Racial Disparities in Inpatient Hospital Outcomes of Primary Sclerosing Cholangitis in United States: Nationwide Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Study Variables
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Trends in PSC-Related Discharges
3.2. Characteristics of Patients with PSC
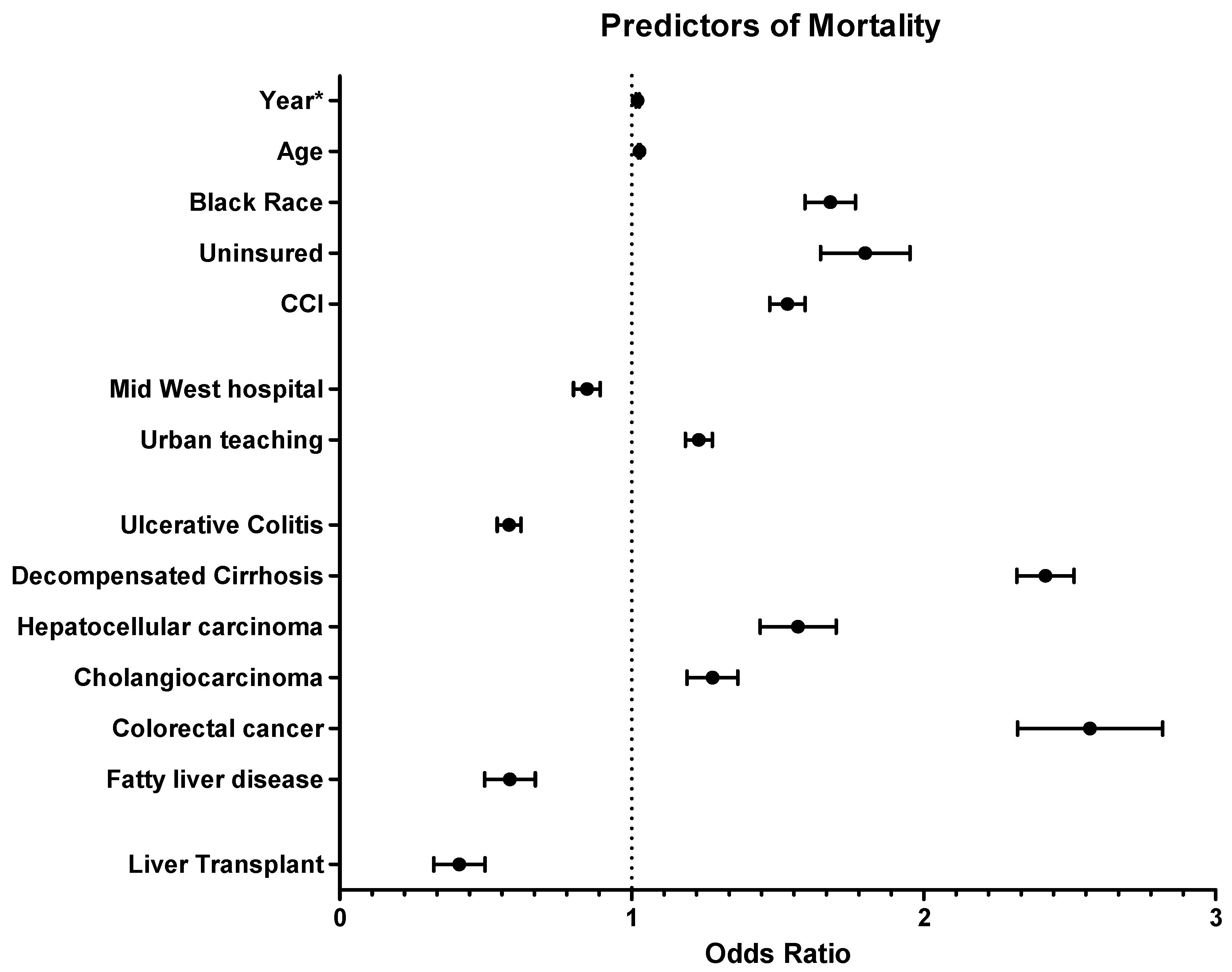
3.3. Independent Predictors of In-Hospital Mortality
3.4. Independent Predictors of Total Charge
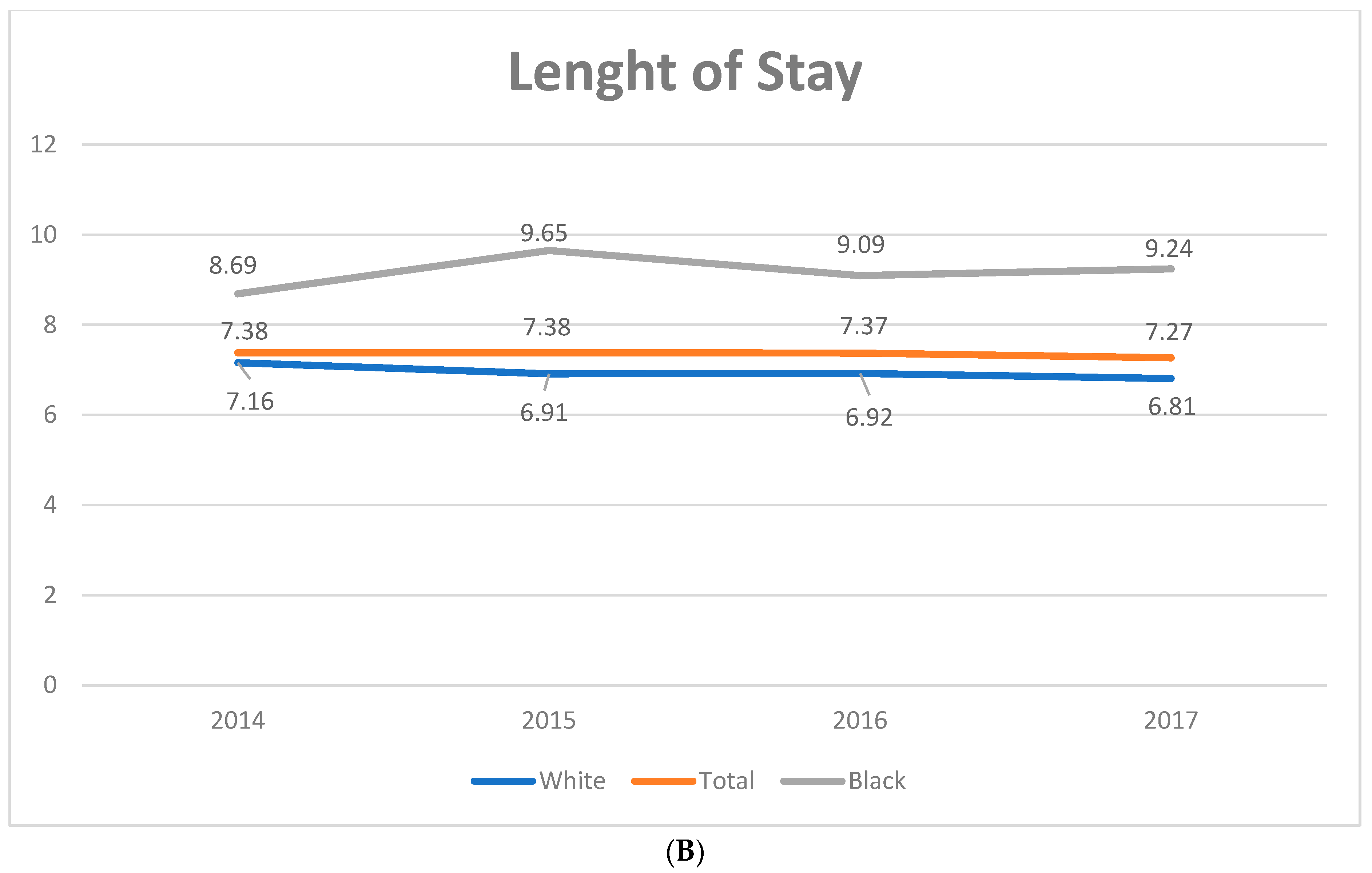
3.5. Independent Predictors of LoS
4. Discussion
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis—A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef] [PubMed]
- Carrijo, I.; Ugrinovich, L.; Simioni, P. Primary sclerosing cholangitis associated with inflammatory bowel disease. Int. Med. 2019, 1, 220. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary sclerosing cholangitis. Lancet 2013, 382, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.N.; Larusso, N.F. Primary Sclerosing Cholangitis. New Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Peerani, F.; Castaneda, D.; Torres, J.; Itzkowitz, S.H. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver 2018, 12, 17–29. [Google Scholar] [CrossRef]
- Lindor, K.D.; Kowdley, K.V.; Harrison, E.M. American College of Gastroenterology. ACG Clinical Guideline: Primary sclerosing cholangitis. Am. J. Gastroenterol. 2015, 110, 646–659. [Google Scholar] [CrossRef]
