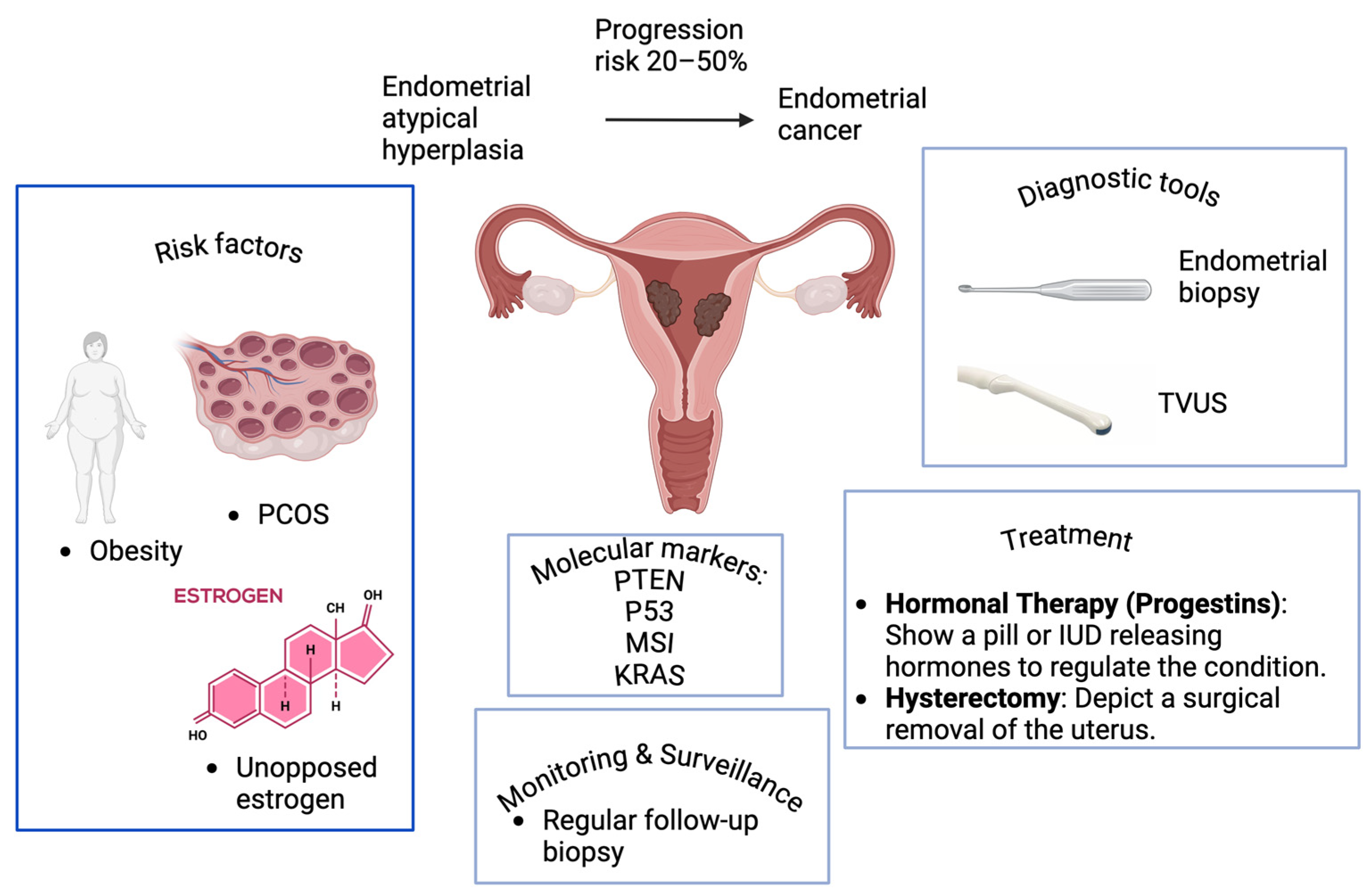
Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer
Abstract
1. Introduction
Study Research Strategy
2. Pathophysiology of Endometrial Atypical Hyperplasia
2.1. Histological Features
- Glandular Crowding: In EAH, there is a marked increase in the number and density of endometrial glands, which also assume irregular shapes. This glandular crowding is accompanied by a loss of the normal gland-to-stroma ratio, as there is an overgrowth of glandular elements relative to the supportive stromal tissue [13].
- Nuclear Atypia: A hallmark of atypical hyperplasia is the presence of nuclear atypia, where the glandular cells exhibit nuclear enlargement, irregular nuclear contours, increased nuclear-to-cytoplasmic ratios, and chromatin clumping. This shift is a critical indicator of disease progression, as it reflects uncontrolled glandular growth suggesting an increased potential for malignancy [13].
- Cellular Architecture: The cellular arrangement is often irregular, with complex, maze-like configurations. There is a disruption in the usual orderly arrangement of cells, with back-to-back glands forming a complex pattern. Loss of cellular polarity is also seen, where the orientation of cells becomes disorganized [13,14].
2.2. Molecular Changes and Genetic Pathways
- PTEN mutations: One of the most common genetic abnormalities associated with both EAH and type I endometrial carcinoma is the loss of function of the tumor suppressor gene phosphatase and tensin homolog (PTEN) [15]. PTEN mutations lead to hyperactivation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway, which promotes cell proliferation and survival [15]. Loss of PTEN function is seen in approximately 55–80% of EAH cases and is considered an early event in endometrial carcinogenesis [16,17].
- KRAS Mutations: Mutations in the Kirsten rat sarcoma viral oncogene homolog (KRAS) gene, which encodes a protein involved in regulating cell growth and division, are also commonly found in EAH [18,19,20]. These mutations activate signaling pathways that promote cellular proliferation, contributing to the progression from hyperplasia to carcinoma. KRAS mutations are detected in about 10–30% of endometrial hyperplasia cases.
- Microsatellite Instability (MSI): MSI is a form of genetic hypermutability resulting from impaired DNA mismatch repair [21]. This abnormality is observed in a subset of EAH cases and is associated with an increased risk of progression to EC. MSI is also frequently observed in Lynch syndrome-associated ECs [22,23].
- Hormonal Imbalances: Unopposed estrogen exposure is a key factor in the development of EAH and its progression to cancer [24,25]. Estrogen stimulates the proliferation of endometrial cells, while progesterone acts by counterbalancing through promotion of cellular differentiation and apoptosis [26]. In cases of prolonged estrogen exposure without sufficient progesterone such as in obesity, PCOS, or anovulatory cycles, the endometrium undergoes unchecked proliferation, increasing the risk of hyperplasia and subsequent neoplastic transformation [27,28]. This hormonal imbalance is a common feature in both EAH and type I endometrioid carcinoma.
