The Microsurgical Resection of an Arteriovenous Malformation in a Patient with Thrombophilia: A Case Report and Literature Review
Abstract
:1. Introduction
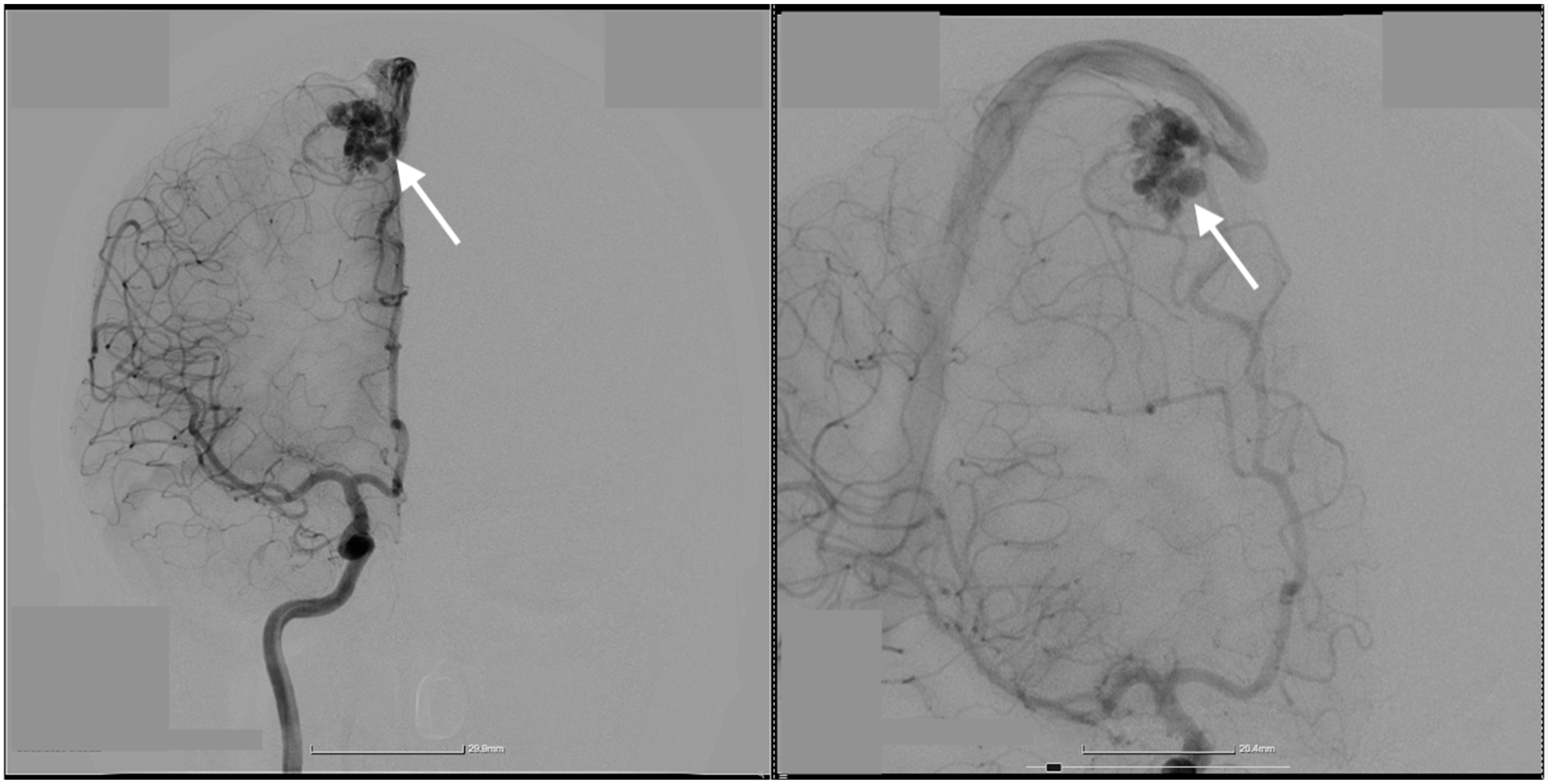
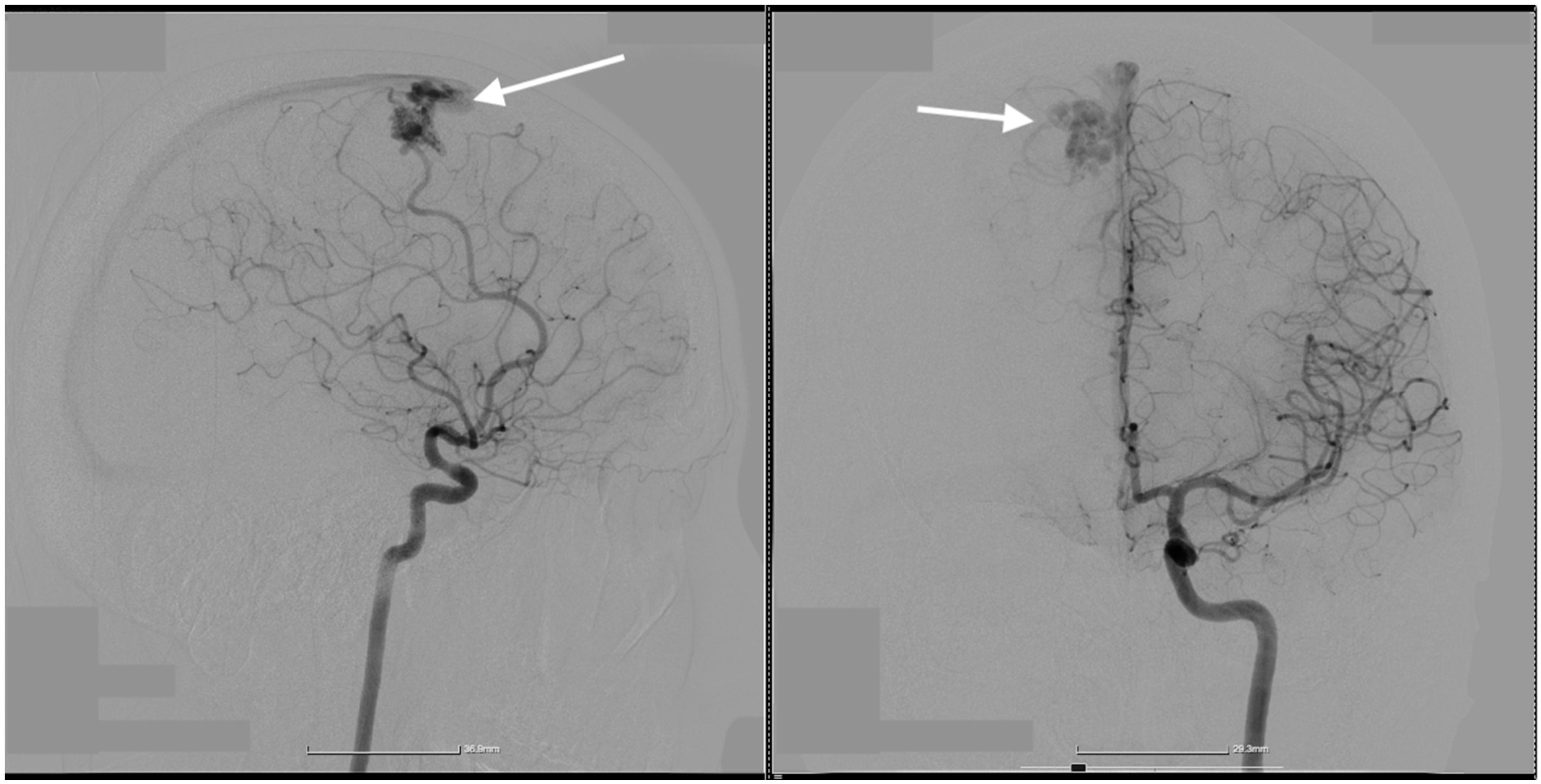
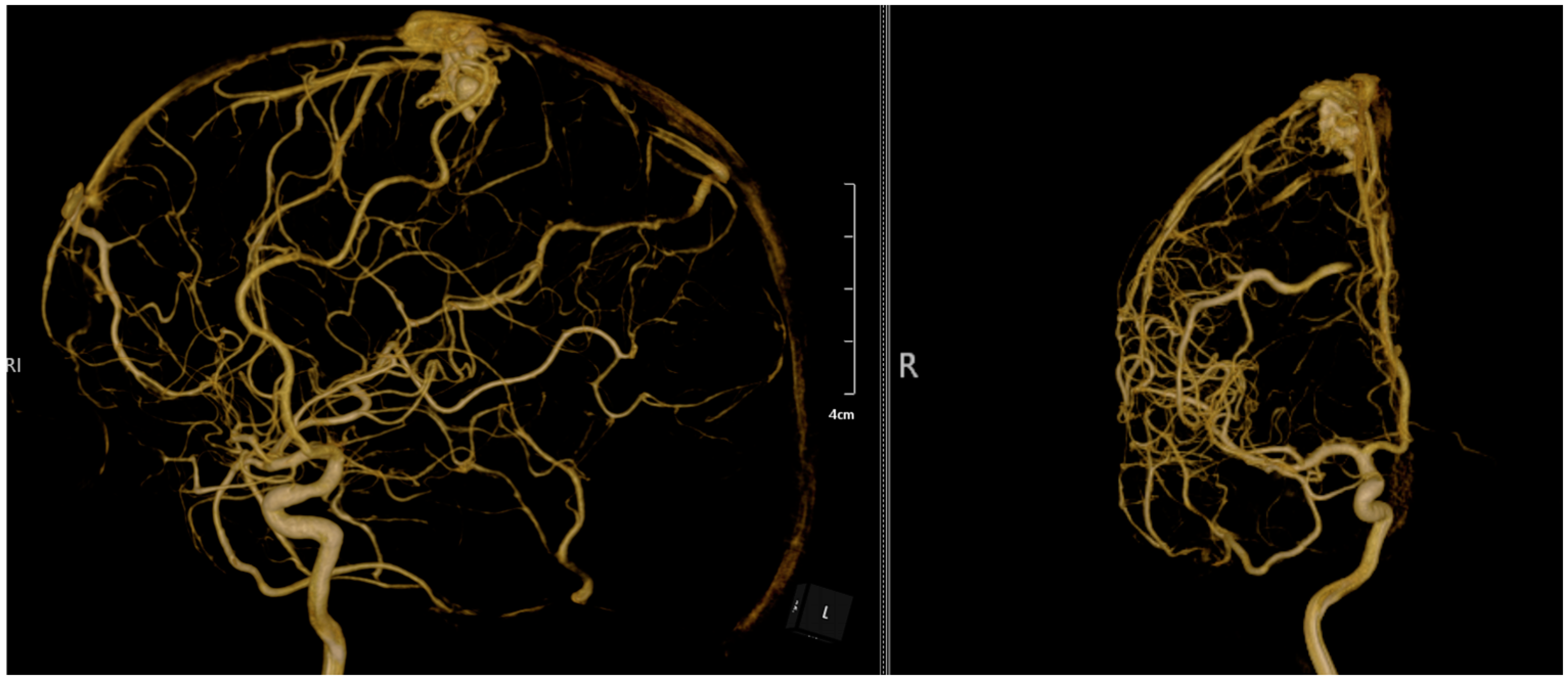
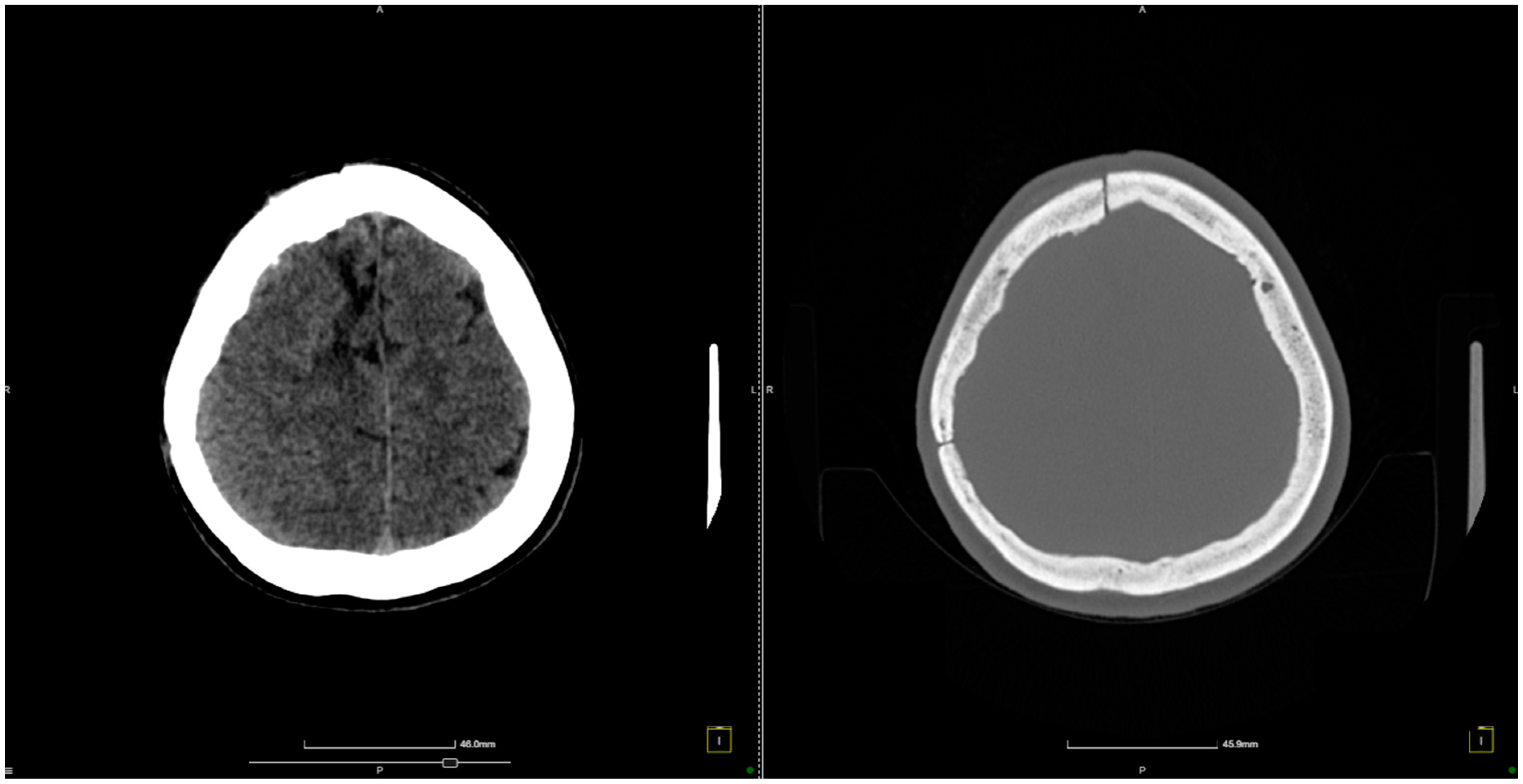
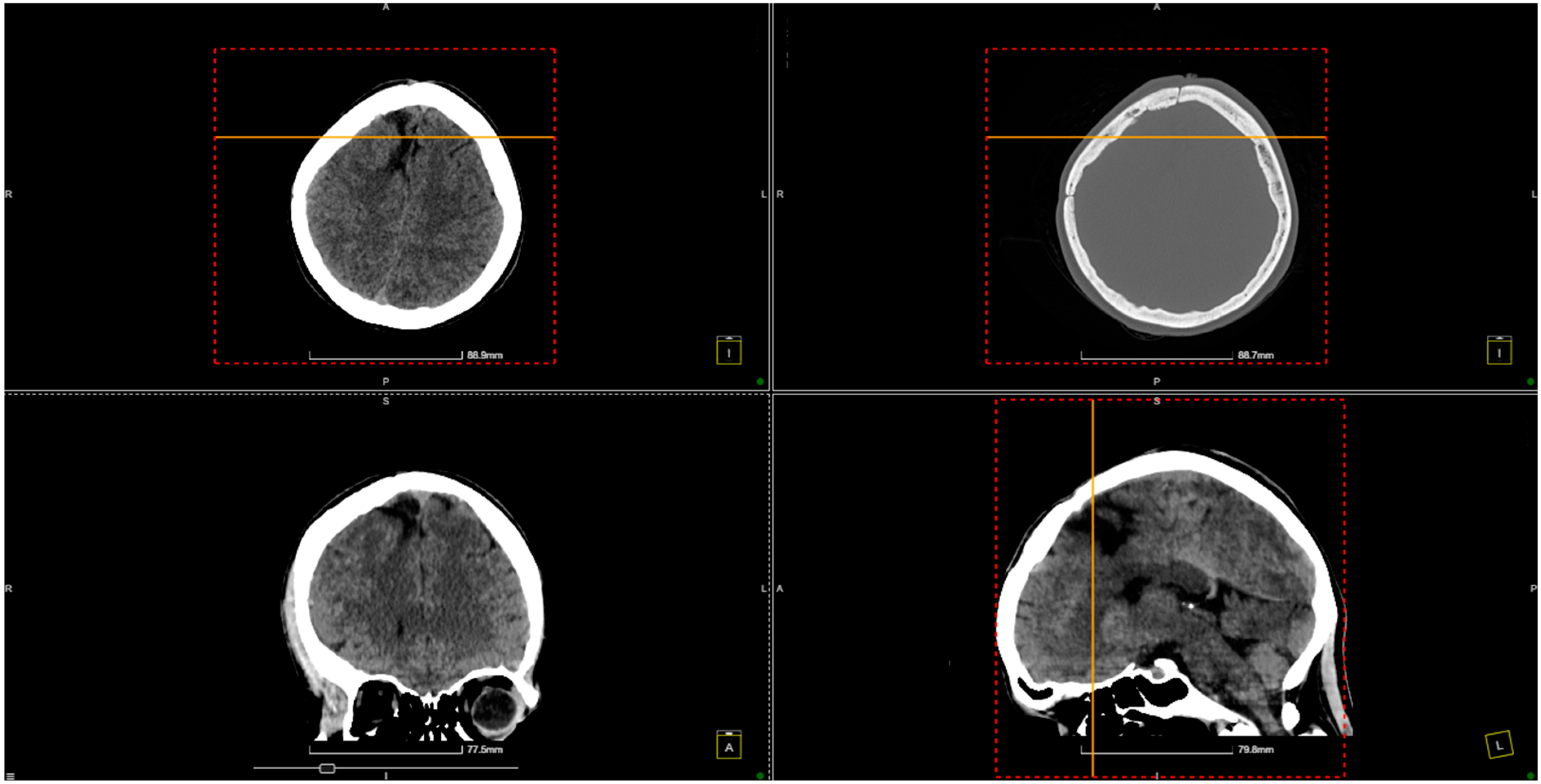
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Chen, P.; Li, R.; Han, H.; Li, Z.; Ma, L.; Yan, D.; Zhang, H.; Lin, F.; Li, R.; et al. Rupture-related quantitative hemodynamics of the supratentorial arteriovenous malformation nidus. J. Neurosurg. 2023, 138, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, K.; Rose, A.; Olukoya, O.; Brown, J.; George, E.J.S. Spontaneous obliteration of brain arteriovenous malformations: Illustrative cases. J. Neurosurg. Case Lessons 2022, 4, CASE22309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, H.; Meng, X.; Jin, H.; Gao, D.; Ma, L.; Li, R.; Li, Z.; Yan, D.; Zhang, H.; et al. Development and Validation of a Scoring System for Hemorrhage Risk in Brain Arteriovenous Malformations. JAMA Netw. Open 2023, 6, e231070. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, D.; Li, Z.; Ma, L.; Zhao, Y.; Wang, H.; Ye, X.; Meng, X.; Jin, H.; Li, Y.; et al. Long-Term Outcomes of Elderly Brain Arteriovenous Malformations After Different Management Modalities: A Multicenter Retrospective Study. Front. Aging Neurosci. 2021, 13, 609588. [Google Scholar] [CrossRef]
