Cytomegalovirus Infections in Hematopoietic Stem Cell Transplant: Moving Beyond Molecular Diagnostics to Immunodiagnostics
Abstract
1. Introduction
1.1. CMV in HSCT
1.2. CMV Diagnosis
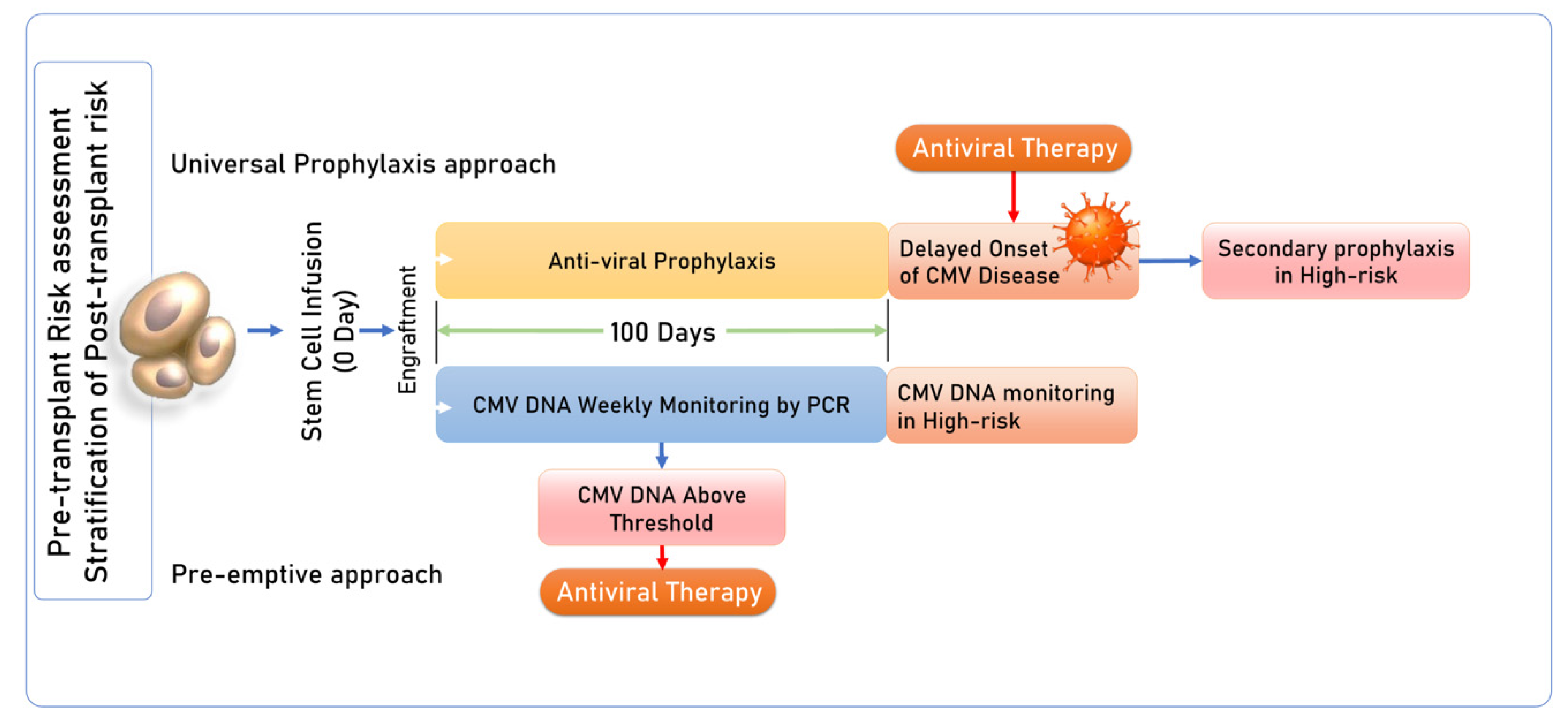
1.3. CMV Management Strategies in HSCT
1.4. CMV Immune Reconstitution and Immune Monitoring in HSCT
2. Role of Genetic Polymorphism
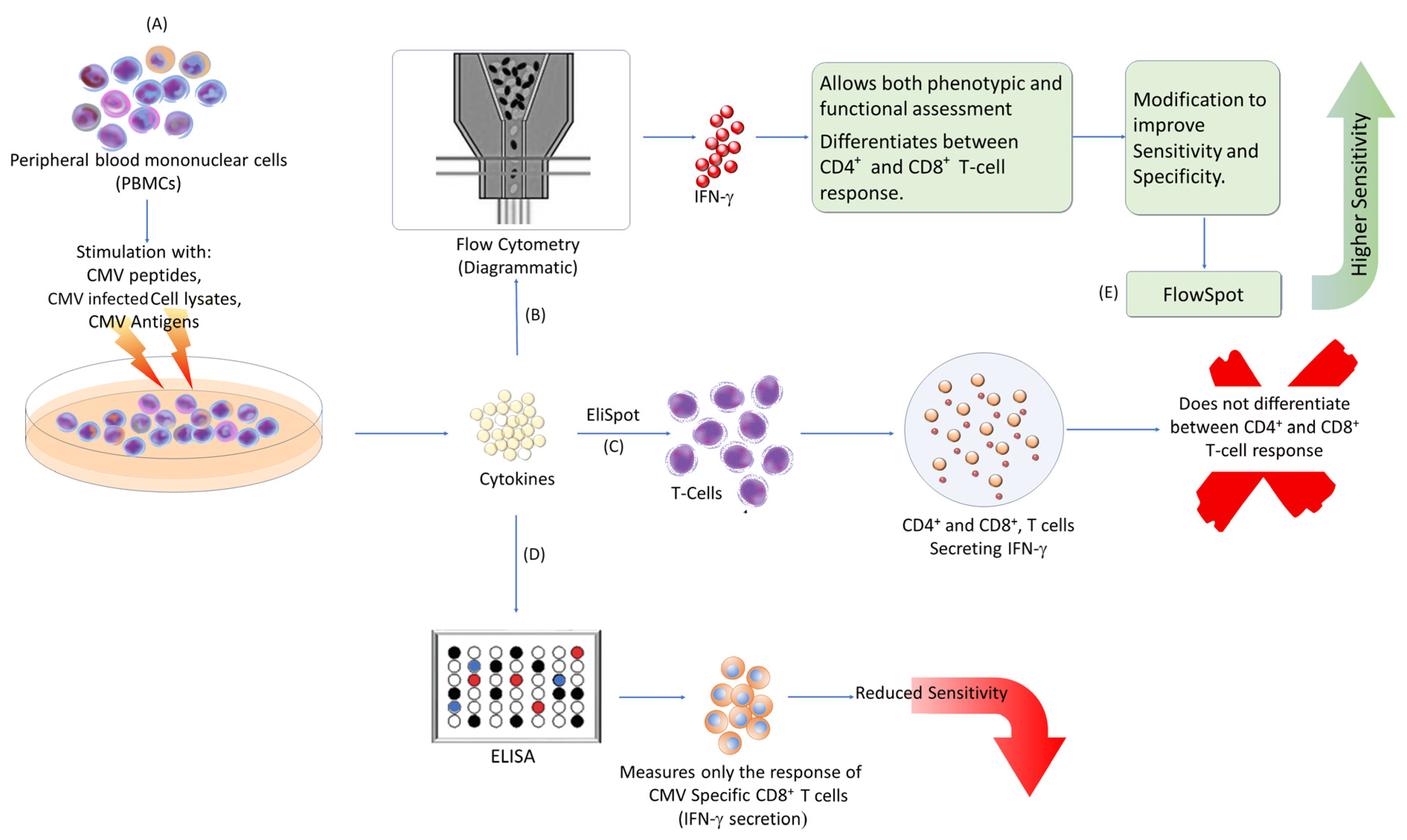
3. Method for Immune Monitoring
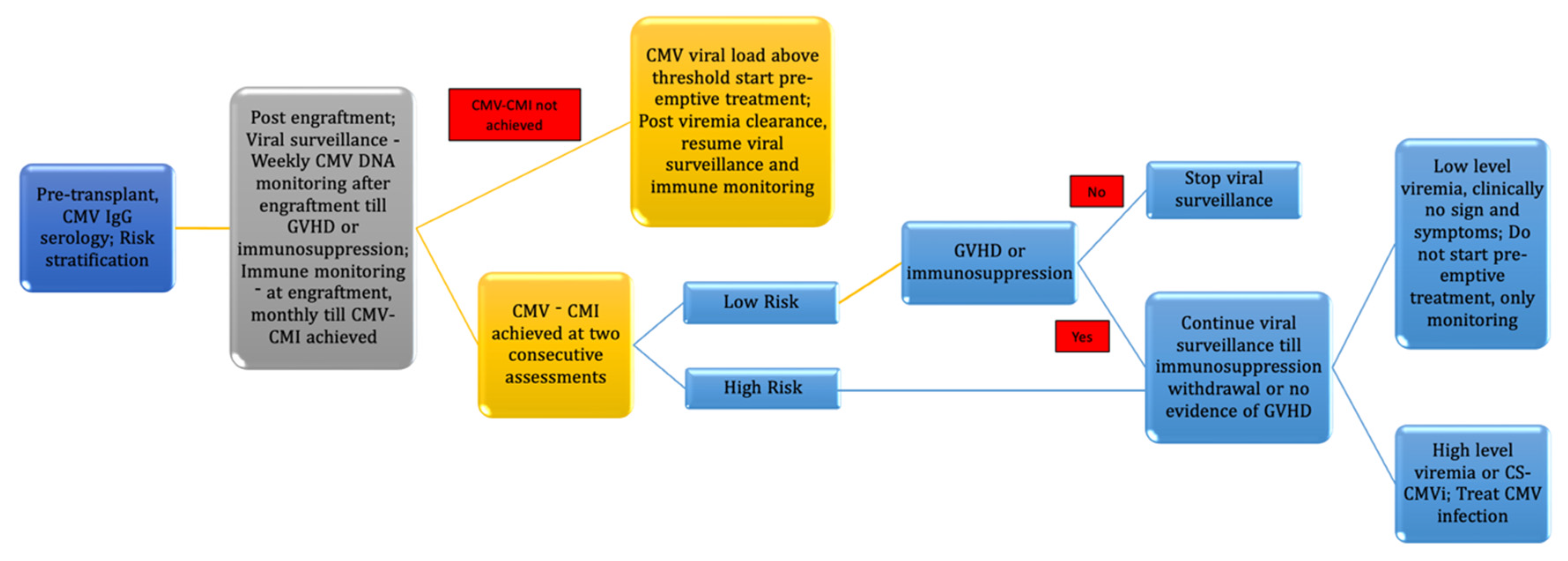
4. Utility of Immune Monitoring in HSCT
5. Functional Immune Monitoring
6. Immune Reconstitution in Haploidentical HSCT
7. Pre-Transplant Immune Monitoring
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bolovan-Fritts, C.; Mocarski, E.; Wiedeman, J. Peripheral Blood CD14+ Cells from Healthy Subjects Carry a Circular Conformation of Latent Cytomegalovirus Genome. Blood 1999, 93, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Pan, C.; Sheng, J.; Liang, H.; Bian, Z.; Liu, Y.; Trang, P.; Wu, J. Human cytomegalovirus reprogrammes hematopoietic progenitor cells into immunosuppressive monocytes to achieve latency. Nat. Microbiol. 2018, 34, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Jackson, S.E.; Wills, M.R. The CD4+ T Cell Response to Human Cytomegalovirus in Healthy and Immunocompromised People. Front. Cell. Infect. Microbiol. 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Sedikides, G.X.; Okecha, G.; Wills, M.R. Generation, maintenance and tissue distribution of T cell responses to human cytomegalovirus in lytic and latent infection. Med. Microbiol. Immunol. 2019, 208, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Munks, M.W.; Rott, K.; Nesterenko, P.A.; Smart, S.M.; Williams, V.; Tatum, A.; Xu, G.; Smith, T.; Murray, S.E.; Hill, A.B. Latent CMV infection of Lymphatic endothelial cells is sufficient to drive CD8 T cell memory inflation. PLoS Pathog. 2023, 19, e1010351. [Google Scholar] [CrossRef]
- van den Brink, M.R.; Velardi, E.; Perales, M.A. Immune reconstitution following stem cell transplantation. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 215–219. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, D.G.; Kim, H.J. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int. J. Mol. Sci. 2019, 20, 2666. [Google Scholar] [CrossRef]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol. Oncol. Clin. N. Am. 2011, 25, 151–169. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Husain, S.; Kwak, E.J.; Silveira, F.P.; Ndirangu, M.; Tran, J.; Shutt, K.A.; Shapiro, R.; Thai, N.; Abu-Elmagd, K.; et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin. Infect. Dis. 2007, 44, 204–212. [Google Scholar] [CrossRef]
- Fishman, J.A.; Emery, V.; Freeman, R.; Pascual, M.; Rostaing, L.; Schlitt, H.J.; Sgarabotto, D.; Torre-Cisneros, J.; Uknis, M.E. Cytomegalovirus in transplantation—Challenging the status quo. Clin. Transpl. 2007, 21, 149–158. [Google Scholar] [CrossRef]
