of B-Type Natriuretic Peptide (Bnp) Levels in Normal and Preeclamptic Women. Am. J. Obstet. Gynecol. 2005, 193, 450–454. [Google Scholar] [CrossRef]
- Sadlecki, P.; Grabiec, M.; Walentowicz-Sadlecka, M. Prenatal Clinical Assessment of NT-ProBNP as a Diagnostic Tool for Preeclampsia, Gestational Hypertension and Gestational Diabetes Mellitus. PLoS ONE 2016, 11, e0162957. [Google Scholar] [CrossRef]
- Borghi, C.; Degli Esposti, D.; Immordino, V.; Cassani, A.; Boschi, S.; Bovicelli, L.; Ambrosioni, E. Relationship of systemic hemodynamics, left ventricular structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am. J. Obstet. Gynecol. 2000, 183, 140–147. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy: Diagnosis and Management; NICE Guideline, No. 133; National Institute for Health and Care Excellence (NICE): London, UK, 2019; ISBN 978-1-4731-3434-8.
- Committee on Obstetric Practice; Society for Maternal-Fetal Medicine. ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet. Gynecol. 2018, 132, e44–e52. [Google Scholar] [CrossRef]
- Tan, M.Y.; Syngelaki, A.; Poon, L.C.; Rolnik, D.L.; O’Gorman, N.; Delgado, J.L.; Akolekar, R.; Konstantinidou, L.; Tsavdaridou, M.; Galeva, S.; et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2018, 52, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; DeMayo, F.J. Animal models of implantation. Reproduction 2004, 128, 679–695. [Google Scholar] [CrossRef]
- Yotsumoto, S.; Shimada, T.; Cui, C.Y.; Nakashima, H.; Fujiwara, H.; Ko, M.S. Expression of adrenomedullin, a hypotensive peptide, in the trophoblast giant cells at the embryo implantation site in mouse. Dev. Biol. 1998, 203, 264–275. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Yee, D.; Magnuson, T.R.; Smithies, O.; Caron, K.M. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J. Clin. Investig. 2006, 116, 2653–2662. [Google Scholar] [CrossRef]
- Gratton, R.J.; Gluszynski, M.; Mazzuca, D.M.; Nygard, K.; Han, V.K. Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2003, 88, 6048–6055. [Google Scholar] [CrossRef][Green Version]
- Nikitenko, L.L.; Brown, N.S.; Smith, D.M.; MacKenzie, I.Z.; Bicknell, R.; Rees, M.C. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol. Hum. Reprod. 2001, 7, 655–664. [Google Scholar] [CrossRef]
- Zhang, X.; Green, K.E.; Yallampalli, C.; Dong, Y.L. Adrenomedullin enhances invasion by trophoblast cell lines. Biol. Reprod. 2005, 73, 619–626. [Google Scholar] [CrossRef]
- Hoeldtke, N.J.; Wagner, R.K.; Calhoun, B.C.; Hume, R.F., Jr. Vasodilatory response of fetoplacental vasculature to adrenomedullin after constriction with the thromboxane sympathomimetic U46619. Am. J. Obstet. Gynecol. 2000, 183, 1573–1578. [Google Scholar] [CrossRef]
- Jerat, S.; Morrish, D.W.; Davidge, S.T.; Kaufman, S. Effect of adrenomedullin on placental arteries in normal and preeclamptic pregnancies. Hypertension 2001, 37, 227–231. [Google Scholar] [CrossRef]
- Ross, G.R.; Yallampalli, U.; Gangula, P.R.; Reed, L.; Sathishkumar, K.; Gao, H.; Chauhan, M.; Yallampalli, C. Adrenomedullin relaxes rat uterine artery: Mechanisms and influence of pregnancy and estradiol. Endocrinology 2010, 151, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghafra, A.; Gude, N.M.; Brennecke, S.P.; King, R.G. Increased adrenomedullin protein content and mRNA expression in human fetal membranes but not placental tissue in pre-eclampsia. Mol. Hum. Reprod. 2006, 12, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Senna, A.A.; Zedan, M.; Abd El Salam, G.E.; El Mashad, A.I. Study of plasma adrenomedullin level in normal pregnancy and preclampsia. Medscape J. Med. 2008, 10, 29. [Google Scholar]
- Boć-Zalewska, A.; Seremak-Mrozikiewicz, A.; Barlik, M.; Kurzawińska, G.; Drews, K. The possible role of adrenomedullin in the etiology of gestational hypertension and preeclampsia. Ginekol. Pol. 2011, 82, 178–184. [Google Scholar]