- Rodrigues, S.G.; Oliveira, R.; Vilela, P.; Oliveira, S.N. Spontaneous thrombosis of cerebral arteriovenous malformation post COVID-19. Neurol. Sci 2021, 42, 4909–4911. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Lin, T.-M.; Wu, H.-M.; Lee, C.-C.; Yang, H.-C.; Luo, C.-B.; Guo, W.-Y.; Chung, W.-Y.; Lin, C.-J. Stagnant venous outflow in ruptured arteriovenous malformations revealed by delayed quantitative digital subtraction angiography. Eur. J. Radiol. 2021, 134, 109455. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Birk, H.; Burkhardt, J.-K.; Chen, X.; Yue, J.K.; Guo, D.; Rutledge, W.C.; Lasker, G.F.; Partow, C.; Tihan, T.; et al. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J. Neurosurg. 2018, 129, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, W.; Jacobs, S.; Sluzewski, M.; van der Pol, B.; Beute, G.; Sprengers, M. Curative Embolization of Brain Arteriovenous Malformations with Onyx: Patient Selection, Embolization Technique, and Results. Am. J. Neuroradiol. 2012, 33, 1299–1304. [Google Scholar] [CrossRef]
- Chen, X.; Lu, X.; Yan, F.; Xu, W.; Gao, L.; Zheng, J.; Yu, J.; Chen, X.; Lu, X.; Yan, F.; et al. Spontaneous thrombosis in main draining veins of unruptured cerebral arteriovenous malformations. Medicine 2019, 98, e15588. [Google Scholar] [CrossRef]