2.3. Progression to Endometrial Cancer
- 20–25% Progression Risk: Lower estimates of progression risk are seen in some studies, particularly among patients with a short duration of untreated hyperplasia or those undergoing close surveillance and management [31]. Women with atypical endometrial hyperplasia have a 32.6% (95% CI: 24.1%, 42.4%) prevalence of concurrent endometrial cancer and an 8.2% (95% CI 3.9%, 17.3%) annual incidence rate of progression to cancer [32]. For EAH, the cumulative risk of progression rose from 8.2% (95% CI, 1.3–14.6%) at 4 years, to 12.4% (95% CI, 3.0–20.8%) at 9 years, and reached 27.5% (95% CI, 8.6–42.5%) at 19 years following diagnosis [29]. The cumulative 20-year progression risk from EAH to carcinoma is 28% [29]. EAH has a 23% risk of progression to carcinoma, compared to 1.6% for hyperplasia without atypia [31]. The study found a 45.9% rate of endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia [33]. The cumulative 20-year progression risk for EAH is estimated at 27.5%, compared to less than 5% for simple EH [34]. For atypical endometrial polyps, the pooled risk estimate of concurrent EC is 5.6%, lower than the 42% risk associated with non-polypoid atypical EH [35].
- The World Health Organization (WHO) system, which considers cytologic atypia, shows a relative risk (RR) of 8.74 for cancer progression, while the EAH with D-score demonstrates a significantly higher RR of 29.22 [36]. Complex hyperplasia without atypia increases cancer risk by 4.90 times compared to simple EH [37]. Women aged ≤39 and ≥50 years with EH have a higher risk of EC progression, but multiple follow-up biopsies increase EC detection [38].
- Progression Risk of 40–50%: Higher estimates are observed in patients with significant risk factors, such as obesity, prolonged unopposed estrogen exposure, and untreated chronic hyperplasia [39]. In such cases, the odds of malignant transformation are substantial. Exogenous estrogen therapy is associated with a 2.5-fold increased risk of endometrial cancer [40]. Nine out of twelve studies demonstrated a significantly increased risk of EC among users of estrogen-only menopausal hormone therapy, with odds ratios/hazard ratios ranging from 1.45 to 4.46 [41].
3. Risk Factors for EAH and Cancer Progression
3.1. Hormonal Imbalance
- Obesity: Adipose tissue is a significant source of estrogen production in postmenopausal women [42]. Obesity leads to increased peripheral conversion of androgens to estrogens in adipose tissue, mediated by the enzyme aromatase [43]. This excessive estrogen is not balanced by progesterone, especially in postmenopausal women or those with anovulatory cycles, promoting endometrial proliferation [44]. Obese women have a 2–4-fold higher risk of developing endometrial hyperplasia and cancer compared to women with normal weight [45].
- PCOS: PCOS is characterized by chronic anovulation, where women often experience irregular menstrual cycles or may not ovulate at all, resulting in prolonged exposure to estrogen without the balancing effect of progesterone [46]. This hormonal environment increases the risk of endometrial hyperplasia and cancer in women with PCOS, particularly those who do not receive treatment to induce ovulation or counteract estrogen with progestins [28].
- Tamoxifen Use: Tamoxifen, a selective estrogen receptor modulator commonly used in the treatment and prevention of breast cancer, has estrogenic effects on the endometrium [47]. This can stimulate endometrial cell proliferation and increase the risk of developing atypical hyperplasia and EC [48]. Women on prolonged tamoxifen therapy are monitored closely for endometrial changes [49].
- Nulliparity: Women who have never given birth (nulliparous) are at higher risk of developing EAH and EC [50]. Pregnancy provides periods of progesterone dominance, which protect the endometrium from estrogenic stimulation [51]. Nulliparous women, especially those with chronic anovulation, lack this protective effect and may be exposed to unopposed estrogen for longer periods [52].
3.2. Genetic Predisposition
- Lynch Syndrome: Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, is an inherited genetic condition caused by mutations in DNA mismatch repair genes such as MLH1, MSH2, MSH6, and PMS2 [53,54]. Women with Lynch syndrome are at a significantly elevated risk of developing EC, with a lifetime risk of 40–60% [55]. Often, EAH is a precursor to malignancy in this population. As a result, women with Lynch syndrome are advised to undergo regular checkups and, in some cases, prophylactic hysterectomy to mitigate cancer risk [56].
- Familial Cancer Syndromes: Other less common familial cancer syndromes may also predispose women to EC, including Cowden syndrome (associated with mutations in the PTEN gene), which can increase the risk of both breast and EC [57]. These genetic predispositions underscore the importance of genetic testing and counseling for women with a family history of cancer.
3.3. Lifestyle and Other Risk Factors
- Obesity: As mentioned earlier, obesity is one of the most significant risk factors for EAH and EC due to increased estrogen production from adipose tissue [58]. Obesity also promotes a state of chronic low-grade inflammation, which may contribute to cancer development [59]. Additionally, obese women are more likely to have insulin resistance, metabolic syndrome, and other endocrine imbalances that can exacerbate cancer risk [60].
- Sedentary Lifestyle: A lack of regular physical activity is closely linked to obesity, insulin resistance, and hormonal imbalances, all of which elevate the risk of developing atypical hyperplasia and EC [61]. Exercise helps reduce body fat and insulin levels, lowering the estrogen burden thereby reducing cancer risk [62,63].
- Diabetes: Women with diabetes have an increased risk of developing EC, even independent of obesity [64]. Hyperinsulinemia and insulin resistance, common in type 2 diabetes, may promote endometrial proliferation and cancer progression through various mechanisms, including increased levels of insulin-like growth factors, which have mitogenic effects on endometrial cells [65,66].
- Hypertension: Hypertension is often part of the metabolic syndrome, which is closely associated with obesity and diabetes [67,68]. While a direct causal relationship between hypertension and EC remains unclear, hypertension may act as a marker for the constellation of metabolic risk factors, including obesity and insulin resistance, that raise cancer risk [69,70].
4. Clinical Presentation and Diagnosis
4.1. Symptoms of Endometrial Atypical Hyperplasia
- Overview of Symptoms
- Abnormal Uterine Bleeding: AUB encompasses heavy, prolonged, or irregular menstrual bleeding in premenopausal women [72]. It may manifest as the following:
- ○
- Menorrhagia: heavy or prolonged menstrual periods.
- ○
- Metrorrhagia: irregular or intermenstrual bleeding.
- ○
- Oligomenorrhea: infrequent menstruation.
- ○
- Polymenorrhea: frequent menstrual periods.
- Women with chronic anovulation, such as those with PCOS, may experience prolonged periods of no bleeding followed by heavy or prolonged menstruation, often indicating endometrial hyperplasia [73].
- Postmenopausal Bleeding: Any bleeding that occurs after a woman has entered menopause is considered abnormal and warrants investigation [74]. Postmenopausal bleeding is a key clinical sign of endometrial hyperplasia or EC, as the endometrium should not be exposed to estrogen stimulation after menopause [75]. Even a single episode of postmenopausal bleeding should prompt diagnostic evaluation.
- Other Clinical Signs: Although less common, women with EAH may experience pelvic discomfort or pain. Occasionally, symptoms such as vaginal discharge or spotting between periods may also be present [76].
