Improving Outcomes of CT-Guided Malignant Lung Lesion Microwave Ablation by Tract Sealing Using Venous Blood Clot
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Patient Profile:
- Patients diagnosed with a primary malignant or metastatic lung tumor.
- Lesions are accessible for microwave ablation at the time of treatment.
- Maximum diameter of the lesion ≤ 3 cm.
- The intent of radical treatment.
- Agreement on curative MWA treatment confirmed by a multidisciplinary team, including an interventional radiologist, radiation therapist, thoracic surgeon, oncologist, and pulmonologist.
- Medical Suitability:
- No contraindications for general anesthesia or sedation.
- No severe coagulopathy or patients must be able and willing to stop antiplatelet medications before the procedure.
- Consent:
- Patient agreement to participate in this study provided via a signed informed consent form.
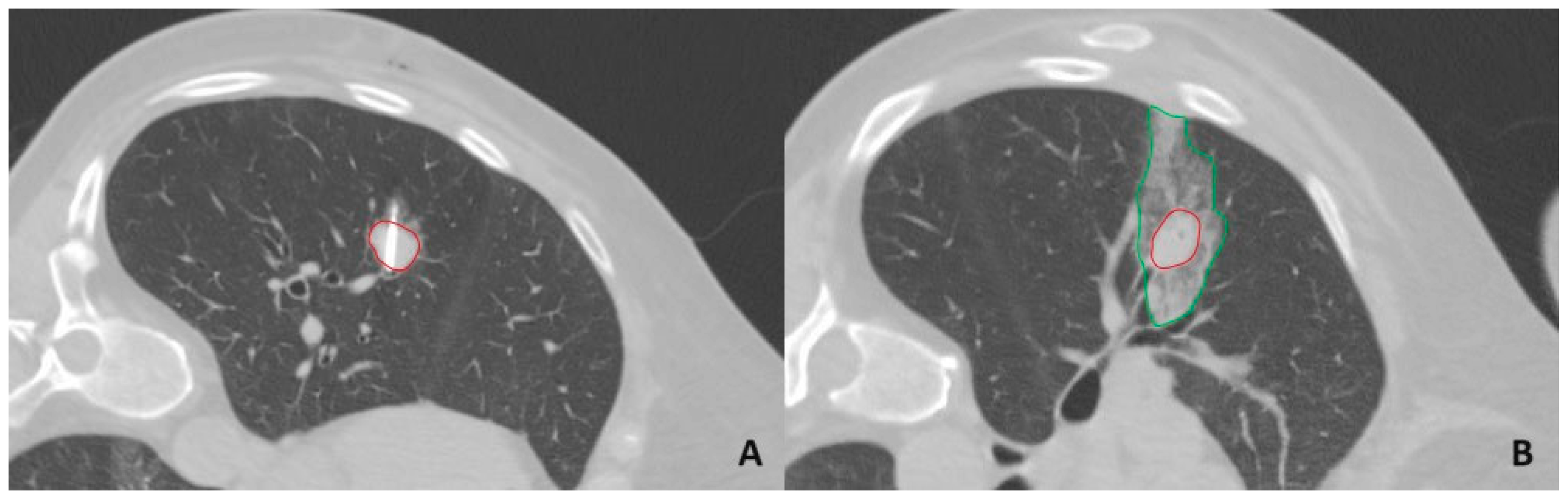
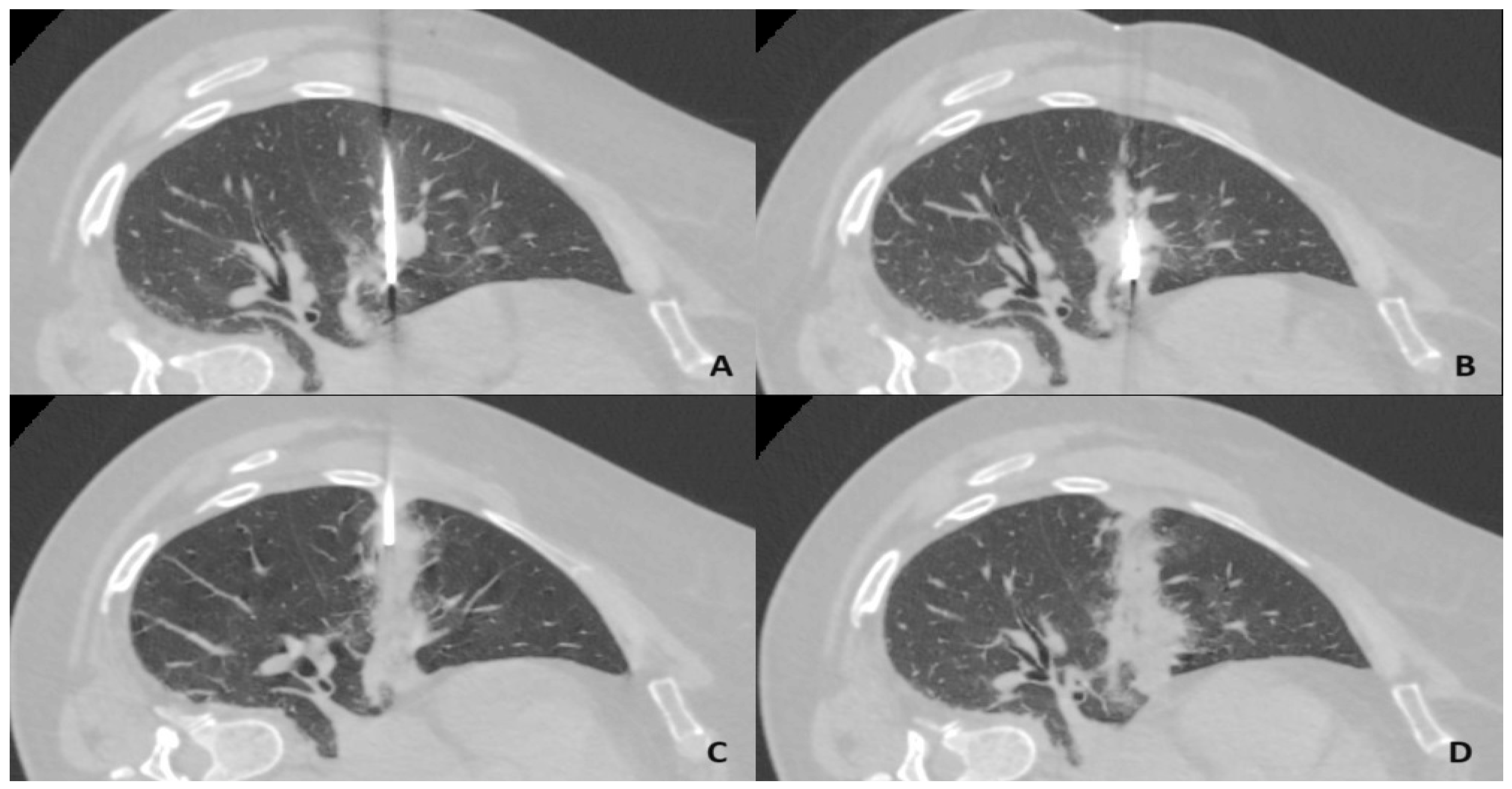
2.2. Microwave Ablation Procedure
2.3. Tract Sealing Using an Autologous Venous Blood Clot
2.4. Assessment of Complications
- Grade 1—complication during the procedure that can be solved within the same session; no additional therapy, no post-procedure sequelae, no deviation from the normal post-therapeutic course.
- Grade 2—prolonged observation including overnight stay (as a deviation from the normal post-therapeutic course < 48 h); no additional post-procedure therapy, no post-procedure sequelae.
- Grade 3—additional post-procedure therapy or prolonged hospital stay (>48 h) required; no post-procedure sequelae.
- Grade 4—complication causing a permanent mild sequela (resuming work and independent living).
- Grade 5—complication causing a permanent severe sequela (requiring ongoing assistance in daily life).
- Grade 6—death.