- Taha, M.; Patel, U.; Wharton, S.B.; Cooper, P.C.; Makris, M. Fatal spontaneous thrombosis of a cerebral arteriovenous malformation in a young patient with a rare heterozygous prothrombin gene mutation. Case report. J. Neurosurg. 2007, 106, 143–146. [Google Scholar] [CrossRef]
- Ulumuddin, M.I.; Sani, A.F.; Kurniawan, D. Spontaneous thrombosis of deep brain arteriovenous malformation in a patient with intraventricular and subarachnoid hemorrhage. Radiol. Case Rep. 2023, 18, 3620–3625. [Google Scholar] [CrossRef]
- Krapf, H.; Siekmann, R.; Freudenstein, D.; Küker, W.; Skalej, M. Spontaneous occlusion of a cerebral arteriovenous maformation: Angiography and MR imaging follow-up and review of the literature. AJNR Am. J. Neuroradiol. 2001, 22, 1556–1560. [Google Scholar] [PubMed]
- Fry, D.L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ. Res. 1968, 22, 165–197. [Google Scholar] [CrossRef]
- Viñuela, F.; Nombela, L.; Roach, M.R.; Fox, A.J.; Pelz, D.M. Stenotic and occlusive disease of the venous drainage system of deep brain AVM’s. J. Neurosurg. 1985, 63, 180–184. [Google Scholar] [CrossRef]
- Quisling, R.; Mickle, J. Venous pressure measurements in vein of Galen aneurysms. AJNR Am. J. Neuroradiol. 1989, 10, 411–417. [Google Scholar]