4.2. Diagnostic Tools
4.2.1. Endometrial Biopsy
- Advantages: It is relatively simple, cost-effective, and can be performed in a clinical setting without the need for general anesthesia [82].
- Limitations: Although the biopsy can accurately diagnose hyperplasia and atypia, it may miss focal lesions or small areas of malignancy due to sampling error, particularly in cases of heterogeneous disease [78].
4.2.2. Transvaginal Ultrasound (TVUS)
- Endometrial Thickness: In postmenopausal women, an endometrial thickness greater than 4–5 mm on TVUS is considered abnormal and warrants further evaluation, as it suggests hyperplasia or cancer [87]. In premenopausal women, the thickness may vary with the menstrual cycle, making it less specific, but a markedly thickened endometrium outside the normal range can still raise suspicion for hyperplasia.
- Identifying Abnormalities: TVUS can also detect other endometrial abnormalities, such as polyps or structural changes, which may be contributing to abnormal bleeding [88].
4.2.3. Hysteroscopy
- Visual Assessment: Hysteroscopy enables clinicians to visually assess the endometrium for abnormalities, such as hyperplasia, polyps, or cancerous lesions, and take targeted biopsies of suspicious areas [71]. It is often considered when other diagnostic tools do not provide sufficient clarity.
- Biopsy during Hysteroscopy: The ability to perform targeted biopsies under direct vision improves diagnostic accuracy and reduces the chance of missing focal lesions, such as early-stage cancers that may be missed with blind endometrial biopsy [97].
4.2.4. Differentiating Atypical Hyperplasia from Carcinoma
- Biopsy Challenges: Endometrial biopsy samples may sometimes be insufficient to differentiate EAH from well-differentiated EC, particularly because both conditions may share features such as glandular crowding and nuclear atypia [101]. Sampling error or focal carcinoma within a background of hyperplasia can complicate diagnosis.
- Hysteroscopy for Clarification: In cases where biopsy results are inconclusive or the pathology report raises suspicion of malignancy, hysteroscopy with directed biopsy can provide additional clarity by targeting abnormal areas that may harbor cancer [102].
5. Management of EAH
5.1. Conservative vs. Definitive Treatment Approaches
- Hormonal Therapy:
- ○
- Indications: Hormonal therapy is typically indicated for women who wish to preserve their fertility or those for whom surgery is contraindicated due to medical comorbidities [105]. It is also used in younger women or in cases of early-stage disease.
- ○
- Progestin Therapy: Progestins, either in oral form or delivered via a levonorgestrel-releasing intrauterine device (LNG-IUD), are the cornerstone of conservative treatment [106]. Progestins counteract the effects of unopposed estrogen, leading to atrophy of the endometrial tissue and regression of hyperplasia [107].
- ▪
- Oral Progestins: Commonly prescribed agents include medroxyprogesterone acetate (MPA) and megestrol acetate. These have shown efficacy in reversing hyperplasia, with a response rate of approximately 70–80% [107]. However, oral progestins can be associated with systemic side effects, such as weight gain, mood changes, and thromboembolic events.
- ▪
- LNG-IUD: The LNG-IUD has gained popularity as it provides a localized, sustained release of progestin with minimal systemic side effects. Studies have shown that the LNG-IUD implantation can lead to outcomes that are similar or even superior to those achieved by oral progestins, with high rates of disease regression (up to 90%) [107]. It is also well-tolerated and offers long-term protection against hyperplasia.
- ○
- Hysterectomy:
- ○
- Indications: Hysterectomy is the definitive treatment for EAH, especially in women who do not desire future fertility [109]. It is particularly recommended in high-risk cases, including those with recurrent hyperplasia, failure of hormonal therapy, or women with significant comorbidities that increase the risk of cancer development.
- ○
- Outcomes: Hysterectomy provides near-total protection against the progression of EAH to EC [109]. In women at high risk, particularly postmenopausal women, or those with concurrent risk factors such as obesity and diabetes, hysterectomy is considered the most reliable treatment option. For women who do not desire children, it is often the preferred course of action.
5.2. Fertility-Sparing Options
- Progestin-Based Therapy: As mentioned, both oral progestins and the LNG-IUD are viable options for treating EAH while maintaining fertility [110]. In such cases, close monitoring is essential to ensure disease regression and to prevent progression. Response to progestin therapy is typically monitored by repeat endometrial biopsies every 3–6 months [2].
- Continuous Follow-Up: For fertility-sparing treatment, continuous and rigorous follow-up is critical. Regular endometrial sampling or imaging is necessary to assess for recurrence or progression, which remains a possibility even after apparent initial regression [2]. If progestin therapy fails or hyperplasia recurs, a hysterectomy may be reconsidered after childbearing is complete [108].
5.3. Follow-Up and Surveillance
- Regular Monitoring: Patients on hormonal therapy require frequent follow-up with serial endometrial biopsies every 3–6 months [2]. This allows for the early detection of persistent hyperplasia or progression to carcinoma. If regression is observed, follow-up intervals may be extended.
- Role of Imaging: In addition to biopsies, TVUS can be used to monitor endometrial thickness, although biopsy remains the gold standard for confirming the presence or absence of hyperplasia [111].
- Recurrence Risk: Despite treatment, EAH carries a notable risk of recurrence, particularly in women treated conservatively. The risk of progression to cancer ranges from 20 to 50%, and regular surveillance is critical for identifying these cases early [2]. In patients who have achieved full fertility, hysterectomy is frequently indicated as a definitive measure to eliminate the risk of progression.
6. Risk Stratification and Prognosis in EAH
6.1. Predicting the Risk of Progression to Endometrial Cancer
- Clinical Factors:
- ○
- Age: Older women, particularly those who are postmenopausal, have a higher risk of progression from atypical hyperplasia to EC [38]. Postmenopausal women often have additional risk factors such as increased cumulative exposure to estrogen.
- ○
- Body Mass Index (BMI): Obesity is a major risk factor for both EAH and EC [112]. Increased adipose tissue leads to higher levels of endogenous estrogen production, contributing to endometrial proliferation without the balancing effect of progesterone [112]. The risk of progression is significantly elevated in obese women.
- ○
- ○
- Unopposed Estrogen Exposure: Women on estrogen replacement therapy without concurrent progestin, those with early menarche or late menopause, and women with nulliparity (no pregnancies) face higher risks due to prolonged estrogen exposure [39].
- Histopathological Features:
- ○
- Nuclear Atypia: The presence of nuclear atypia in endometrial hyperplasia is a key predictor of progression to cancer [2]. The more severe the atypia, the higher is the risk.
- ○
- Glandular Complexity: Increased glandular crowding and complexity in endometrial tissue can indicate a greater likelihood of malignant transformation [2]. Atypical hyperplasia with cellular architectural complexity is more likely to progress than simple hyperplasia without atypia.
- Risk Models: Several risk models incorporate clinical and histopathological features to predict progression. These models often weigh the significance of factors such as age, BMI, and the presence of nuclear atypia [113]. However, they are not infallible, and their predictive accuracy is limited, requiring continued research on more advanced, molecular-based risk stratification methods [114].