- Boonstra, K.; Beuers, U.; Ponsioen, C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J. Hepatol. 2012, 56, 1181–1188. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Kareemi, H.; Parab, R.; Barkema, H.W.; Quan, H.; Myers, R.P.; Kaplan, G.G. Incidence of primary sclerosing cholangitis: A systematic review and metaanalysis. Hepatology 2011, 53, 1590–1599. [Google Scholar] [CrossRef]
- Overview of the National (Nationwide) Inpatient Sample (NIS). In NIS Overview; Agency for Healthcare Research and Quality, Rockville, USA: 2019. Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed on 8 July 2020).
- Rockville, M. The healthcare cost and utilization project: An overview. Eff. Clin. Pract. 2002, 5, 143–151. [Google Scholar]
- 2016 Introduction to the NIS. In Healthcare Cost and Utilization Project (HCUP); Agency for Healthcare Research and Quality Rockville, USA: 2018. Available online: www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2016.jsp (accessed on 8 July 2020).
- Technical Supplement 13. In Comparative Analysis of HCUP and NHDS Inpatient Discharge Data; Agency for Healthcare Research and Quality:, Rockville, USA 2012. Available online: http://www.ahrq.gov/research/data/hcup/nhds/niscomp.html (accessed on 11 March 2020).
- HCUP Quality Control Procedures. In Healthcare Cost and Utilization Project; Agency for Healthcare Research and Quality: Rockville, USA 2019. Available online: https://www.hcup-us.ahrq.gov/db/quality.pdf (accessed on 11 March 2020).