- Humar, A.; Snydman, D. AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplant recipients. Am. J. Transpl. 2009, 9, S78–S86. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, M.; Nichols, W.G. Immunosuppressive effects of beta-herpesviruses. Herpes J. IHMF 2003, 10, 12–16. [Google Scholar]
- Razonable, R. Direct and indirect effects of cytomegalovirus: Can we prevent them? Enferm. Infec. Microbiol. Clin. 2010, 28, 1–5. [Google Scholar] [CrossRef]
- Razonable, R.R.; Inoue, N.; Pinninti, S.G.; Boppana, S.B.; Lazzarotto, T.; Gabrielli, L.; Simonazzi, G.; Pellett, P.E.; Schmid, D.S. Clinical Diagnostic Testing for Human Cytomegalovirus Infections. J. Infect. Dis. 2020, 221 (Suppl. 1), S74–S85. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Åsberg, A.; Chou, S.; Danziger-Isakov, L.; Humar, A. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013, 96, 333–360. [Google Scholar] [CrossRef]
- Emery, V.; Zuckerman, M.; Jackson, G.; Aitken, C.; Osman, H.; Pagliuca, A.; Potter, M.; Peggs, K.; Clark, A. Management of cytomegalovirus infection in hematopoietic stem cell transplantation. Br. J. Haematol. 2013, 162, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Boeckh, M.; Singh, N. Cytomegalovirus Infection in Solid Organ and Hematopoietic Cell Transplantation: State of the Evidence. J. Infect. Dis. 2020, 221, S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Lazzarotto, T.; Bonifazi, F.; Patriarca, F.; Irrera, G.; Ciceri, F.; Aversa, F.; Citterio, F.; Cillo, U.; Cozzi, E.; et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin. Transplant. 2019, 33, e13666. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Robin, C.; Thiebaut, A.; Alain, S.; de Fontbrune, F.S.; Berceanu, A.; d’Aveni, M.; Ceballos, P.; Redjoul, R.; Nguyen-Quoc, S.; Bénard, N.; et al. Letermovir for secondary prophylaxis of cytomegalovirus infection and disease after allogeneic hematopoietic cell transplantation: Results from the French Compassionate Program. Biol. Blood Marrow Transplant. 2020, 26, 978–984. [Google Scholar] [CrossRef]
- Yanir, A.; Schulz, A.; Lawitschka, A.; Nierkens, S.; Eyrich, M. Immune Reconstitution After Allogeneic Haematopoietic Cell Transplantation: From Observational Studies to Targeted Interventions. Front. Pediatr. 2022, 9, 786017. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, K.; Sun, Y.; Wen, R.; Zhang, X.; Li, X.; Long, B. Diagnosis and treatment for the early stage of cytomegalovirus infection during hematopoietic stem cell transplantation. Front. Immunol. 2022, 13, 971156. [Google Scholar] [CrossRef]
- Simons, L.; Cavazzana, M.; André, I. Concise Review: Boosting T-Cell Reconstitution Following Allogeneic Transplantation-Current Concepts and Future Perspectives. Stem. Cells Transl. Med. 2019, 8, 650–657. [Google Scholar] [CrossRef]