- Lauria, M.R.; Standley, C.A.; Sorokin, Y.; Yelian, F.D.; Cotton, D.B. Adrenomedullin levels in normal and preeclamptic pregnancy at term. J. Soc. Gynecol. Investig. 1999, 6, 318–321. [Google Scholar] [CrossRef]
- Kanenishi, K.; Kuwabara, H.; Ueno, M.; Sakamoto, H.; Hata, T. Immunohistochemical adrenomedullin expression is decreased in the placenta from pregnancies with pre-eclampsia. Pathol. Int. 2000, 50, 536–540. [Google Scholar] [CrossRef]
- Knerr, I.; Dachert, C.; Beinder, E.; Metzler, M.; Dötsch, J.; Repp, R.; Rascher, W. Adrenomedullin, calcitonin gene-related peptide and their receptors: Evidence for a decreased placental mRNA content in preeclampsia and HELLP syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 47–53. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Dong, N.; Lou, J.; Srinivasan, D.K.; Cheng, W.; Huang, X.; Liu, M.; Fang, C.; Peng, J.; et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012, 484, 246–250. [Google Scholar] [CrossRef]
- Tokudome, T.; Kishimoto, I.; Yamahara, K.; Osaki, T.; Minamino, N.; Horio, T.; Sawai, K.; Kawano, Y.; Miyazato, M.; Sata, M.; et al. Impaired recovery of blood flow after hind-limb ischemia in mice lacking guanylyl cyclase-A, a receptor for atrial and brain natriuretic peptides. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1516–1521. [Google Scholar] [CrossRef]
- Yamahara, K.; Itoh, H.; Chun, T.H.; Ogawa, Y.; Yamashita, J.; Sawada, N.; Fukunaga, Y.; Sone, M.; Yurugi-Kobayashi, T.; Miyashita, K.; et al. Significance and therapeutic potential of the natriuretic peptides/cGMP/cGMP-dependent protein kinase pathway in vascular regeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 3404–3409. [Google Scholar] [CrossRef]
- Woodard, G.E.; Rosado, J.A. Natriuretic peptides in vascular physiology and pathology. Int. Rev. Cell Mol. Biol. 2008, 268, 59–293. [Google Scholar] [CrossRef] [PubMed]
- Degrelle, S.A.; Chissey, A.; Stepanian, A.; Fournier, T.; Guibourdenche, J.; Mandelbrot, L.; Tsatsaris, V. Placental Overexpression of Soluble CORIN in Preeclampsia. Am. J. Pathol. 2020, 190, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Sugulle, M.; Herse, F.; Hering, L.; Mockel, M.; Dechend, R.; Staff, A.C. Cardiovascular Biomarker Midregional Proatrial Natriuretic Peptide During and After Preeclamptic Pregnancies. Hypertension 2012, 59, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.I.; van der Meer, M.A.; Saleh, L.; van den Berg, S.A.; van den Meiracker, A.H.; Danser, A.H.; Visser, W. Copeptin and mid-regional pro-atrial natriuretic peptide in women with suspected or confirmed pre-eclampsia: Comparison with sFlt-1/PlGF ratio Ultrasound. Obstet. Gynecol. 2020, 56, 872–878. [Google Scholar] [CrossRef]
- Zaki, M.A.; Beard, S.; de Alwis, N. Plasma soluble corin and N-terminal pro-atrial natriuretic peptide levels in pregnancy induced hypertension. Pregnancy Hypertens. 2012, 2, 48–52. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishizawa, H.; Kambayashi, A.; Ito, M.; Noda, Y.; Terasawa, S.; Kato, T.; Miyamura, H.; Shiogama, K.; Sekiya, T.; et al. Increased levels of soluble corin in pre-eclampsia and fetal growth restriction. Placenta 2016, 48, 20–25. [Google Scholar] [CrossRef]
- Birdir, C.; Janssen, K.; Stanescu, A.D.; Enekwe, A.; Kasimir-Bauer, S.; Gellhaus, A.; Kimmig, R.; Köninger, A. Maternal serum copeptin, MR-proANP and procalcitonin levels at 11–13 weeks gestation in the prediction of preeclampsia. Matern. Fetal Med. 2015, 292, 1033–1042. [Google Scholar] [CrossRef]
- Marinoni, E.; Di Iorio, R.; Letizia, C.; Villaccio, B.; Alberini, A.; Cosmi, E.V. Amniotic fluid concentrations of adrenomedullin in preterm labor. Obstet. Gynecol. 1999, 93, 964–967. [Google Scholar] [CrossRef]
- Di Iorio, R.; Marinoni, E.; Letizia, C.; Alò, P.; Villaccio, B.; Poverini, R.; Cosmi, E.V. Influence of labor on fetoplacental adrenomedullin concentrations. Am. J. Obstet. Gynecol. 2001, 185, 697–702. [Google Scholar] [CrossRef]