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- McCormick, P.J.; Lin, H.M.; Deiner, S.G.; Levin, M.A. Validation of the All Patient Refined Diagnosis Related Group (APR-DRG) Risk of Mortality and Severity of Illness Modifiers as a Measure of Perioperative Risk. J. Med. Syst. 2018, 42, 81. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2010, 30, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Manne, S.; Shick, J.; Lissoos, T.; Dolin, P. Incidence, prevalence, and natural history of primary sclerosing cholangitis in the United Kingdom. Medicine 2017, 96, e7116. [Google Scholar] [CrossRef]
- Barner-Rasmussen, N.; Pukkala, E.; Jussila, A.; Färkkilä, M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: A population-based study in Finland. Scand. J. Gastroenterol. 2020, 55, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bambha, K.; Kim, W.; Talwalkar, J.; Torgerson, H.; Benson, J.T.; Therneau, T.M.; Loftus, E.V.; Yawn, B.P.; Dickson, E.R.; Melton, L.J. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003, 125, 1364–1369. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Laupland, K.B.; Butzner, D.; Urbanski, S.J.; Lee, S.S. The Burden of Large and Small Duct Primary Sclerosing Cholangitis in Adults and Children: A Population-Based Analysis. Am. J. Gastroenterol. 2007, 102, 1042–1049. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Takakura, W.R.; Tabibian, J.H.; Bowlus, C.L. The evolution of natural history of primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2017, 33, 71–77. [Google Scholar] [CrossRef]
- Charatcharoenwitthaya, P.; Enders, F.B.; Halling, K.C.; Lindor, K.D. Utility of serum tumor markers, imaging, and biliary cytology for detecting CCA in primary sclerosing cholangitis. Hepatology 2008, 48, 1106–1117. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J.; Lindor, K.D. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011, 54, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Merion, R.M.; Shearon, T.H.; Berg, C.L.; Everhart, J.E.; Abecassis, M.M.; Shaked, A.; Fisher, R.A.; Trotter, J.F.; Brown, R.S.; Terrault, N.A.; et al. Hospitalization Rates Before and After Adult-to-Adult Living Donor or Deceased Donor Liver Transplantation. Ann. Surg. 2010, 251, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Bowlus, C.L.; Li, C.