- Lilleri, D.; Gerna, G.; Zelini, P.; Chiesa, A.; Rognoni, V.; Mastronuzzi, A.; Giorgiani, G.; Zecca, M.; Locatelli, F. Monitoring of human cytomegalovirus and virus-specific T-cell response in young patients receiving allogeneic hematopoietic stem cell transplantation. PLoS ONE 2012, 7, e41648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheinberg, P.; Melenhorst, J.J.; Brenchley, J.M.; Hill, B.J.; Hensel, N.F.; Chattopadhyay, P.K.; Roederer, M.; Picker, L.J.; Price, D.A.; Barrett, A.J.; et al. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood 2009, 114, 5071–5080. [Google Scholar] [CrossRef]
- Luo, X.H.; Huang, X.J.; Liu, K.Y.; Xu, L.P.; Liu, D.H. Protective immunity transferred by infusion of CMV-specific CD8+ T cells within donor grafts its associations with CMV reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation CMV-specific CD8+ T cells within donor grafts. Biol. Blood Marrow Transplant. 2010, 16, 994–1004. [Google Scholar] [CrossRef]
- Gandhi, M.K.; Wills, M.R.; Okecha, G.; Day, E.K.; Hicks, R.; Marcus, R.E.; Sissons, J.G.P.; Carmichael, A.J. Late diversification in the clonal composition of human cytomegalovirus-specific CD8+ T cells following allogeneic hemopoietic stem cell transplantation. Blood 2003, 102, 3427–3438. [Google Scholar] [CrossRef] [PubMed]
- Sellar, R.S.; Vargas, F.A.; Henry, J.Y.; Verfuerth, S.; Charrot, S.; Beaton, B.; Chakraverty, R.; Quezada, S.A.; Mackinnon, S.; Thomson, K.J.; et al. CMV promotes recipient T-cell immunity following reduced-intensity T-cell-depleted HSCT, significantly modulating chimerism status. Blood 2015, 125, 731–739. [Google Scholar] [CrossRef]
- Stevanović, S.; van Bergen, C.A.; van Luxemburg-Heijs, S.A.; van der Zouwen, B.; Jordanova, E.S.; Kruisselbrink, A.B.; van de Meent, M.; Harskamp, J.C.; Claas, F.H.; Marijt, E.W.; et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood 2013, 122, 1963–1973. [Google Scholar] [CrossRef]
- Vallejo, M.; Muñiz, P.; Kwon, M.; Solán, L.; Bailén, R.; Carbonell, D.; Chicano, M.; Suárez-González, J.; Catalán, P.; Bellón, J.M.; et al. Risk prediction of CMV reactivation after allogeneic stem cell transplantation using five non-HLA immunogenetic polymorphisms. Ann. Hematol. 2022, 101, 1567–1576. [Google Scholar] [CrossRef]
- Annibali, O.; Piccioni, L.; Tomarchio, V.; Circhetta, E.; Sarlo, C.; Franceschini, L.; Cantonetti, M.; Rizzo, E.; Angeletti, S.; Tirindelli, M.C.; et al. Impact of IFN lambda 3/4 single nucleotide polymorphisms on the cytomegalovirus reactivation in autologous stem cell transplant patients. PLoS ONE 2018, 13, e0200221. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.F.; Leite, L.; Pereira, P.; Vaz, C.P.; Branca, R.; Campilho, F.; Freitas, F.; Ligeiro, D.; Marques, A.; Torrado, E.; et al. PTX3 Polymorphisms Influence Cytomegalovirus Reactivation After Stem-Cell Transplantation. Front. Immunol. 2019, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Blyth, E.; Withers, B.; Clancy, L.; Gottlieb, D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence 2016, 7, 967–980. [Google Scholar] [CrossRef]