- Sundquist, K.; Wang, X.; Svensson, P.J.; Sundquist, J.; Hedelius, A.; Lönn, S.L.; Zöller, B.; Memon, A.A. Plasminogen activator inhibitor-1 4G/5G polymorphism, factor V Leiden, prothrombin mutations and the risk of VTE recurrence. Thromb. Haemost. 2015, 114, 1156–1164. [Google Scholar] [CrossRef]
- Favaloro, E.J. Genetic Testing for Thrombophilia-Related Genes: Observations of Testing Patterns for Factor V Leiden (G1691A) and Prothrombin Gene ‘Mutation’ (G20210A). Semin. Thromb. Hemost. 2019, 45, 730–742. [Google Scholar] [CrossRef]
- Marchiori, A.; Mosena, L.; Prins, M.H.; Prandoni, P. The risk of recurrent venous thromboembolism among heterozygous carriers of factor V Leiden or prothrombin G20210A mutation. A systematic review of prospective studies. Haematologica 2007, 92, 1107–1114. [Google Scholar] [CrossRef]
- Ren, Q.; He, M.; Zeng, Y.; Liu, Z.; Liu, H.; Xu, J. Microsurgery for intracranial arteriovenous malformation: Long-term outcomes in 445 patients. PLoS ONE 2017, 12, e0174325. [Google Scholar] [CrossRef] [PubMed]
- Tonchev, N.; Pinchuk, A.; Dumitru, C.A.; Neyazi, B.; Swiatek, V.M.; Stein, K.P.; Sandalcioglu, I.E.; Rashidi, A. Postoperative Acute Intracranial Hemorrhage and Venous Thromboembolism in Patients with Brain Metastases Receiving Acetylsalicylic Acid Perioperatively. Curr. Oncol. 2024, 31, 4599–4612. [Google Scholar] [CrossRef]