-S.; Karlsen, T.H.; Lie, B.A.; Selmi, C. Primary sclerosing cholangitis in genetically diverse populations listed for liver transplantation: Unique clinical and human leukocyte antigen associations. Liver Transplant. 2010, 16, 1324–1330. [Google Scholar] [CrossRef]
- Wilder, J.; Henson, J.; Patel, Y.; Zheng, J.; Chow, S.-C.; Muir, A. Racial Differences in Phenotype and Outcome Among Patients with Primary Sclerosing Cholangitis Listed for Liver Transplant: 2016 ACG Fellows-In-Training Award (Liver Category). Am. J. Gastroenterol. 2016, 111, S341–S342. [Google Scholar] [CrossRef]
- Wiencke, K.; Boberg, K. Current consensus on the management of primary sclerosing cholangitis. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 786–791. [Google Scholar] [CrossRef]
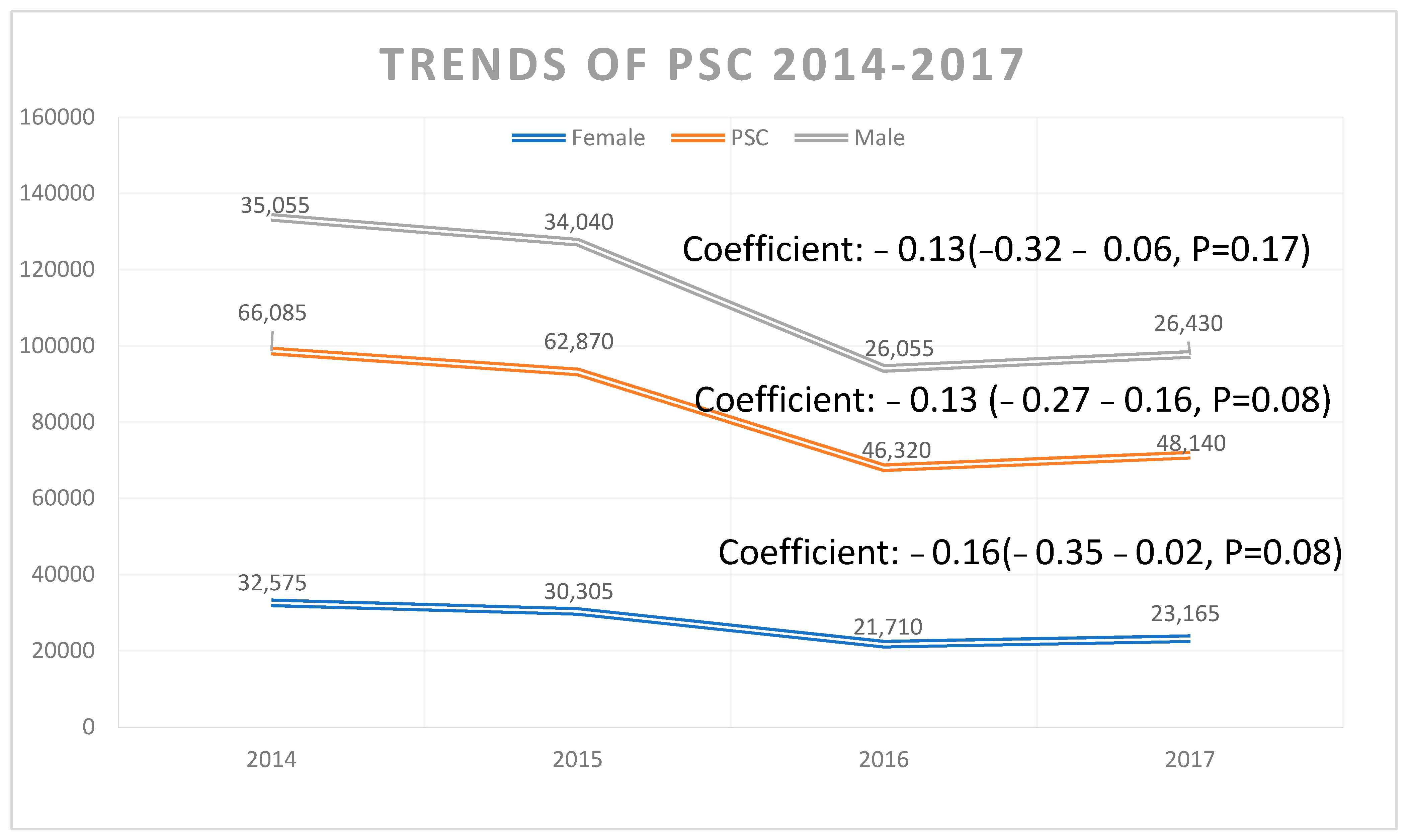
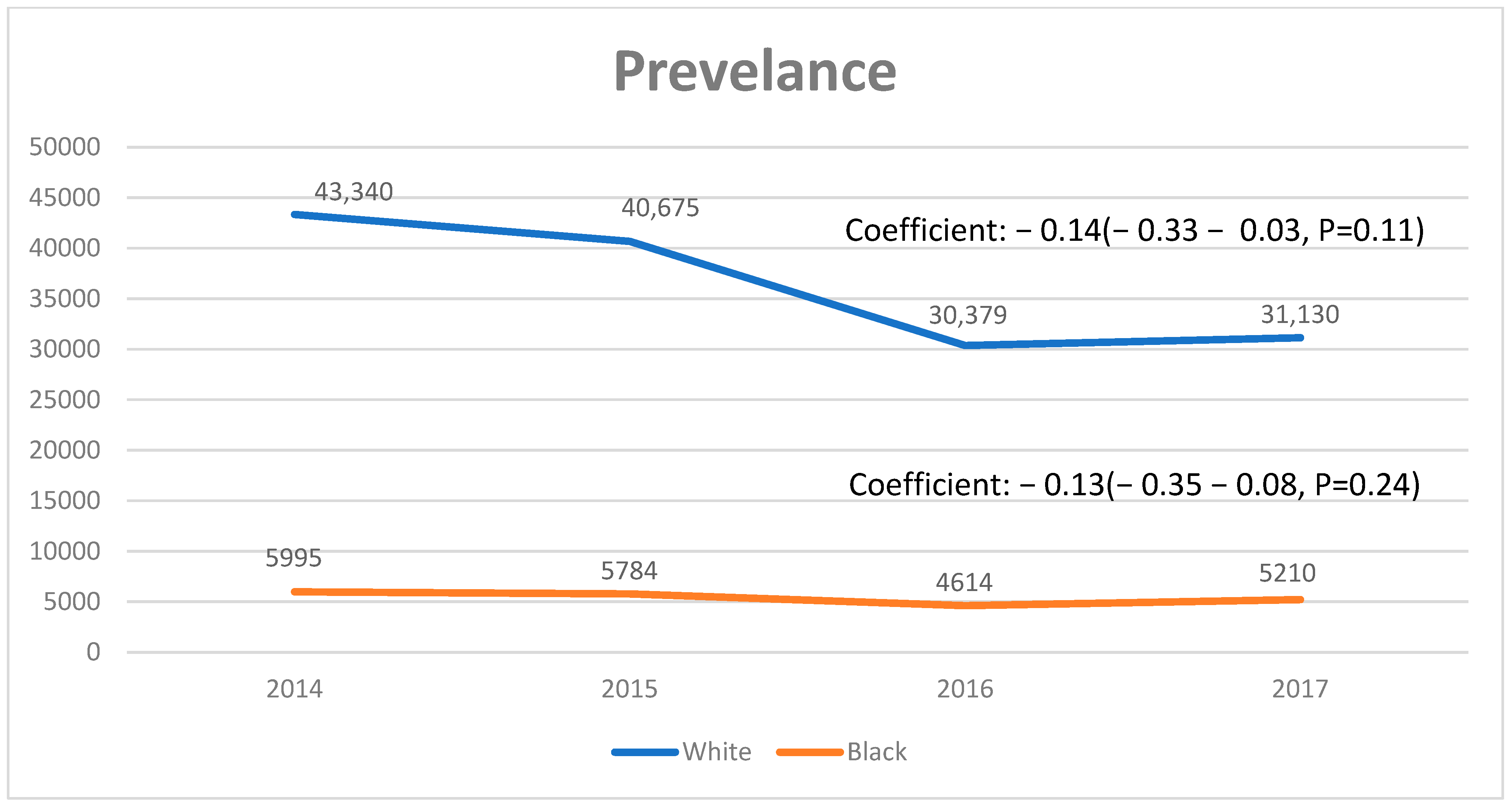
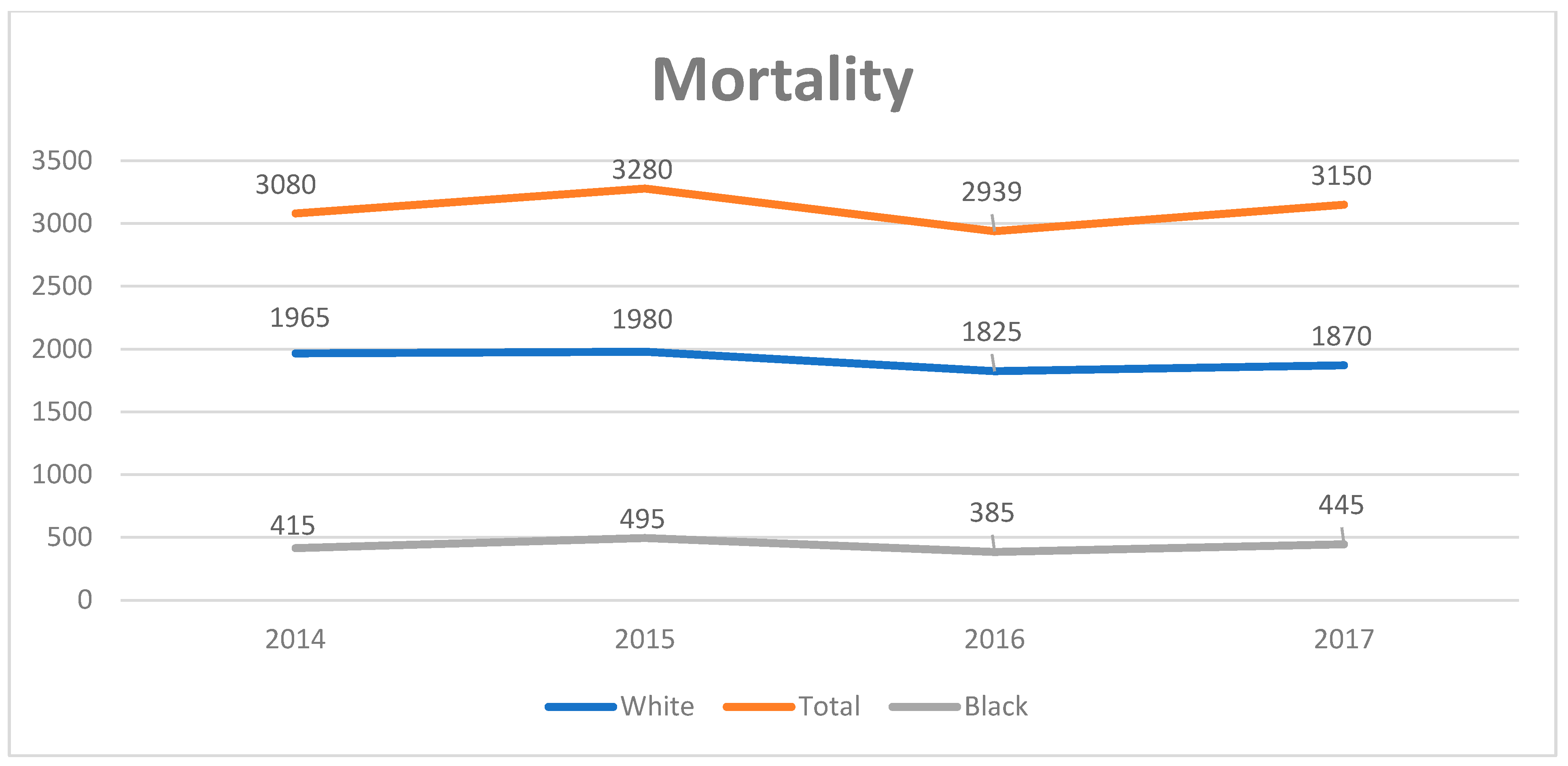
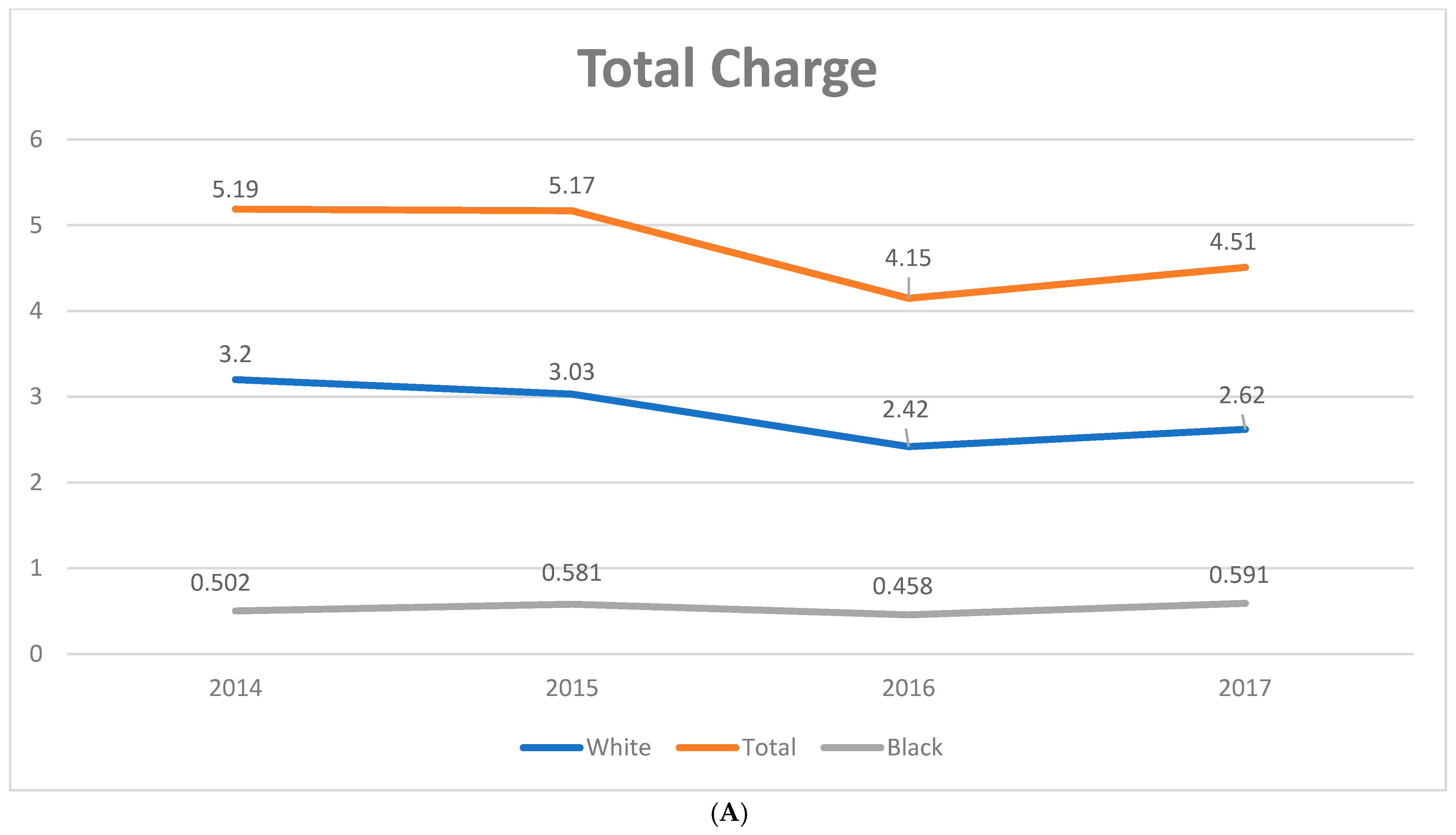
| Values Listed as Mean (95% CI) or % (N/Total) Unless Otherwise Listed | 2008–2017 (Weighted) | 2008 (Weighted) | 2017 (Weighted) | Coefficient (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age, years | 63.29 ± 0.37 | 63.29 ± 0.70 | 62.15 ± 0.37 | −0.14 (−0.27–−0.01) | 0.030 |
| Race, % | |||||
| White | 63.73 ± 0.53 | 58.09 ± 2.11 | 64.02 ± 1.14 | Reference | |
| Black | 9.06 ± 0.18 | 6.36 ± 0.55 | 11.05 ± 0.49 | 0.23 (0.10–0.37) | <0.01 |
| Hispanic | 10.81 ± 0.27 | 8.98 ± 0.96 | 11.56 ± 0.58 | 0.07 (−0.10–0.23) | 0.425 |
| Other † | 16.41 ± 0.48 | 26.67 ± 2.15 | 13.36 ± 1.15 | −0.61 (−0.82–−0.40) | <0.01 |
| Male, % | 52.30 ± 0.19 | 50.85 ± 0.73 | 53.29 ± 0.56 | 0.10 (0.05–0.13) | <0.01 |
| Primary Payer, % | |||||
| Medicare | 54.82 ± 0.31 | 52.69 ± 1.28 | 53.26 ± 0.69 | Reference | |
| Medicaid | 10.39 ± 0.18 | 9.63 ± 0.75 | 12.09 ± 0.44 | 0.20 (0.08–0.33) | 0.002 |
| Private | 28.40 ± 0.28 | 31.63 ± 1.04 | 29.44 ± 0.72 | −0.06 (−0.14–0.03) | 0.210 |
| Uninsured | 6.39 ± 1.38 | 6.06 ± 0.44 | 5.21 ± 0.28 | −0.27 (−0.39–−0.14) | <0.01 |
| Elective admission, % | 9.33 ± 0.25 | 10.62 ± 0.73 | 8.06 ± 0.41 | −0.28 (−0.41–−0.16) | <0.01 |
| Disposition status, % | |||||