- Tey, S.K.; Kennedy, G.A.; Cromer, D.; Davenport, M.P.; Walker, S.; Jones, L.I.; Crough, T.; Durrant, S.T.; Morton, J.A.; Butler, J.P.; et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV assay to identify high-risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS ONE 2013, 8, e74744. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, Y.J.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Kang, E.S. Clinical Usefulness of Monitoring Cytomegalovirus-Specific Immunity by Quantiferon-CMV in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Ann. Lab. Med. 2017, 37, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, R.F.; El Haddad, L.; Winston, D.J.; Rowley, S.D.; Mulane, K.M.; Chandrasekar, P.; Avery, R.K.; Hari, P.; Peggs, K.S.; Kumar, D.; et al. Cytomegalovirus (CMV) Cell-Mediated Immunity and CMV Infection After Allogeneic Hematopoietic Cell Transplantation: The REACT Study. Clin. Infect. Dis. 2020, 71, 2365–2374. [Google Scholar] [CrossRef]
- Seo, E.; Choi, E.S.; Kim, J.H.; Kim, H.; Koh, K.-N.; Im, H.J.; Lee, J. Immunologic monitoring of cytomegalovirus (CMV) enzyme-linked immune absorbent spot (ELISPOT) for controlling clinically significant CMV infection in pediatric allogeneic hematopoietic stem cell transplant recipients. PLoS ONE 2021, 16, e0246191. [Google Scholar] [CrossRef]
- Nesher, L.; Shah, D.P.; Ariza-Heredia, E.J.; Azzi, J.M.; Siddiqui, H.K.; Ghantoji, S.S.; Marsh, L.Y.; Michailidis, L.; Makedonas, G.; Rezvani, K.; et al. Utility of the enzyme-linked immunospot interferon-γ–release assay to predict the risk of cytomegalovirus infection in hematopoietic cell transplant recipients. J. Infect. Dis. 2016, 213, 1701–1707. [Google Scholar] [CrossRef]
- Naik, S.; Vasileiou, S.; Aguayo-Hiraldo, P.; Mukhi, S.; Sasa, G.; Martinez, C.; Krance, R.A.; Gottschalk, S.; Leen, A. Toward Functional Immune Monitoring in Allogeneic Stem Cell Transplant Recipients. Biol. Blood Marrow Transplant. 2020, 26, 911–919. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, J.; Chen, M.; Nie, W.; Zhang, H.; Su, X.; Ling, L.; Liu, X.; Liu, L.; Wang, C.; et al. Interferon-gamma FlowSpot assay for the measurement of the T-cell response to cytomegalovirus. Heliyon 2023, 9, e16792. [Google Scholar] [CrossRef]
- Barabas, S.; Spindler, T.; Kiener, R.; Tonar, C.; Lugner, T.; Batzilla, J.; Bendfeldt, H.; Rascle, A.; Asbach, B.; Wagner, R.; et al. An optimized IFN-γ ELISpot assay for the sensitive and standardized monitoring of CMV protein-reactive effector cells of cell-mediated immunity. BMC Immunol. 2017, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Tassi, E.; Noviello, M.; De Simone, P.; Lupo-Stanghellini, M.T.; Doglio, M.; Serio, F.; Abbati, D.; Beretta, V.; Valtolina, V.; Oliveira, G.; et al. Cytomegalovirus-specific T cells restricted for shared and donor human leukocyte antigens differentially impact on cytomegalovirus reactivation risk after allogeneic hematopoietic stem cell transplantation. Haematologica 2023, 108, 1530–1543. [Google Scholar] [CrossRef]