- Lin, T.M.; Yang, H.C.; Lee, C.C.; Wu, H.M.; Hu, Y.S.; Luo, C.B.; Guo, W.Y.; Kao, Y.H.; Chung, W.Y.; Lin, C.J. Stasis index from hemodynamic analysis using quantitative DSA correlates with hemorrhage of supratentorial arteriovenous malformation: A cross-sectional study. J. Neurosurg. 2019, 132, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.K.; Hu, Y.-S.; Lin, T.-M.; Lin, C.-J.; Lirng, J.-F.; Wu, H.-M.; Yang, H.-C.; Lee, C.-C.; Luo, C.-B.; Guo, W.-Y. Shortened cerebral circulation time correlates with seizures in brain arteriovenous malformation: A cross-sectional quantitative digital subtraction angiography study. Eur. Radiol. 2022, 32, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Rämö, J.T.; Jurgens, S.J.; Niiranen, T.; Sanna-Cherchi, S.; Bauer, K.A.; Haj, A.; Choi, S.H.; Palotie, A.; Daly, M.; et al. Thrombosis risk in single- and double-heterozygous carriers of factor V Leiden and prothrombin G20210A in FinnGen and the UK Biobank. Blood 2024, 143, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Bauer, K.A. Managing thromboembolic risk in patients with hereditary and acquired thrombophilias. Blood 2020, 135, 344–350. [Google Scholar] [CrossRef]
- Colucci, M.; Binetti, B.M.; Tripodi, A.; Chantarangkul, V.; Semeraro, N. Hyperprothrombinemia associated with prothrombin G20210A mutation inhibits plasma fibrinolysis through a TAFI-mediated mechanism. Blood 2004, 103, 2157–2161. [Google Scholar] [CrossRef]