6.2. Role of Biomarkers in Risk Stratification
- PTEN Loss:
- ○
- ○
- The loss of PTEN has been proposed as a marker for increased risk of progression [116]. Studies have shown that women with PTEN loss in endometrial hyperplasia are more likely to develop EC [17]. Research shows that PTEN expression is significantly higher in normal proliferative endometrium and simple hyperplasia compared to complex atypical hyperplasia [117]. However, a meta-analysis found that PTEN loss was not significantly associated with therapy outcomes in EH and EEC treated with progestins [118]. Recent studies have demonstrated that loss of PTEN expression may reflect progression to endometrial carcinoma, with negative PTEN immunoexpression indicating poor prognosis and higher recurrence probability [119]. Additionally, PTEN expression is downregulated in atypical hyperplastic and neoplastic endometrial tissues, showing an inverse relationship with tumor grade, stage, and myometrial invasion [120]. These findings suggest that PTEN may have potential as a screening tool for precancerous endometrial lesions.
- ○
- This biomarker may be particularly useful in identifying high-risk patients who might benefit from more aggressive treatment, such as hysterectomy, even in the absence of severe clinical or histological risk features.
- p53 Mutations:
- ○
- ○
- While p53 mutations are less common in atypical hyperplasia, their presence may indicate a more aggressive disease course [123]. Some studies suggest that p53 abnormalities could serve as a biomarker for progression in hyperplasia cases, especially when detected alongside other molecular changes such as PTEN loss or KRAS mutations.
- ○
- P53 pathway markers, including p21, mdm2, and phospho-p63, have shown promise in refining molecular classifications and predicting clinical outcomes [124]. Expression of estrogen and progesterone receptors correlates with simple EH and well-differentiated tumors, while p53 and Ki-67 overexpression indicates a more malignant phenotype [125]. Positive expression of p53 in endometrial hyperplasia may indicate progression to carcinoma [126]. Elevated p53 expression was associated with poor differentiation in EC. P53 expression was significantly higher in cases with FIGO stages III and IV compared to stages I and II (100% vs. 18.1%, p = 0.0016) and in grade 3 tumors compared to grades 1 and 2 (50% vs. 0%, p = 0.0116) [127]. These findings suggest that molecular markers can improve risk stratification and guide personalized treatment approaches in endometrial pathologies.
- Microsatellite Instability:
- ○
- ○
- The presence of MSI in endometrial hyperplasia could be a significant predictor of progression, particularly in patients with known genetic predispositions such as Lynch syndrome. Screening for MSI or MMR deficiency in patients with atypical hyperplasia could help identify those at higher risk for developing EC [129].
- ○
- Studies have shown that EH, particularly EAH, often exhibits MSI and loss of MMR protein expression, which are precursors to endometrial carcinoma [130,131]. The progression from EAH to carcinoma is associated with an increase in unstable microsatellite loci and tumor mutational burden [131]. MLH1 promoter methylation is an early event in this process, although it may not be required for MLH1 silencing and MMR loss [132]. MSI analysis of EAH in young patients (≤50 years) may serve as a prognostic marker for potential progression to MSI-high endometrial carcinomas [133]. Combined MSI and immunohistochemistry analysis can help identify hereditary nonpolyposis colorectal cancer patients among young women with EC and EAH [133].
- KRAS Mutations:
- ○
- ○
- KRAS mutations are associated with type I EC and may be involved in early carcinogenesis [18]. Studies have found KRAS mutations in both cancerous and non-cancerous endometrial tissues, suggesting their potential as early indicators of malignancy risk [136]. Other biomarkers, such as PTEN loss, increased stromal p16 expression, and decreased PAX2 expression, have been associated with the transition from EAH to EC [137]. KRAS mutation is common in endometrial cancer and may be involved in disease progression, but its role in fertility-sparing treatment outcomes is not yet known [134].
- ○
- While KRAS mutations alone do not necessarily predict poor outcomes, their presence alongside other mutations, such as PTEN loss, may indicate a higher risk of progression to carcinoma [16]. Further studies are needed to determine the prognostic significance of KRAS mutations in EAH.
6.3. Prognostic Implications of Biomarkers
- High-Risk Patients: Those identified with molecular abnormalities such as PTEN loss or MSI may benefit from early definitive treatment, such as hysterectomy, even if they have not yet developed overt carcinoma [139].
- Low-Risk Patients: Women without significant molecular changes could be candidates for more conservative management, such as progestin therapy, with close follow-up, reducing the need for aggressive interventions in all cases of atypical hyperplasia.
7. EAH in Special Populations
7.1. Premenopausal Women
- Fertility-Preserving Options:
- ○
- For women of reproductive age, especially those desiring future pregnancies, conservative management is preferred. The standard treatment involves progestin-based therapy, either through oral progestins or the use of an LNG-IUD [140].
- ○
- Oral progestins such as MPA and megestrol acetate are commonly prescribed for premenopausal women [108]. Progestins help counterbalance the effects of unopposed estrogen and lead to regression of hyperplasia [2]. However, these women require frequent follow-up with repeat biopsies to monitor for regression or progression.
- ○
- LNG-IUD: For fertility preservation, the LNG-IUD is often favored because of its local effect on the endometrium and lower systemic side effects [141]. The LNG-IUD has been shown to be highly effective, with response rates reaching up to 90% in premenopausal women.
- ○
- If progestin therapy successfully leads to regression, patients can attempt pregnancy [142]. However, once childbearing is complete, definitive treatment such as hysterectomy may be considered to eliminate the risk of progression to EC.
- Management of Coexisting PCOS:
- ○
- PCOS is a common comorbidity in premenopausal women with EAH, contributing to prolonged anovulation and unopposed estrogen exposure [143]. In these women, treating the underlying hormonal imbalance is critical.
- ○
- Weight management and metformin may be considered to improve insulin resistance and reduce the hyperandrogenic state seen in PCOS [144,145]. Additionally, combined oral contraceptives can help regulate menstrual cycles and reduce estrogen-driven endometrial proliferation in women with mild hyperplasia without atypia [146].
- ○
- For women with atypical hyperplasia and PCOS, progestin-based therapy remains the cornerstone of treatment, but the underlying metabolic and hormonal imbalances should be addressed to reduce future risks [147].
- Surveillance:
- ○
- Continuous follow-up is essential, including regular endometrial biopsies every 3–6 months [109]. While fertility-sparing treatment can be effective, the risk of recurrence or progression remains significant, and patients must be counseled on the need for long-term monitoring.
7.2. Postmenopausal Women
- Higher Risk of Progression:
- ○
- Postmenopausal women are at a substantially increased risk of progressing from EAH to EC, with studies showing up to a 50% progression rate in untreated cases [148]. This is due in part to the absence of regular menstrual shedding and the prolonged, often unopposed exposure to estrogen in women with obesity or those on estrogen-only HT [149].
- ○
- Definitive treatment in the form of a hysterectomy is typically recommended for postmenopausal women, especially those who are high-risk or have comorbidities such as obesity, diabetes, or hypertension [150]. Hysterectomy offers definitive protection against progression and is often the preferred approach once the diagnosis of atypical hyperplasia is confirmed.