| Routine | 59.55 ± 0.46 | 60.11 ± 0.84 | 54.89 ± 0.67 | Reference | |
| Short-term hospital | 4.69 ± 0.08 | 4.74 ± 0.33 | 5.15 ± 0.27 | 0.11 (−0.03–0.24) | 0.128 |
| SNF/ICF/Other | 13.33 ± 0.15 | 13.88 ± 0.57 | 13.98 ± 0.42 | 0.07 (−0.02–0.16) | 0.121 |
| Home healthcare | 16.92 ± 0.19 | 15.55 ± 0.65 | 18.88 ± 0.48 | 0.24 (0.15–0.33) | <0.01 |
| Against medical advice | 0.36 ± 0.02 | 0.51 ± 0.07 | 0.69 ± 0.08 | 0.17 (−0.13–0.47) | 0.266 |
| Died ‡ | 5.15 ± 0.07 | 5.21 ± 0.24 | 6.41 ± 0.25 | 0.25 (0.15–0.36) | <0.01 |
| Resource utilization | |||||
| Number of procedures | |||||
| LoS, days | 7.52 ± 0.05 | 8.07 ± 0.18 | 7.27 ± 0.13 | −0.08 (−0.12–−0.04) | <0.01 |
| Charge, $ § | 75,703 ± 931 | 61,873 ± 2567 | 91,262 ± 2961 | 3185 (2502–3867) | <0.01 |
| Cost, USD §|| | 22,007 ± 234 | 21,338 ± 695 | 23,330 ± 757 | 242 (78–407) | 0.004 |
| Values Listed as Mean (95% CI) or % (N/Total) Unless Otherwise Listed | 2008–2017 (Weighted) | 2008 (Weighted) | 2017 (Weighted) | Coefficient (95% CI) | p Value |
|---|---|---|---|---|---|
| Number diagnosed | |||||
| Severity of illness, % | |||||
| Minor/moderate loss of function | 16.36 ± 0.19 | 26.44 ± 0.52 | 17.25 ± 0.42 | Reference | |
| Major/extreme loss of function | 83.64 ± 0.19 | 73.56 ± 0.52 | 82.75 ± 0.42 | 0.21 (0.13–0.28) | <0.01 |
| Risk of dying, % | |||||
| Minor/moderate loss of function | 36.44 ± 0.37 | 54.73 ± 0.66 | 39.07 ± 0.52 | Reference | |
| Major/extreme loss of function | 63.56 ± 0.37 | 45.27 ± 0.66 | 60.93 ± 0.53 | 0.15 (0.08–0.23) | <0.01 |
| Severity of Cirrhosis | |||||
| Compensated cirrhosis, % | 8.35 ± 0.13 | 6.63 ± 0.49 | 10.91 ± 0.46 | 0.44 (0.34–0.55) | <0.01 |
| Primary biliary cirrhosis, % | 0.97 ± 0.04 | 1.11 ± 0.17 | 0.60 ± 0.08 | −0.42 (−0.21–−0.62) | <0.01 |
| Decompensated cirrhosis, % | 18.27 ± 0.18 | 14.18 ± 0.70 | 22.33 ± 0.55 | 0.45 (0.37–0.52) | <0.01 |
| Coexisting malignancy | |||||
| Hepatocellular carcinoma, % | 2.38 ± 0.07 | 1.37 ± 0.17 | 7.92 ± 0.39 | 2.13 (1.96–2.84) | <0.01 |
| Gall bladder carcinoma, % | 0.89 ± 0.03 | 0.74 ± 0.09 | 1.13 ± 0.11 | 0.28 (0.09–0.48) | 0.004 |
| Cholangiocarcinoma, % | 4.96 ± 0.10 | 5.12 ± 0.35 | 2.79 ± 0.17 | −0.36 (−0.25–−0.46) | <0.01 |