- Yong, M.K.; Cameron, P.U.; Slavin, M.; Morrissey, C.O.; Bergin, K.; Spencer, A.; Ritchie, D.; Cheng, A.C.; Samri, A.; Carcelain, G.; et al. Identifying cytomegalovirus complications using the Quantiferon-CMV assay after allogeneic hematopoietic stem cell transplantation. J. Infect. Dis. 2017, 215, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, A.; Ackermann, J.; Goitowski, B.; Trenschel, R.; Ditschkowski, M.; Timm, J.; Ottinger, H.; Beelen, D.W.; Grüner, N.; Fiedler, M. Assessing the risk of CMV reactivation and reconstitution of antiviral immune response post bone marrow transplantation by the QuantiFERON-CMV assay and real-time PCR. J. Clin. Virol. 2018, 100, 61–66. [Google Scholar] [CrossRef]
- Król, L.; Stuchlý, J.; Hubáček, P.; Keslová, P.; Sedláček, P.; Starý, J.; Hrušák, O.; Kalina, T. Signature profiles of CMV-specific T-cells in patients with CMV reactivation after hematopoietic SCT. Bone Marrow Transplant. 2010, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Roosnek, E.; Rufer, N.; Sinzger, C.; Riegler, S.; Löffler, J.; Grigoleit, U.; Moris, A.; Rammensee, H.-G.; Kanz, L.; et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002, 99, 3916–3922. [Google Scholar] [CrossRef]
- Boeckh, M.; Ljungman, P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009, 113, 5711–5719. [Google Scholar] [CrossRef]
- Wagner-Drouet, E.; Teschner, D.; Wolschke, C.; Janson, D.; Schäfer-Eckart, K.; Gärtner, J.; Mielke, S.; Schreder, M.; Kobbe, G.; Kondakci, M.; et al. Standardized monitoring of cytomegalovirus-specific immunity can improve risk stratification of recurrent cytomegalovirus reactivation after hematopoietic stem cell transplantation. Haematologica 2021, 106, 363–374. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Zheng, R.; Yu, M.; Lin, Z.; Wang, C.; McCluskey, J.; Yang, J.; Chen, Z.; Corbett, A.J.; et al. The establishment of a cytomegalovirus -specific CD8+ T-cell threshold by kinetic modeling for the prediction of post-hemopoietic stem cell transplant reactivation. iScience 2022, 25, 105340. [Google Scholar] [CrossRef]
- Mena-Romo, J.D.; Romero, P.P.; Martín-Gandul, C.; Gentil, M.; Suárez-Artacho, G.; Lage, E.; Sánchez, M.; Cordero, E. CMV-specific T-cell immunity in solid organ transplant recipients at low risk of CMV infection. Chronology and applicability in preemptive therapy. J. Infect. 2017, 75, 336–345. [Google Scholar]
- Hall, V.G.; Humar, A.; Kumar, D. Utility of Cytomegalovirus Cell-Mediated Immunity Assays in Solid Organ Transplantation. J. Clin. Microbiol. 2022, 60, e0171621. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Ljungman, P.T.; Boeckh, M.J. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Jakharia, N.; Howard, D.; Riedel, D.J. CMV Infection in Hematopoietic Stem Cell Transplantation: Prevention and Treatment Strategies. Curr. Treat. Options Infect. Dis. 2021, 13, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-H.; Zhu, Y.; Chen, Y.-T.; Shui, L.-P.; Liu, L. CMV Infection and CMV-Specific Immune Reconstitution Following Haploidentical Stem Cell Transplantation: An Update. Front. Immunol. 2021, 12, 732826. [Google Scholar] [CrossRef] [PubMed]