- Implications of Hormone Therapy:
- ○
- ○
- Women diagnosed with EAH while on HT may need to discontinue estrogen-only therapy or switch to combined HT, which includes a progestin component to counteract the effects of estrogen on the endometrium.
- ○
- In some cases, women who cannot tolerate combined HT may be candidates for LNG-IUD placement, which provides localized progestin to protect the endometrium from hyperplasia while allowing for systemic estrogen therapy [151].
- Optimal Management Strategies:
- ○
- Hysterectomy remains the most reliable and often preferred option for postmenopausal women with atypical hyperplasia, as it eliminates the risk of progression and recurrence [152]. Women who are poor surgical candidates due to medical comorbidities may be treated with progestin-based therapy, but the risk of recurrence and progression remains higher than that associated with surgical management [153].
- ○
- For women who opt for conservative management, continuous follow-up with serial endometrial biopsies or TVUS is mandatory. However, long-term conservative management is not ideal for most postmenopausal women due to their higher cancer risk.
- Surveillance:
- ○
- Postmenopausal women on conservative treatment should undergo frequent endometrial surveillance, similar to premenopausal women [154]. However, the threshold for transitioning to hysterectomy is generally lower in this population due to their elevated risk of cancer progression.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ring, K.L.; Mills, A.M.; Modesitt, S.C. Endometrial Hyperplasia. Obstet. Gynecol. 2022, 140, 1061–1075. [Google Scholar] [CrossRef]
- Nees, L.K.; Heublein, S.; Steinmacher, S.; Juhasz-Böss, I.; Brucker, S.; Tempfer, C.B.; Wallwiener, M. Endometrial Hyperplasia as a Risk Factor of Endometrial Cancer. Arch. Gynecol. Obstet. 2022, 306, 407–421. [Google Scholar] [CrossRef] [PubMed]
- De Rocco, S.; Buca, D.; Oronzii, L.; Petrillo, M.; Fanfani, F.; Nappi, L.; Liberati, M.; D’Antonio, F.; Scambia, G.; Leombroni, M.; et al. Reproductive and Pregnancy Outcomes of Fertility-Sparing Treatments for Early-Stage Endometrial Cancer or Atypical Hyperplasia: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 273, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fan, Y.; Li, X.; Wang, Y.; Wang, J.; Tian, L. Metabolic Syndrome Is an Independent Risk Factor for Time to Complete Remission of Fertility-Sparing Treatment in Atypical Endometrial Hyperplasia and Early Endometrial Carcinoma Patients. Reprod. Biol. Endocrinol. 2022, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.S.; Chin, S.H.M.; Goh, C.; Hui, L.X.; Mathur, M.; Kuei, T.L.Y.; Xian, F.C.H. Non-Atypical Endometrial Hyperplasia: Risk Factors for Occult Endometrial Atypia and Malignancy in Patients Managed with Hysterectomy. Obstet. Gynecol. Sci. 2021, 64, 300–308. [Google Scholar] [CrossRef]
- Lee, N.; Lee, K.-B.; Kim, K.; Hong, J.H.; Yim, G.W.; Seong, S.J.; Lee, B.; Lee, J.-M.; Cho, J.; Lim, S.; et al. Risk of Occult Atypical Hyperplasia or Cancer in Women with Nonatypical Endometrial Hyperplasia. J. Obstet. Gynaecol. Res. 2020, 46, 2505–2510. [Google Scholar] [CrossRef]
- Uccella, S.; Zorzato, P.C.; Dababou, S.; Bosco, M.; Torella, M.; Braga, A.; Frigerio, M.; Gardella, B.; Cianci, S.; Laganà, A.S.; et al. Conservative Management of Atypical Endometrial Hyperplasia and Early Endometrial Cancer in Childbearing Age Women. Medicina 2022, 58, 1256. [Google Scholar] [CrossRef]
- Contreras, N.-A.; Sabadell, J.; Verdaguer, P.; Julià, C.; Fernández-Montolí, M.-E. Fertility-Sparing Approaches in Atypical Endometrial Hyperplasia and Endometrial Cancer Patients: Current Evidence and Future Directions. Int. J. Mol. Sci. 2022, 23, 2531. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.-B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II Endometrial Cancers: Have They Different Risk Factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Chia, V.M. Endometrial Hyperplasia and the Risk of Progression to Carcinoma. Maturitas 2009, 63, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Management of Endometrial Intraepithelial Neoplasia or Atypical Endometrial Hyperplasia: ACOG Clinical Consensus No. 5. Obstet. Gynecol. 2023, 142, 735–744. [CrossRef] [PubMed]
- Sobczuk, K.; Sobczuk, A. New Classification System of Endometrial Hyperplasia WHO 2014 and Its Clinical Implications. Prz. Menopauzalny 2017, 16, 107–111. [Google Scholar] [CrossRef]
- D’Angelo, E.; Espinosa, I.; Cipriani, V.; Szafranska, J.; Barbareschi, M.; Prat, J. Atypical Endometrial Hyperplasia, Low-Grade: “Much ADO about Nothing”. Am. J. Surg. Pathol. 2021, 45, 988–996. [Google Scholar] [CrossRef]
- Chen, H.; Strickland, A.L.; Castrillon, D.H. Histopathologic Diagnosis of Endometrial Precancers: Updates and Future Directions. Semin. Diagn. Pathol. 2022, 39, 137–147. [Google Scholar] [CrossRef]
- Li, L.; Yue, P.; Song, Q.; Yen, T.-T.; Asaka, S.; Wang, T.-L.; Beavis, A.L.; Fader, A.N.; Jiao, Y.; Yuan, G.; et al. Genome-Wide Mutation Analysis in Precancerous Lesions of Endometrial Carcinoma. J. Pathol. 2021, 253, 119–128. [Google Scholar] [CrossRef]
- Yang, H.P.; Meeker, A.; Guido, R.; Gunter, M.J.; Huang, G.S.; Luhn, P.; d’Ambrosio, L.; Wentzensen, N.; Sherman, M.E. PTEN Expression in Benign Human Endometrial Tissue and Cancer in Relation to Endometrial Cancer Risk Factors. Cancer Causes Control 2015, 26, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B.; Hennessy, B.T.; Li, J.; Barkoh, B.A.; Luthra, R.; Mills, G.B.; Broaddus, R.R. Clinical Assessment of PTEN Loss in Endometrial Carcinoma: Immunohistochemistry Outperforms Gene Sequencing. Mod. Pathol. 2012, 25, 699–708. [Google Scholar] [CrossRef]