2.5. Statistical Analysis
2.6. Control Group
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang, Z.; Wang, J.; Liu, K.; Wu, J.; Ge, Y.; Zhu, G.; Cao, L.; Ma, X.; Li, J. Global, Regional, and National Burden of Tracheal, Bronchus, and Lung Cancer and its Risk Factors from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. eClinicalMedicine 2024, 75, 102804. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and Occupational Determinants of Lung Cancer. Transl. Lung Cancer Res. 2019, 8, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Stella, G.M.; Kolling, S.; Benvenuti, S.; Bortolotto, C. Lung-Seeking Metastases. Cancers 2019, 11, 1010. [Google Scholar] [CrossRef]
- Tafti, B.A.; Genshaft, S.; Suh, R.; Abtin, F. Lung Ablation: Indications and Techniques. Semin. Interv. Radiol. 2019, 36, 163–175. [Google Scholar] [CrossRef]
- Murphy, M.; Wrobel, M.; Fisher, D.; Cahalane, A.M.; Fintelmann, F.J. Update on Image-Guided Thermal Lung Ablation: Society Guidelines, Therapeutic Alternatives, and Postablation Imaging Findings. Am. J. Roentgenol. 2022, 219, 471–485. [Google Scholar] [CrossRef]
- Jiang, B.; Mcclure, M.; Chen, T.; Chen, S. Efficacy and safety of thermal ablation of lung malignancies: A Network meta-analysis. Ann. Thorac. Med. 2018, 13, 243–250. [Google Scholar]
- Han, X.; Wei, Z.; Zhao, Z.; Yang, X.; Ye, X. Cost and effectiveness of microwave ablation versus video-assisted thoracoscopic surgical resection for ground-glass nodule lung adenocarcinoma. Front. Oncol. 2022, 12, 962630. [Google Scholar] [CrossRef]
- Tan, C.; Ho, A.; Robinson, H.; Huang, L.; Ravindran, P.; Chan, D.L.; Alzahrani, N.; Morris, D.L. A Systematic Review of Microwave Ablation for Colorectal Pulmonary Metastases. Anticancer Res. 2023, 43, 2899–2907. [Google Scholar] [CrossRef]
- Yang, X.; Jin, Y.; Lin, Z.; Li, X.; Huang, G.; Ni, Y.; Li, W.; Han, X.; Meng, M.; Chen, J.; et al. Microwave ablation for the treatment of peripheral ground–glass nodule-like lung cancer: Long-term results from a multi-center study. J. Cancer Res. Ther. 2023, 19, 1001–1010. [Google Scholar] [CrossRef]
- Hu, H.; Zhai, B.; Liu, R.; Chi, J.C. Microwave Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Adjacent to the Pericardium: Propensity Score Analyses of Long-term Outcomes. Cardiovasc. Interv. Radiol. 2021, 44, 237–246. [Google Scholar] [CrossRef]
- Lokhandwala, T.; Bittoni, M.A.; Dann, R.A.; D’Souza, A.O.; Johnson, M.; Nagy, R.J.; Lanman, R.B.; Merritt, R.E.; Carbone, D.P. Costs of Diagnostic Assessment for Lung Cancer: A Medicare Claims Analysis. Clin. Lung Cancer 2017, 18, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Macionis, A.; Zemaitis, M.; Maziliauskiene, G.; Dubeikaite, R.; Vajauskas, D. Reduction of complication rate following CT-guided percutaneous transthoracic lung biopsy by sealing biopsy tract with patient’s blood clot. In Proceedings of the ECR 2024 “Next Generation Radiology”, Vienna, Austria, 28 February–3 March 2024; p. C-24651. [Google Scholar]
- Malone, L.; Stanfill, R.; Wang, H.; Fahey, K.M.; Bertino, R.E. Effect of Intraparenchymal Blood Patch on Rates of Pneumothorax and Pneumothorax Requiring Chest Tube Placement After Percutaneous Lung Biopsy. AJR Am. J. Roentgenol. 2013, 200, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.D.; Elicker, B.M.; Ordovas, K.G.; Kohi, M.P.; Nguyen, J.; Naeger, D.M. Nonclotted Blood Patch Technique Reduces Pneumothorax and Chest Tube Placement Rates After Percutaneous Lung Biopsies. J. Thorac. Imaging 2016, 31, 243–246. [Google Scholar] [CrossRef]