- Hakki, M.; Riddell, S.R.; Storek, J.; Carter, R.A.; Stevens-Ayers, T.; Sudour, P.; White, K.; Corey, L.; Boeckh, M. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: Impact of host factors, drug therapy, and subclinical reactivation. Blood 2003, 102, 3060–3067. [Google Scholar] [CrossRef]
- Gabanti, E.; Borsani, O.; Colombo, A.A.; Zavaglio, F.; Binaschi, L.; Caldera, D.; Sciarra, R.; Cassinelli, G.; Alessandrino, E.P.; Bernasconi, P.; et al. Human Cytomegalovirus-Specific T-Cell Reconstitution and Late-Onset Cytomegalovirus Infection in Hematopoietic Stem Cell Transplantation Recipients following Letermovir Prophylaxis. Transplant. Cell. Ther. 2022, 28, 211.e1–211.e9. [Google Scholar] [CrossRef]
- Sperotto, A.; Candoni, A.; Gottardi, M.; Facchin, G.; Stella, R.; De Marchi, R.; Michelutti, A.; Cavallin, M.; Rosignoli, C.; Patriarca, F.; et al. Cytomegalovirus prophylaxis versus pre-emptive strategy: Different CD4+ and CD8+ T cell reconstitution after allogeneic hematopoietic stem cell transplantation. Transplant. Cell. Ther. 2021, 27, 518.e1–518.e4. [Google Scholar] [CrossRef]
- Zamora, D.; Duke, E.R.; Xie, H.; Edmison, B.C.; Akoto, B.B.; Kiener, R.; Stevens-Ayers, T.; Wagner, R.; Mielcarek, M.; Leisenring, W.M.; et al. Cytomegalovirus-specific T-cell reconstitution following Letermovir prophylaxis after hematopoietic cell transplantation. Blood 2021, 138, 34–43. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Karami, S.; Nazari, H.G.; Sankanian, G.; Hamidpour, M.; Hajifathali, A. Immunotherapy with adoptive cytomegalovirus-specific T cells transfer: Summarizing latest gene engineering techniques. Health Sci. Rep. 2021, 4, e322. [Google Scholar] [CrossRef]
- Bae, S.; Jung, J.; Kim, S.-M.; Kang, Y.-A.; Lee, Y.-S.; Chong, Y.P.; Sung, H.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; et al. The Detailed Kinetics of Cytomegalovirus-specific T cell Responses after Hematopoietic Stem Cell Transplantation: 1 Year Follow-up Data. Immune Netw. 2018, 18, e2. [Google Scholar] [CrossRef]
| S. No. | Diagnostic Test |
|---|---|
| 1. | Serology: Antibody detection of anti-CMV immunoglobulin (IgM and IgG), does not indicate active viral replication. |
| 2. | Viral culture: Isolation of virus from multiple samples, including whole blood, plasma, sterile body fluids, urine, and biopsy using tissue cultures that use human embryonic fibroblast cells. However, detection of CMV in culture only indicates the presence of the virus or shedding of the virus and does not confirm active CMV disease. Moreover, these methods are time-consuming, lack sensitivity, and are rarely used nowadays. |
| 3. | Histopathology: Histopathology is the gold standard for the diagnosis of tissue-invasive CMV disease by detecting cytomegalic cells and viral-specific inclusion bodies in a tissue biopsy specimen but requires invasive procedures like bronchoscopy or endoscopy to obtain a tissue sample. |