- Sideris, M.; Emin, E.I.; Abdullah, Z.; Hanrahan, J.; Stefatou, K.M.; Sevas, V.; Emin, E.; Hollingworth, T.; Odejinmi, F.; Papagrigoriadis, S.; et al. The Role of KRAS in Endometrial Cancer: A Mini-Review. Anticancer Res. 2019, 39, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sang, Z.-Y.; Ma, J.; Zhu, Y.-P.; Wu, S.-F. KRAS, YWHAE, SP1 and MSRA as Biomarkers in Endometrial Cancer. Transl. Cancer Res. 2021, 10, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Priya, N.D.; Nanda Kumar, K.V.; Charani, M.S.; Bindu, D.; Reddy, K.L.; Gowthami, Y. Biomarker Panel for Early Detection in Uterine Cancer: A Review. Asian Pac. J. Canc. Biol. 2024, 9, 201–208. [Google Scholar] [CrossRef]
- Kavun, A.; Veselovsky, E.; Lebedeva, A.; Belova, E.; Kuznetsova, O.; Yakushina, V.; Grigoreva, T.; Mileyko, V.; Fedyanin, M.; Ivanov, M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed]
- Kanopiene, D.; Vidugiriene, J.; Valuckas, K.P.; Smailyte, G.; Uleckiene, S.; Bacher, J. Endometrial Cancer and Microsatellite Instability Status. Open Med. 2015, 10, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Westin, S.N.; Coleman, R.L. Microsatellite Instability in Endometrial Cancer: New Purpose for an Old Test. Cancer 2019, 125, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Brinton, L.A.; Felix, A.S. Menopausal Hormone Therapy and Risk of Endometrial Cancer. J. Steroid Biochem. Mol. Biol. 2014, 142, 83–89. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone Action in Endometrial Cancer, Endometriosis, Uterine Fibroids, and Breast Cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Shetty, C.; Rizvi, S.M.H.A.; Sharaf, J.; Williams, K.-A.D.; Tariq, M.; Acharekar, M.V.; Guerrero Saldivia, S.E.; Unnikrishnan, S.N.; Chavarria, Y.Y.; Akindele, A.O.; et al. Risk of Gynecological Cancers in Women with Polycystic Ovary Syndrome and the Pathophysiology of Association. Cureus 2023, 15, e37266. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chen, W.; Wang, J.-H.; Lin, S.-Z. Association between Polycystic Ovarian Syndrome and Endometrial, Ovarian, and Breast Cancer. Medicine 2018, 97, e12608. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.V., Jr.; Sherman, M.E.; Rush, B.B.; Ronnett, B.M.; Ioffe, O.B.; Duggan, M.A.; Glass, A.G.; Richesson, D.A.; Chatterjee, N.; Langholz, B. Absolute Risk of Endometrial Carcinoma during 20-Year Follow-up among Women with Endometrial Hyperplasia. J. Clin. Oncol. 2010, 28, 788–792. [Google Scholar] [CrossRef]
- Giannella, L.; Grelloni, C.; Bernardi, M.; Cicoli, C.; Lavezzo, F.; Sartini, G.; Natalini, L.; Bordini, M.; Petrini, M.; Petrucci, J.; et al. Atypical Endometrial Hyperplasia and Concurrent Cancer: A Comprehensive Overview on a Challenging Clinical Condition. Cancers 2024, 16, 914. [Google Scholar] [CrossRef]
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The Behavior of Endometrial Hyperplasia. A Long-Term Study of “Untreated” Hyperplasia in 170 Patients. Cancer 1985, 56, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.T.; Sanni, O.B.; Coleman, H.G.; Cardwell, C.R.; McCluggage, W.G.; Quinn, D.; Wylie, J.; McMenamin, Ú.C. Concurrent and Future Risk of Endometrial Cancer in Women with Endometrial Hyperplasia: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0232231. [Google Scholar] [CrossRef]
- Pennant, S.; Manek, S.; Kehoe, S. Endometrial Atypical Hyperplasia and Subsequent Diagnosis of Endometrial Cancer: A Retrospective Audit and Literature Review. J. Obstet. Gynaecol. 2008, 28, 632–633. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Ioffe, O.B.; Ronnett, B.M.; Rush, B.B.; Richesson, D.A.; Chatterjee, N.; Langholz, B.; Glass, A.G.; Sherman, M.E. Endometrial Carcinoma Risk among Women Diagnosed with Endometrial Hyperplasia: The 34-Year Experience in a Large Health Plan. Br. J. Cancer 2008, 98, 45–53. [Google Scholar] [CrossRef]
- de Rijk, S.R.; Steenbergen, M.E.; Nieboer, T.E.; Coppus, S.F. Atypical Endometrial Polyps and Concurrent Endometrial Cancer. Obstet. Gynecol. 2016, 128, 519–525. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Saccone, G.; Insabato, L.; Mollo, A.; De Placido, G.; Zullo, F. Endometrial Hyperplasia and Progression to Cancer: Which Classification System Stratifies the Risk Better? A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2019, 299, 1233–1242. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Saccone, G.; Mollo, A.; De Placido, G.; Mascolo, M.; Insabato, L.; Zullo, F. Complexity of Glandular Architecture Should Be Reconsidered in the Classification and Management of Endometrial Hyperplasia. APMIS 2019, 127, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Hwang, S.O.; Lee, B.; Kim, K.; Kim, Y.B.; Park, S.H.; Choi, H.Y. Risk Factors of Progression to Endometrial Cancer in Women with Endometrial Hyperplasia: A Retrospective Cohort Study. PLoS ONE 2020, 15, e0243064. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.; Roberts, H.; Marjoribanks, J.; Lethaby, A. Hormone Therapy in Postmenopausal Women and Risk of Endometrial Hyperplasia. Cochrane Database Syst. Rev. 2012, 3, CD000402. [Google Scholar] [CrossRef]
- Allen, N.E.; Tsilidis, K.K.; Key, T.J.; Dossus, L.; Kaaks, R.; Lund, E.; Bakken, K.; Gavrilyuk, O.; Overvad, K.; Tjønneland, A.; et al. Menopausal Hormone Therapy and Risk of Endometrial Carcinoma among Postmenopausal Women in the European Prospective Investigation Into Cancer and Nutrition. Am. J. Epidemiol. 2010, 172, 1394–1403. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Hilal, Z.; Kern, P.; Juhasz-Boess, I.; Rezniczek, G.A. Menopausal Hormone Therapy and Risk of Endometrial Cancer: A Systematic Review. Cancers 2020, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Hetemäki, N.; Mikkola, T.S.; Tikkanen, M.J.; Wang, F.; Hämäläinen, E.; Turpeinen, U.; Haanpää, M.; Vihma, V.; Savolainen-Peltonen, H. Adipose Tissue Estrogen Production and Metabolism in Premenopausal Women. J. Steroid Biochem. Mol. Biol. 2021, 209, 105849. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.-Y.; Xu, X.-L.; Li, J.; Fu, X.-W.; Deng, S.-L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.R.; Gill, P.; Lensen, S.; Thompson, J.M.D.; Farquhar, C.M. Body Mass Index Trumps Age in Decision for Endometrial Biopsy: Cohort Study of Symptomatic Premenopausal Women. Am. J. Obstet. Gynecol. 2016, 215, 598.e1–598.e8. [Google Scholar] [CrossRef]