| Colorectal cancer, % | 1.26 ± 0.04 | 1.15 ± 0.13 | 1.58 ± 0.12 | 0.34 (0.17–0.52) | <0.01 |
| Overlap with autoimmune diseases | |||||
| Inflammatory bowel disease, % | 6.77 ± 0.13 | 6.30 ± 0.53 | 8.11 ± 0.44 | 0.22 (0.09–0.35) | 0.001 |
| Ulcerative colitis, % | 4.69 ± 0.11 | 4.36 ± 0.47 | 5.66 ± 0.36 | 0.22 (0.07–0.38) | 0.004 |
| Autoimmune hepatitis, % | 1.11 ± 0.04 | 0.37 ± 0.07 | 1.94 ± 0.17 | 0.84 (0.61–1.06) | <0.01 |
| Autoimmune disease, % † | 0.22 ± 0.01 | 0.27 ± 0.05 | 0.33 ± 0.06 | 0.13 (−0.26–0.52) | 0.510 |
| Fatty liver disease | 2.61 ± 0.05 | 1.41 ± 0.11 | 3.11 ± 2.76 | 0.55 (0.43–0.66) | <0.01 |
| Clostridium difficile | 2.15 ± 0.05 | 2.09 ± 0.16 | 1.98 ± 0.14 | 0.03 (−0.10–0.16) | 0.650 |
| Marijuana use | 0.47 ± 0.02 | 0.17 ± 0.04 | 0.84 ± 0.09 | 1.18 (1.05–1.42) | <0.01 |
| Liver transplant, % | 0.86 ± 0.05 | 0.86 ± 0.17 | 1.42 ± 0.17 | 0.46 (0.06–0.85) | 0.022 |
| CCI | 2.34 ± 0.01 | 2.13 ± 0.04 | 3.12 ± 0.03 | ||
| Secondary cholangitis | 17.88 ± 0.16 | 21.39 ± 0.66 | 15.91 ± 0.43 | −0.33 (−0.40–−0.27) | <0.01 |
| CBD stone with or without GB stone | 15.97 ± 0.19 | 18.21 ± 0.82 | 16.75 ± 0.43 | −0.33 (−0.47–−27) | <0.01 |
| Mechanical complication of bile duct prosthesis | 2.81 ± 0.06 | 3.04 ± 0.22 | 1.86 ± 0.15 | −0.40 (–0.53–−0.28) | <0.01 |
| Pancreatitis | 17.42 ± 0.15 | 16.93 ± 0.51 | 12.44 ± 0.38 | −0.20 (−0.26–−0.14) | <0.01 |
| Complications, % | |||||
| Ascites | 8.21 ± 0.01 | 6.84 ± 0.46 | 10.99 ± 0.42 | 0.46 (0.36–0.55) | <0.01 |
| Hepatic encephalopathy | 3.82 ± 0.08 | 2.06 ± 0.21 | 9.60 ± 0.38 | 1.46 (1.32–1.59) | <0.01 |
| Portal hypertension | 4.40 ± 0.10 | 3.00 ± 0.29 | 7.11 ± 0.38 | 0.73 (0.58–0.88) | <0.01 |
| Gastroesophageal varices | 3.07 ± 0.08 | 2.43 ± 0.24 | 4.06 ± 0.27 | 0.43 (0.28–0.58) | <0.01 |
| ICU admission | 6.87 ± 0.09 | 6.18 ± 0.29 | 7.29 ± 0.29 | 0.12 (0.05–0.20) | 0.002 |
| Characteristics | 2008–2017 (Weighted) | 2008 (Weighted) | 2017 (Weighted) | Coefficient (95% CI) | p Value |
|---|---|---|---|---|---|
| Hospital Region, % | |||||
| Northeast | 21.89 ± 0.67 | 23.60 ± 3.50 | 20.95 ± 1.59 | Reference | |
| Midwest | 22.35 ± 0.68 | 19.98 ± 2.52 | 22.89 ± 1.75 | 0.07 (−0.22–0.37) | 0.614 |