| 4. | Antigen detection: This involves the detection of pp65 in peripheral blood leucocytes by using specific fluorescently labelled monoclonal antibodies in peripheral blood polymorph nuclear leukocytes. This technique is sensitive, specific, and quantitative. The antigen detection can also be used for monitoring therapeutic response. However, being labour-intensive is not used widely. |
| 5. | Molecular assay: PCR is highly sensitive as it can detect minute amounts of nucleic acid in various clinical samples, determine viral load, and can be used for therapeutic monitoring. |
| Studies | Study Design | Study Population | CMV Cell-Mediated Immune Assessment | Method for CMV Assessment | Results |
|---|---|---|---|---|---|
| Chemaly et al., React Study, 2020 [36] | Multicentric 13 centers USA, Canada, UK, Sweden | 241 adult patients (>18 years) Allogenic HSCT R+ | Pre-transplant: d = 14, Post-transplant: d +14, +28, every 15 d up to 6 months | CMV pp65 and IE1-specific ELISPOT assay | CMV-CMI, independent predictor of CS-CMVi (p = 0.04) CMV-CMI low in patients who experienced CS-CMVi (94%), and high CMV-CMI has less CS-CMVi (p < 0.0001) |
| Seo et al., 2021 [37] | Australia | 52 pediatric patients Allogenic HSCT D+/R− status: 45 D−/R− status: 4 D−/R− status: 3 | Pre-transplant: once Post-transplant: 1, 2, 3, 4, 5, 6, 9, 12 months Monitoring terminated when CMV-CMI recovered at two consecutive evaluations | CMV pp65 and IE1-specific ELISpot assay | Pre-HSCT CMV-specific CMI > 5 SFC/2 × 105 cells significant predictive factor CMV-CMI recovery post-HSCT, but not CS-CMVi; Recovery of CMV-CMI > 50 SFC/2 × 105 cells Post-HSCT protective factor for CS-CMVi (aOR = 0.13; 95% CI = 0.22–0.71) |
| Lilleri et al., 2012 [24] | Italy | 131 adult and pediatric patients Allogenic HSCT D+/R+ status: 51 D−/R+ status: 38 D+/R− status: 42 | Post-transplant— monthly until day 180, then every 3 months until the detection of CMV-specific CD4+ and CD8+ T cells | Flow cytometric analysis for CMV-specific CD4+ and CD8+ T cells producing IFN-γ and IL-2 | In the absence of GvHD, virus-specific T cell immune response remained stable after recovery, and the patients did not require treatment for CMV reactivation after achieving protective immunity |
| Nesher et al. [38] | USA | 63 adult patients Allogeneic HSCT D+/R+ status: 41 D−/R+ status: 22 | Pre-transplant Post-transplant d 30 (±7 d), d 60 (±7 d), and d 100 (±14 d) | CMV-specific IE-1 and pp65 antigens ELISpot assay | Thresholds (50 spots/250,000 cells for IF-1; 100 spots /250,000 cells for pp65) identified patients who were protected against CMV infection CMV-specific ELISpot response above the determined thresholds only significant factor for preventing CMV reactivation (aHR—0.21; 95% confidence interval, p = 0.046) |