- Rababa’h, A.M.; Matani, B.R.; Yehya, A. An Update of Polycystic Ovary Syndrome: Causes and Therapeutics Options. Heliyon 2022, 8, e11010. [Google Scholar] [CrossRef]
- Oceguera-Basurto, P.; Topete, A.; Oceguera-Villanueva, A.; Rivas-Carrillo, J.; Paz-Davalos, M.; Quintero-Ramos, A.; Del Toro-Arreola, A.; Daneri-Navarro, A. Selective Estrogen Receptor Modulators in the Prevention of Breast Cancer in Premenopausal Women: A Review. Transl. Cancer Res. 2020, 9, 4444–4456. [Google Scholar] [CrossRef]
- Ryu, K.-J.; Kim, M.S.; Lee, J.Y.; Nam, S.; Jeong, H.G.; Kim, T.; Park, H. Risk of Endometrial Polyps, Hyperplasia, Carcinoma, and Uterine Cancer after Tamoxifen Treatment in Premenopausal Women with Breast Cancer. JAMA Netw. Open 2022, 5, e2243951. [Google Scholar] [CrossRef]
- AlZaabi, A.; AlAmri, H.; ALAjmi, G.; Allawati, M.; Muhanna, F.; Alabri, R.; AlBusaidi, F.; AlGhafri, S.; Al-Mirza, A.A.; Al Baimani, K. Endometrial Surveillance in Tamoxifen and Letrozole Treated Breast Cancer Patients. Cureus 2021, 13, e20030. [Google Scholar] [CrossRef]
- Yang, H.P.; Cook, L.S.; Weiderpass, E.; Adami, H.-O.; Anderson, K.E.; Cai, H.; Cerhan, J.R.; Clendenen, T.V.; Felix, A.S.; Friedenreich, C.M.; et al. Infertility and Incident Endometrial Cancer Risk: A Pooled Analysis from the Epidemiology of Endometrial Cancer Consortium (E2C2). Br. J. Cancer 2015, 112, 925–933. [Google Scholar] [CrossRef]
- Pocobelli, G.; Doherty, J.A.; Voigt, L.F.; Beresford, S.A.; Hill, D.A.; Chen, C.; Rossing, M.A.; Holmes, R.S.; Noor, Z.S.; Weiss, N.S. Pregnancy History and Risk of Endometrial Cancer. Epidemiology 2011, 22, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Iwasaki, M.; Abe, S.K.; Islam, M.R.; Rahman, M.S.; Saito, E.; Merritt, M.A.; Choi, J.-Y.; Shin, A.; Sawada, N.; et al. Reproductive Factors and Endometrial Cancer Risk Among Women. JAMA Netw. Open 2023, 6, e2332296. [Google Scholar] [CrossRef]
- Biller, L.H.; Syngal, S.; Yurgelun, M.B. Recent Advances in Lynch Syndrome. Fam. Cancer 2019, 18, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Lynch Syndrome-Associated Colorectal Cancer. N. Engl. J. Med. 2018, 379, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, L.; Zang, Y.; Liu, W.; Liu, S.; Teng, F.; Xue, F.; Wang, Y. Endometrial Cancer in Lynch Syndrome. Int. J. Cancer 2022, 150, 7–17. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Kalin, A.; Merideth, M.A.; Regier, D.S.; Blumenthal, G.M.; Dennis, P.A.; Stratton, P. Management of Reproductive Health in Cowden Syndrome Complicated by Endometrial Polyps and Breast Cancer. Obstet. Gynecol. 2013, 121, 461–464. [Google Scholar] [CrossRef]
- Clontz, A.D.; Gan, E.; Hursting, S.D.; Bae-Jump, V.L. Effects of Weight Loss on Key Obesity-Related Biomarkers Linked to the Risk of Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2197. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Nino, M.E. The Role of Chronic Inflammation in Obesity-Associated Cancers. ISRN Oncol. 2013, 2013, 697521. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical Activity, Obesity and Sedentary Behavior in Cancer Etiology: Epidemiologic Evidence and Biologic Mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Spanoudaki, M.; Giaginis, C.; Karafyllaki, D.; Papadopoulos, K.; Solovos, E.; Antasouras, G.; Sfikas, G.; Papadopoulos, A.N.; Papadopoulou, S.K. Exercise as a Promising Agent against Cancer: Evaluating Its Anti-Cancer Molecular Mechanisms. Cancers 2023, 15, 5135. [Google Scholar] [CrossRef]
- Thomas, R.; Kenfield, S.A.; Yanagisawa, Y.; Newton, R.U. Why Exercise Has a Crucial Role in Cancer Prevention, Risk Reduction and Improved Outcomes. Br. Med. Bull. 2021, 139, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.; Agnew, H.J.; Crosbie, E.J. Impact of Type 2 Diabetes Mellitus on Endometrial Cancer Survival: A Prospective Database Analysis. Front. Oncol. 2022, 12, 899262. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Jóźwik, M.; Niemira, M.; Krętowski, A. Insulin Resistance and Endometrial Cancer: Emerging Role for microRNA. Cancers 2020, 12, 2559. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Tan, J.; Xu, Y.; Yi, C. Diabetes Mellitus and Endometrial Carcinoma: Risk Factors and Etiological Links. Medicine 2022, 101, e30299. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, S.; Rusu, E.; Miricescu, D.; Radu, A.C.; Axinia, B.; Vrabie, A.M.; Ionescu, R.; Jinga, M.; Sirbu, C.A. Links between Metabolic Syndrome and Hypertension: The Relationship with the Current Antidiabetic Drugs. Metabolites 2023, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- El Meouchy, P.; Wahoud, M.; Allam, S.; Chedid, R.; Karam, W.; Karam, S. Hypertension Related to Obesity: Pathogenesis, Characteristics and Factors for Control. Int. J. Mol. Sci. 2022, 23, 12305. [Google Scholar] [CrossRef]
- Habeshian, T.S.; Peeri, N.C.; De Vivo, I.; Schouten, L.J.; Shu, X.-O.; Cote, M.L.; Bertrand, K.A.; Chen, Y.; Clarke, M.A.; Clendenen, T.V.; et al. Data from Hypertension and Risk of Endometrial Cancer: A Pooled Analysis in the Epidemiology of Endometrial Cancer Consortium (E2C2). Am. Assoc. Cancer Res. 2024, 33, 788–795. [Google Scholar]