| South | 31.23 ± 0.63 | 29.31 ± 2.68 | 32.60 ± 1.69 | 0.14 (−0.11–0.38) | 0.266 |
| West | 24.51 ± 0.63 | 27.10 ± 3.04 | 23.54 ± 1.48 | −0.01 (−0.29–0.27) | 0.961 |
| Hospital bed size, % | |||||
| Small | 11.98 ± 0.29 | 8.44 ± 0.95 | 14.80 ± 0.88 | Reference | |
| Medium | 23.84 ± 0.48 | 22.33 ± 2.10 | 24.49 ± 1.33 | −0.34 (−0.51–−0.16) | <0.01 |
| Large | 64.18 ± 0.60 | 69.22 ± 2.47 | 60.71 ± 1.65 | −0.65 (−0.83–−0.47) | <0.01 |
| Hospital location/teaching, % | |||||
| Rural or urban nonteaching | 34.60 ± 0.63 | 44.19 ± 3.16 | 20.66 ± 1.06 | Reference | |
| Urban teaching | 65.40 ± 0.63 | 55.80 ± 3.16 | 79.34 ± 1.06 | 1.04 (1.02–1.20) | <0.01 |
| Characteristics | In-Hospital Mortality * OR (95% CI) | Charge ‡ IRR (95% CI) | LoS ‡ IRR (95% CI) |
|---|---|---|---|
| Calendar year | 1.02 (1.01–1.03) | 3742 (3077–4406) | −0.13 (−0.09–−0.16) |
| Age, years | 1.03 (1.02–1.03) | −28 (−83–28) | −0.002 (−0.008–0.004) |
| Race | |||
| White | Reference | Reference | Reference |
| Black | 1.68 (1.53–1.83) | 17,849 (14,203–21,495) | 1.78 (1.51–2.05) |
| Hispanic | 1.03 (0.93–1.13) | 11,740 (9024–14,455) | 0.67 (0.44–0.90) |
| Other † | 1.04 (0.94–1.13) | 1105 (−1237–3447) | 0.41 (0.24–0.59) |
| Male | 1.02 (1.01–1.04) | 3314 (2326–4302) | 0.39 (0.27–0.50) |
| Primary Payer | |||
| Medicare | Reference | Reference | Reference |
| Medicaid | 1.46 (1.31–1.63) | −3080 (−5825–−335) | 0.83 (0.55–1.11) |
| Private | 1.22 (0.88–1.34) | 2525 (83–4219) | −0.98 (−1.15–−0.81) |
| Uninsured | 1.79 (1.54–2.07) | −3926 (−6460–−1391) | −0.59 (−0.87–−0.31) |
| Hospital Region | |||
| Northeast | Reference | Reference | Reference |
| Midwest | 0.84 (0.77–0.93) | −5669 (−9991–−1347) | −1.14 (−1.36–−0.91) |
| South | 1.03 (0.95–1.12) | −4043 (−8284–197) | −0.18 (−0.40–0.04) |
| West | 1.02 (0.93–1.12) | 28,680 (23,054–34,305) | −0.66 (−0.93–−0.40) |
| Hospital bed size | |||
| Small | Reference | Reference | Reference |
| Medium | 1.05 (0.94–1.31) | 1499 (−1091–4088) | 0.71 (0.47–0.95) |
| Large | 1.26 (0.94–1.34) | 5449 (2829–8068) | 1.29 (1.07–1.51) |
| Hospital location/teaching | |||
| Rural/urban nonteaching | Reference | Reference | Reference |
| Urban teaching | 1.23 (1.15–1.31) | 1298 (1247–3744) | 1.33 (1.18–1.48) |