| Tey et al., 2013 [34] | Australia | 41 adult patients (>18 years) Allogenic HSCT D+/R− status: 14 D−/R− status: 24 D−/R− status: 3 | Post-transplant weekly between 3 and 14 weeks, 6 and 12 months | CMV-specific peptides pp65, IE1, epitopes from pp50, IE2 and gB; QuantiFERON CMV assay | The median time to stable CMV-specific immune reconstitution was 59 days, incidence of CMV reactivation lower in patients who developed this than those who did not (27% versus 65%; p = 0.031) Failure to reconstitute CMV-specific immunity soon after the onset of CMV viremia associated with higher peak viral loads (5685 copies/mL versus 875 copies/mL; p = 0.002) |
| Naik et al. [39] | USA | 23 pediatric patients >2 years Allogenic HSCT | Pre-transplant: once Post-transplant: 1, 2, 3, 4, 5, 6, 9, 12 months | CMV pp65, IE1-specific IFNg ELISpot Longitudinal assessment of CD3+ T cells by FACS | Recipients with CD3+ counts >300 cells/microL exhibited potent functionally protective levels of CMV-directed T cell activity (defined as >30 antigen-specific SFCs/5 × 105), rapidly cleared viral reactivation |
| Lee et al. [35] | Republic of Korea | 33 pediatric patients Allo-HSCT D+/R+ status: 16 D+/R− status: 7 D−/R− status: 2 | Baseline at 4 weeks post-HSCT Every time with CMV antigenemia (pp65) till the end of CMV treatment, 7 d after CMV antigenemia becomes negative | QuantiFERON CMV assay | Patients who had positive QF-CMV results after CMV reactivation had no recurrent infections thereafter, patients with indeterminate or negative results had recurrent CMV infections. Established CMV-specific T cell immunity following initial CMV infection to prevent recurrent CMV infection episodes |
| 1 | Predict CMV reactivation |
| 2 | Shorten the duration of pre-emptive therapy |
| 3 | Predict those who could clear the virus spontaneously |
| 4 | Predict those who could contract an end-organ disease |
| 5 | Predict recurrent CMV reactivation following the first reactivation episode |
| 6 | To determine the need for adoptive T cell therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, C.; Mundan, N.G.; Das, S.; Jawed, A.; Dar, S.A.; Dailah, H.G. Cytomegalovirus Infections in Hematopoietic Stem Cell Transplant: Moving Beyond Molecular Diagnostics to Immunodiagnostics. Diagnostics 2024, 14, 2523. https://doi.org/10.3390/diagnostics14222523
Gupta C, Mundan NG, Das S, Jawed A, Dar SA, Dailah HG. Cytomegalovirus Infections in Hematopoietic Stem Cell Transplant: Moving Beyond Molecular Diagnostics to Immunodiagnostics. Diagnostics. 2024; 14(22):2523. https://doi.org/10.3390/diagnostics14222523
Chicago/Turabian StyleGupta, Chhavi, Netto George Mundan, Shukla Das, Arshad Jawed, Sajad Ahmad Dar, and Hamad Ghaleb Dailah. 2024. "Cytomegalovirus Infections in Hematopoietic Stem Cell Transplant: Moving Beyond Molecular Diagnostics to Immunodiagnostics" Diagnostics 14, no. 22: 2523. https://doi.org/10.3390/diagnostics14222523
APA StyleGupta, C., Mundan, N. G., Das, S., Jawed, A., Dar, S. A., & Dailah, H. G. (2024). Cytomegalovirus Infections in Hematopoietic Stem Cell Transplant: Moving Beyond Molecular Diagnostics to Immunodiagnostics. Diagnostics, 14(22), 2523. https://doi.org/10.3390/diagnostics14222523