| Study | Number of Patients | Sex Ratio | AVM Localization | Rupture Status | AVM Diameter | Associated Complications | Arterial Feeder(s) | Venous Outflow(s) | Size of Nidus (cm) | Surgical Approach | Treatment Method | Characteristics of Thrombophilia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2023) [20] | 4 | Mixed | Deep brain regions | All ruptured | 0.79–2.56 cm | Hemorrhage, neurological deficits | Tiny arterial branches | Single draining vein | 0.79–2.56 | Transvenous embolization | Onyx embolization | Thrombophilia not detailed, but hemorrhage risks increased in hypercoagulable states |
| Massoud et al. (2022) [1] | 4 | Mixed | Supratentorial | Ruptured | 0.79–2.56 cm | Hemorrhage, embolization-induced rupture | Small feeders with tortuous paths | Single draining vein | 0.79–2.56 | Transvenous embolization | Onyx-based TVE | TVE management helped control complications associated with thrombosis risks |
| Chen et al. (2021) [4] | 5 | Mixed | Deep brain | Ruptured | Variable | Neurological deficits, hemorrhage | Variable deep feeders | Single draining vein | Variable | Transvenous embolization | Combined approach (arterial and venous) | No direct thrombophilia analysis, but TVE showed good outcomes in hemorrhage control |