| Ulcerative colitis | 0.61 (0.50–0.63) | −7577 (−10,155–−4998) | −1.90 (−2.18–−1.62) |
| Comorbidities | |||
| Decompensated cirrhosis | 2.41 (2.25–2.59) | 3958 (1737–6178) | 1.49 (1.29–1.70) |
| Hepatocellular carcinoma | 1.56 (1.35–1.80) | −237 (−4944–4471) | −0.04 (−0.38–0.31) |
| Gall bladder carcinoma | 1.11 (0.87–1.42) | 5095 (−2249–12,439) | 0.47 (0.04–0.89) |
| Cholangiocarcinoma | 1.27 (1.13–1.43) | 2279 (−913–5470) | 0.52 (0.28–0.77) |
| Colorectal cancer | 2.55 (2.15–3.01) | 3613 (−2130–9357) | 1.44 (1.03–1.93) |
| Autoimmune hepatitis | 1.34 (0.75–1.35) | 238 (−8407–8884) | −1.68 (−2.13–−1.23) |
| Liver transplant | 0.39 (0.27–0.57) | 274,448 (244,522–304,374) | 17.38 (15.20–19.57) |
| Fatty liver | 0.57 (0.44–0.74) | −430 (−2652–1792) | −0.37 (−0.61–−0.13) |
| Marijuana use | 0.93 (0.54–1.56) | −2383 (−8076–3309) | −0.87 (−1.49–−0.25) |
| ICU admission | 16.16 (14.98–17.42) | 59,501 (53,430–65,572) | 10.26 (9.76–10.76) |
| CCI > 1 | 1.53 (1.43–1.64) | 4904 (4479–5328) | 0.30 (0.27–0.32) |
| LoS, days | 1.03 (1.02–1.04) | 10,158 (9518–10,799) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohra, I.; Gopakumar, H.; Dahiya, D.S.; Kahaleh, M.; Sharma, N. Racial Disparities in Inpatient Hospital Outcomes of Primary Sclerosing Cholangitis in United States: Nationwide Analysis. Diagnostics 2024, 14, 2493. https://doi.org/10.3390/diagnostics14222493
Vohra I, Gopakumar H, Dahiya DS, Kahaleh M, Sharma N. Racial Disparities in Inpatient Hospital Outcomes of Primary Sclerosing Cholangitis in United States: Nationwide Analysis. Diagnostics. 2024; 14(22):2493. https://doi.org/10.3390/diagnostics14222493
Chicago/Turabian StyleVohra, Ishaan, Harishankar Gopakumar, Dushyant Singh Dahiya, Michel Kahaleh, and Neil Sharma. 2024. "Racial Disparities in Inpatient Hospital Outcomes of Primary Sclerosing Cholangitis in United States: Nationwide Analysis" Diagnostics 14, no. 22: 2493. https://doi.org/10.3390/diagnostics14222493
APA StyleVohra, I., Gopakumar, H., Dahiya, D. S., Kahaleh, M., & Sharma, N. (2024). Racial Disparities in Inpatient Hospital Outcomes of Primary Sclerosing Cholangitis in United States: Nationwide Analysis. Diagnostics, 14(22), 2493. https://doi.org/10.3390/diagnostics14222493