- Graffy, P.; Loomis, S.B.; Pickhardt, P.J.; Lubner, M.G.; Kitchin, D.R.; Lee, F.T.; Hinshaw, J.L. Pulmonary Intraparenchymal Blood Patching Decreases the Rate of Pneumothorax-Related Complications following Percutaneous CT–Guided Needle Biopsy. J. Vasc. Interv. Radiol. 2017, 28, 608–613. [Google Scholar] [CrossRef]
- Billich, C.; Muche, R.; Brenner, G.; Schmidt, S.A.; Kruger, S.; Brambs, H.; Pauls, S. CT-guided lung biopsy: Incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur. Radiol. 2008, 18, 1146–1152. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.; Lou, T.Y.; Yang, H.F.; Yu, J.H.; Xu, X.X.; Zheng, H.J.; Li, B. Usefulness of normal saline for sealing the needle track after CT-guided lung biopsy. Clin. Radiol. 2015, 70, 1192–1197. [Google Scholar] [CrossRef]
- Tran, A.A.; Brown, S.B.; Rosenberg, J.; Hovsepian, D.M. Tract Embolization With Gelatin Sponge Slurry for Prevention of Pneumothorax After Percutaneous Computed Tomography-Guided Lung Biopsy. Cardiovasc. Interv. Radiol. 2014, 37, 1546–1553. [Google Scholar] [CrossRef]
- Renier, H.; Gerard, L.; Lamborelle, P.; Cousin, F. Efficacy of the tract embolization technique with gelatin sponge slurry to reduce pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy. Cardiovasc. Interv. Radiol. 2020, 43, 597–603. [Google Scholar] [CrossRef]
- Baadh, A.S.; Hoffmann, J.C.; Fadl, A.; Danda, D.; Bhat, V.R.; Georgiou, N.; Hon, M. Utilization of the track embolization technique to improve the safety of percutaneous lung biopsy and/or fiducial marker placement. Clin. Imaging 2016, 40, 1023–1028. [Google Scholar] [CrossRef]
- Zaetta, J.M.; Licht, M.O.; Fisher, J.S.; Avelar, R.L. A Lung Biopsy Tract Plug for Reduction of Postbiopsy Pneumothorax and Other Complications: Results of a Prospective, Multicenter, Randomized, Controlled Clinical Study. J. Vasc. Interv. Radiol. 2010, 21, 1235–1243. [Google Scholar] [CrossRef]
- Grage, R.A.; Naveed, M.A.; Keogh, S.; Wang, D. Efficacy of a Dehydrated Hydrogel Plug to Reduce Complications Associated With Computed Tomography–guided Percutaneous Transthoracic Needle Biopsy. Thorac. Imaging 2017, 32, 57–62. [Google Scholar] [CrossRef]
- Ahrar, J.; Gupta, S.; Ensor, J.; Mahvash, A.; Sabir, S.; Steele, J.; McRae, S.; Avritscher, R.; Huang, S.Y.; Odisio, B.; et al. Efficacy of a Self-expanding Tract Sealant Device in the Reduction of Pneumothorax and Chest Tube Placement Rates After Percutaneous Lung Biopsy: A Matched Controlled Study Using Propensity Score Analysis. Cardiovasc. Interv. Radiol. 2017, 40, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Engeler, C.E.; Hunter, D.W.; Castaneda-Zuniga, W.; Tashjian, J.H.; Yedlicka, J.W.; Amplatz, K. Pneumothorax after lung biopsy: Prevention with transpleural placement of compressed collagen foam plugs. Radiology 1992, 184, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Petsas, T.; Siamblis, D.; Giannakenas, C.; Tepetes, K.; Dougenis, D.; Spiropoulos, K.; Fezoulis, I.; Dimopoulos, I. Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: Part II--Clinical study. Cardiovasc. Interv. Radiol. 1995, 18, 378–382. [Google Scholar] [CrossRef]