| Rodrigues et al. (2020) [5] | 1 | Male | Parieto-temporal | Thrombosed (spontaneous) | Not specified | Seizures, focal neurological deficits | Anterior cerebral artery | Parieto-temporal cortical vein | Not specified | Conservative management | Anticoagulation, dexamethasone | Thrombosis linked to hypercoagulable state induced by COVID-19, leading to spontaneous AVM thrombosis |
| Lin et al. (2019) [21] | 45 | Mixed | Supratentorial | 36% ruptured | 1.8–6.2 cm | Hemorrhage, seizures | MCA feeders | Multiple venous drainers | 3.2 | Superselective catheterization | Endovascular embolization | Thrombophilia not directly associated with but implicated in hemorrhage risk |
| Burkhardt et al. (2018) [7] | 24 | Mixed | Supratentorial | Mixed | Variable | Venous drainage issues | Small arterial feeders | Single draining vein | Variable | Transvenous embolization | Combined transarterial and transvenous embolization | No thrombophilia data, but complications noted with venous thrombosis |
| Rooij et al. (2011) [8] | 24 | Mixed | Frontal, occipital, parietal, temporal | Mixed | 1–3 cm | Hemorrhage, seizures | MCA and ACA feeders | Single superficial draining vein | 1–3 | Curative embolization | Onyx embolization | Thrombophilia not directly analyzed, but embolization was complicated by venous reflux |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, C.; Brehar, F.