- Yamashiro, C.; Kanenishi, K.; Akiyama, M.; Tanaka, H.; Shiota, A.; Hata, T. Adrenomedullin concentrations in early 2nd-trimester amniotic fluid: Relation to preterm delivery and fetal growth at birth. Gynecol. Obstet. Investig. 2002, 54, 99–104. [Google Scholar] [CrossRef]
- Iavazzo, C.; Tassis, K.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Hadjithomas, A.; Vrachnis, N.; Malamitsi-Puchner, A. Adrenomedullin concentration in second trimester amniotic fluid cannot be used as a predictor of preterm delivery. In Vivo 2009, 23, 1021–1036. [Google Scholar] [PubMed]
- Wellmann, S.; Benzing, J.; Fleischlin, S.; Morgenthaler, N.; Fouzas, S.; Bührer, C.A.; Szinnai, G.; Burkhardt, T.; Lapaire, O. Cardiovascular biomarkers in preeclampsia at triage. Fetal Diagn. Ther. 2014, 36, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dong, Y.B.; Liu, Y.R.; Zhang, Y.; Li, H.Y.; Song, W. Correlation between corin, N-terminal pro-atrial natriuretic peptide and neonatal adverse prognostic in hypertensive disorders of pregnancy. Pregnancy Hypertens. 2021, 23, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Dockree, S.; Brook, J.; Shine, B.; James, T.; Vatish, M. Pregnancy-specific Reference Intervals for BNP and NT-pro BNP-Changes in Natriuretic Peptides Related to Pregnancy. J. Endocr. Soc. 2021, 5, bvab091. [Google Scholar] [CrossRef]
- Moungmaithong, S.; Wang, X.; Lau, C.S.; Tse, A.W.; Lee, N.M.; Leung, H.H.; Poon, L.C.; Sahota, D.S. Glycosylated fibronectin improves first-trimester prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2023, 62, 512–521. [Google Scholar] [CrossRef]
- Hauspurg, A.; Marsh, D.J.; McNeil, R.B.; Merz, C.N.; Greenland, P.; Straub, A.C.; Rouse, C.E.; Grobman, W.A.; Pemberton, V.L.; Silver, R.M.; et al. Association of N-Terminal Pro–Brain Natriuretic Peptide Concentration in Early Pregnancy with Development of Hypertensive Disorders of Pregnancy and Future Hypertension. JAMA Cardiol. 2022, 7, 268–276. [Google Scholar] [CrossRef]
| Non-GH (n = 41) | GH (n = 18) | p Value | |
|---|---|---|---|
| Age (years) | 30 | 33 | p < 0.001 |
| Weight (kg) | 65 ± 1.53 | 78.2 ± 3.5 | p < 0.001 |
| BMI (kg/m2) | 22 ± 0.55 | 28 ± 1.34 | p < 0.001 |
| First birth (%) | 42.4% | 50% | - |
| Second birth (%) | 48.5% | 50% | - |
| Third birth (%) | 9.1% | - | - |
| Pregnancy duration (weeks) | 38.8 ± 0.49 | 36.6 ± 0.55 | p < 0.0016 |
| Natural childbirth (%) | 68.5% | 30% | p < 0.001 |
| Cesarean section (%) | 31.5% | 70% | p < 0.001 |
| Newborn’s body weight (g) | 3389 ± 99 | 3016 ± 199 | p < 0.045 |
| Neonatal Apgar Score | 9.97 ± 0.03 | 9.30 ± 0.24 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagodzinska, A.; Wsol, A.; Gondek, A.; Cudnoch-Jedrzejewska, A. High Serum Adrenomedullin and Mid-Regional Pro-Atrial Natriuretic Peptide Concentrations in Early Pregnancy Predict the Development of Gestational Hypertension. Diagnostics 2024, 14, 2670. https://doi.org/10.3390/diagnostics14232670
Jagodzinska A, Wsol A, Gondek A, Cudnoch-Jedrzejewska A. High Serum Adrenomedullin and Mid-Regional Pro-Atrial Natriuretic Peptide Concentrations in Early Pregnancy Predict the Development of Gestational Hypertension. Diagnostics. 2024; 14(23):2670. https://doi.org/10.3390/diagnostics14232670
Chicago/Turabian StyleJagodzinska, Aleksandra, Agnieszka Wsol, Agata Gondek, and Agnieszka Cudnoch-Jedrzejewska. 2024. "High Serum Adrenomedullin and Mid-Regional Pro-Atrial Natriuretic Peptide Concentrations in Early Pregnancy Predict the Development of Gestational Hypertension" Diagnostics 14, no. 23: 2670. https://doi.org/10.3390/diagnostics14232670
APA StyleJagodzinska, A., Wsol, A., Gondek, A., & Cudnoch-Jedrzejewska, A. (2024). High Serum Adrenomedullin and Mid-Regional Pro-Atrial Natriuretic Peptide Concentrations in Early Pregnancy Predict the Development of Gestational Hypertension. Diagnostics, 14(23), 2670. https://doi.org/10.3390/diagnostics14232670