- Dassa, M.; Izaaryene, J.; Daidj, N.; Piana, G. Efficacy of Tract Embolization After Percutaneous Pulmonary Radiofrequency Ablation. Cardiovasc. Interv. Radiol. 2021, 44, 903–910. [Google Scholar] [CrossRef]
- Izaaryene, J.; Mancini, J.; Louis, G.; Chaumoitre, K.; Bartoli, J.; Vidal, V.; Gaubert, J. Embolisation of pulmonary radio frequency pathway—A randomised trial. Int. J. Hyperth. 2017, 33, 814–819. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Yao, W.; Lu, M.; Fan, W.; Huang, J.; Gu, Y.; Gao, F.; Wang, Y.; Li, J.; Zhu, Z. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: A propensity score analysis. Int. J. Hyperth. 2018, 34, 1329–1336. [Google Scholar] [CrossRef]
- Lee, H.N.; Lee, S.M.; Choe, J.; Lee, S.M.; Chae, E.J.; Do, K.; Seo, J.B. Diagnostic Performance of CT-Guided Percutaneous Transthoracic Core Needle Biopsy Using Low Tube Voltage (100 kVp): Comparison with Conventional Tube Voltage (120 kVp). Acta Radiol. 2017, 59, 425–433. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Meng, H.; Lv, W.; Jia, H. Efficacy and Radiation Exposure of Ultra-Low-Dose Chest CT at 100 kVp with Tin Filtration in CT-Guided Percutaneous Core Needle Biopsy for Small Pulmonary Lesions Using a Third-Generation Dual-Source CT Scanner. J. Vasc. Interv. Radiol. 2019, 30, 95–102. [Google Scholar] [CrossRef]
- De Baere, T. Pneumothorax and Lung Thermal Ablation: Is It a Complication? Is It Only About Tract Sealing? Cardiovasc. Interv. Radiol. 2021, 44, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Bie, Z.; Su, F.; Sun, J.; Li, X. Effects of tract embolization on pneumothorax rate after percutaneous pulmonary microwave ablation: A rabbit study. Int. J. Hyperth. 2023, 40, 2165728. [Google Scholar] [CrossRef]
- Topal, U.; Berkman, Y.M. Effect of needle tract bleeding on occurrence of pneumothorax after transthoracic needle biopsy. Eur. J. Radiol. 2005, 53, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Q.; Li, W.; Liu, Y. Autologous Blood Patch Intraparenchymal Injection Reduces the Incidence of Pneumothorax and the Need for Chest Tube Placement Following CT-Guided Lung Biopsy: A Systematic Review and Meta-analysis. Eur. J. Med. Res. 2024, 29, 108. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.A.; Milovanovic, L.; Dao, D.; Farrokhyar, F.; Midia, M. Risk Factors for Pneumothorax Complicating Radiofrequency Ablation for Lung Malignancy: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2014, 25, 1671–1681. [Google Scholar] [CrossRef]
- Zhao, H.; Steinke, K. Long-term outcome following microwave ablation of early-stage non-small cell lung cancer. J. Med. Imaging Radiat. Oncol. 2020, 64, 787–793. [Google Scholar] [CrossRef]
- Xu, S.; Bie, Z.X.; Li, Y.M.; Li, B.; Guo, R.Q.; Li, X.G. Computed tomography-guided microwave ablation for the treatment of non-small cell lung cancer patients with and without adjacent lobe invasion: A comparative study. Thorac. Cancer 2021, 12, 2780–2788. [Google Scholar] [CrossRef]
- Zheng, A.; Wang, X.; Yang, X.; Wang, W.; Huang, G.; Gai, Y.; Ye, X. Major Complications After Lung Microwave Ablation: A Single-Center Experience on 204 Sessions. Ann. Thorac. Surg. 2014, 98, 243–248. [Google Scholar] [CrossRef]