-M.; Radoi, M.P.; Serban, M.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Ciurea, A.V.; Dobrin, N. The Microsurgical Resection of an Arteriovenous Malformation in a Patient with Thrombophilia: A Case Report and Literature Review. Diagnostics 2024, 14, 2613. https://doi.org/10.3390/diagnostics14232613
Toader C, Brehar F-M, Radoi MP, Serban M, Covache-Busuioc R-A, Glavan L-A, Ciurea AV, Dobrin N. The Microsurgical Resection of an Arteriovenous Malformation in a Patient with Thrombophilia: A Case Report and Literature Review. Diagnostics. 2024; 14(23):2613. https://doi.org/10.3390/diagnostics14232613
Chicago/Turabian StyleToader, Corneliu, Felix-Mircea Brehar, Mugurel Petrinel Radoi, Matei Serban, Razvan-Adrian Covache-Busuioc, Luca-Andrei Glavan, Alexandru Vlad Ciurea, and Nicolaie Dobrin. 2024. "The Microsurgical Resection of an Arteriovenous Malformation in a Patient with Thrombophilia: A Case Report and Literature Review" Diagnostics 14, no. 23: 2613. https://doi.org/10.3390/diagnostics14232613
APA StyleToader, C., Brehar, F.-M., Radoi, M. P., Serban, M., Covache-Busuioc, R.-A., Glavan, L.-A., Ciurea, A. V., & Dobrin, N. (2024). The Microsurgical Resection of an Arteriovenous Malformation in a Patient with Thrombophilia: A Case Report and Literature Review. Diagnostics, 14(23), 2613. https://doi.org/10.3390/diagnostics14232613