| Characteristic | Number of Lesions (%) |
|---|---|
| Primary lung cancer | 18 (60) |
| Adenocarcinoma (including adenocarcinoma in situ) | 15 (50) |
| Squamous cell carcinoma | 3 (10) |
| Metastatic lung lesions | 12 (40) |
| Hemangioendothelioma | 4 (13.3) |
| Intestinal adenocarcinoma | 3 (10) |
| Hemangiopericytoma | 2 (6.7) |
| Renal carcinoma | 2 (6.7) |
| Melanoma | 1 (3.3) |
| Repeatedly ablated tumors | 3 |
| Total | 30 |
| Pneumothorax Grade | Number of Patients |
|---|---|
| Grade 1 | 4 |
| Grade 2 | 0 |
| Grade 3 | 1 |
| Grade 4 | 0 |
| Grade 5 | 0 |
| Grade 6 | 0 |
| Complication | Pneumothorax | p-Value | Chest Tube Insertion | p-Value | |
|---|---|---|---|---|---|
| Group | |||||
| Biopsy without tract sealing | 31.4% (33/105) | 0.220 | 10.5% (11/105) | 0.458 | |
| MWA with tract sealing | 19.2% (5/26) | 3.8% (1/26) | |||
| Characteristics | Overall | Pneumothorax | No Pneumothorax | p-Value | ||
|---|---|---|---|---|---|---|
| Number of Sessions | 26 | 5 | 21 | |||
| Patient-related | Age (y) | 66.0 ± 8.8 | 62.6 ± 5.0 | 66.8 ± 9.4 | 0.308 | |
| Sex | Men | 14 | 5 (100%) | 9 (42.9%) | 0.042 | |
| Women | 12 | 0 (0%) | 12 (57.1%) | |||
| Lung emphysema, bullae | Yes | 8 | 3 (60%) | 5 (23.8%) | 0.281 | |
| No | 18 | 2 (40%) | 16 (76.2%) | |||
| Procedure-related | Number of lesions ablated per session | 1 | 22 | 4 (80%) | 18 (85.7%) | 1.000 |
| 2 | 4 | 1 (20%) | 3 (14.3%) | |||
| Number of pleural punctures | 1 | 20 | 5 (100%) | 15 (71.4%) | 0.298 | |
| 2 | 6 | 0 (0%) | 6 (28.6%) | |||
| Number of lesions | 30 | 5 | 25 | |||
| Lesion-related | Contact with pleura | Yes | 4 | 1 (20%) | 3 (12%) | 0.538 |
| No | 26 | 4 (80%) | 22 (88%) | |||
| Length of aerated lung traversed (mm) | 41.4 ± 12.3 | 36.6 ± 15.3 | 42.4 ± 12.0 | 0.300 | ||
| Duration of ablation (min) | 20.0 ± 10.2 | 18.4 ± 7.6 | 20.4 ± 10.8 | 0.829 | ||
| Lesion’s maximum diameter (mm) | 14.0 ± 6.6 | 11.4 ± 6.8 | 14.5 ± 6.6 | 0.327 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mačionis, A.; Maziliauskienė, G.; Dubeikaitė, R.; Vajauskas, D.; Adukauskienė, D.; Nedzelskienė, I.; Žemaitis, M. Improving Outcomes of CT-Guided Malignant Lung Lesion Microwave Ablation by Tract Sealing Using Venous Blood Clot. Diagnostics 2024, 14, 2631. https://doi.org/10.3390/diagnostics14232631
Mačionis A, Maziliauskienė G, Dubeikaitė R, Vajauskas D, Adukauskienė D, Nedzelskienė I, Žemaitis M. Improving Outcomes of CT-Guided Malignant Lung Lesion Microwave Ablation by Tract Sealing Using Venous Blood Clot. Diagnostics. 2024; 14(23):2631. https://doi.org/10.3390/diagnostics14232631
Chicago/Turabian StyleMačionis, Aurimas, Gertrūda Maziliauskienė, Rūta Dubeikaitė, Donatas Vajauskas, Dalia Adukauskienė, Irena Nedzelskienė, and Marius Žemaitis. 2024. "Improving Outcomes of CT-Guided Malignant Lung Lesion Microwave Ablation by Tract Sealing Using Venous Blood Clot" Diagnostics 14, no. 23: 2631. https://doi.org/10.3390/diagnostics14232631
APA StyleMačionis, A., Maziliauskienė, G., Dubeikaitė, R., Vajauskas, D., Adukauskienė, D., Nedzelskienė, I., & Žemaitis, M. (2024). Improving Outcomes of CT-Guided Malignant Lung Lesion Microwave Ablation by Tract Sealing Using Venous Blood Clot. Diagnostics, 14(23), 2631. https://doi.org/10.3390/diagnostics14232631