- Sun, L.-M.; Kuo, H.-T.; Jeng, L.-B.; Lin, C.-L.; Liang, J.-A.; Kao, C.-H. Hypertension and Subsequent Genitourinary and Gynecologic Cancers Risk: A Population-Based Cohort Study. Medicine 2015, 94, e753. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Carugno, J.; Chiofalo, B.; Vilos, G.A.; Cianci, S.; Budak, M.S.; Lasmar, B.P.; Raffone, A.; Kahramanoglu, I. Hysteroscopy in the Management of Endometrial Hyperplasia and Cancer in Reproductive Aged Women: New Developments and Current Perspectives. Transl. Cancer Res. 2020, 9, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, L.; Critchley, H.O.D. Abnormal Uterine Bleeding. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, V.; Tahrani, A.A.; Helmy, A.; Gupta, J.K.; Singhal, R. Polycystic Ovary Syndrome and Endometrial Hyperplasia: An Overview of the Role of Bariatric Surgery in Female Fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Shen, W.-J.; Zhang, Y. Pathological Pattern of Endometrial Abnormalities in Postmenopausal Women with Bleeding or Thickened Endometrium. World J. Clin. Cases 2022, 10, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.M.; Sparidaens, E.M.; van den Brink, J.-W.; Breijer, M.C.; Boss, E.A.; Veersema, S.; Siebers, A.G.; Bulten, J.; Pijnenborg, J.M.A.; Bekkers, R.L.M. Long-Term Risk of Endometrial Cancer Following Postmenopausal Bleeding and Reassuring Endometrial Biopsy. Acta Obstet. Gynecol. Scand. 2016, 95, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Q.; Zhu, Z.; Dai, H.; Hu, H. The Value of Vaginal Microbiome in Patients with Endometrial Hyperplasia. J. Healthc. Eng. 2021, 2021, 4289931. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Liang, X.-D.; Zhou, R.; Sun, X.-L.; Wang, J.-L.; Wei, L.-H. Microscale Endometrial Sampling Biopsy in Detecting Endometrial Cancer and Atypical Hyperplasia in a Population of 1551 Women: A Comparative Study with Hysteroscopic Endometrial Biopsy. Chin. Med. J. 2020, 134, 193–199. [Google Scholar] [CrossRef]
- Johnson Alegbeleye, B. Endometrial Sampling for Endometrial Cancer: Still the Gold Standard? Am. J. Biomed. Sci. Res. 2019, 2, 463–468. [Google Scholar] [CrossRef]
- Ilavarasi, C.R.; Jyothi, G.S.; Alva, N.K. Study of the Efficacy of Pipelle Biopsy Technique to Diagnose Endometrial Diseases in Abnormal Uterine Bleeding. J. Midlife. Health 2019, 10, 75–80. [Google Scholar]
- De Leon, M.B.; Howard, W.; Del Priore, G. Novel Approach to Outpatient Endometrial Biopsy to Detect Endometrial Cancer. J. Clin. Oncol. 2013, 31, e16522. [Google Scholar] [CrossRef]
- Turan, G.; Yalçın Bahat, P.; Aslan Çetin, B.; Topbaş Selçuki, N.F. How Compatible Are Hysterectomy Pathology Results with Endometrial Biopsy in Abnormal Uterine Bleeding Women? Kafkas J. Med. Sci. 2020, 10, 104–109. [Google Scholar] [CrossRef]
- Terzic, M.M.; Aimagambetova, G.; Terzic, S.; Norton, M.; Bapayeva, G.; Garzon, S. Current Role of Pipelle Endometrial Sampling in Early Diagnosis of Endometrial Cancer. Transl. Cancer Res. 2020, 9, 7716–7724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Peng, B.; Jiang, J.; Fang, L.; Weng, W.; Wang, W.; Wang, S.; Zhu, X. Automatic Measurement of Endometrial Thickness from Transvaginal Ultrasound Images. Front. Bioeng. Biotechnol. 2022, 10, 853845. [Google Scholar] [CrossRef] [PubMed]
- Shokouhi, B. Role of Transvaginal Ultrasonography in Diagnosing Endometrial Hyperplasia in Pre- and Post-Menopause Women. Niger. Med. J. 2015, 56, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Poliakova, Y.M.; Lutsenko, N.S.; Haidai, N.V. Diagnosis of Endometrial Hyperplasia in Routine Gynecological Practice. Zaporozhye Med. J. 2019, 1, 155836. [Google Scholar] [CrossRef]
- Metin, M.R.; Aydın, H.; Ünal, Ö.; Akçay, Y.; Duymuş, M.; Türkyılmaz, E.; Avcu, S. Differentiation between Endometrial Carcinoma and Atypical Endometrial Hyperplasia with Transvaginal Sonographic Elastography. Diagn. Interv. Imaging 2016, 97, 425–431. [Google Scholar] [CrossRef]
- Park, Y.R.; Lee, S.W.; Kim, Y.; Bae, I.Y.; Kim, H.-K.; Choe, J.; Kim, Y.-M. Endometrial Thickness Cut-off Value by Transvaginal Ultrasonography for Screening of Endometrial Pathology in Premenopausal and Postmenopausal Women. Obstet. Gynecol. Sci. 2019, 62, 445–453. [Google Scholar] [CrossRef]
- Soljačić Vraneš, H.; Djaković, I.; Kraljević, Z.; Nakić Radoš, S.; Leniček, T.; Kuna, K. Clinical Value of Transvaginal Ultrasonography in Comparison to Hysteroscopy with Histopathologic Examination in Diagnosing Endometrial Abnormalities. Acta Clin. Croat. 2019, 58, 249–254. [Google Scholar] [CrossRef]
- Valino, M.C.; Yen, C.-F.; Huang, K.-G.; Uwais, A. Hysteroscopy as a Tool for Identification of Uterine Endocervical Lesion. Gynecol. Minim. Invasive Ther. 2018, 7, 88–89. [Google Scholar] [PubMed]
- Spadoto-Dias, D.; Bueloni-Dias, F.N.; Elias, L.V.; Leite, N.J.; Modotti, W.P.; Lasmar, R.B.; Dias, R. The Value of Hysteroscopic Biopsy in the Diagnosis of Endometrial Polyps. Women’s Health 2016, 12, 412–419. [Google Scholar] [CrossRef]
- Huang, C.; Hong, M.-K.; Ding, D.-C. Endometrial Adenomyoma Polyp Caused Postmenopausal Bleeding Mimicking Uterine Malignancy. Gynecol. Minim. Invasive Ther. 2017, 6, 129–131. [Google Scholar] [CrossRef]
- De Franciscis, P.; Riemma, G.; Schiattarella, A.; Cobellis, L.; Guadagno, M.; Vitale, S.G.; Mosca, L.; Cianci, A.; Colacurci, N. Concordance between the Hysteroscopic Diagnosis of Endometrial Hyperplasia and Histopathological Examination. Diagnostics 2019, 9, 142. [Google Scholar] [CrossRef]
- Elbareg, A.M.; Elmahashi, M.O.; Essadi, F.M. Evaluation of Intrauterine Pathology: Efficacy of Diagnostic Hysteroscopy in Comparison to Histopathological Examination. Reprod. Syst. Sex. Disord. 2015, 4, 149. [Google Scholar] [CrossRef]
- Garuti, G.; Sagrada, P.F.; Mirra, M.; Fornaciari, O.; Centinaio, G.; Finco, A.; Soligo, M. Hysteroscopic-View of Endometrial Atypical Hyperplasia. A Helpful Diagnostic Tool in the Care and Treatment Process? Eur. J. Gynaecol. Oncol. 2024, 45, 8. [Google Scholar]
- Harika, B.; Subbaiah, M.; Maurya, D.K. Diagnostic Accuracy of Hysteroscopic Scoring System in Predicting Endometrial Malignancy and Atypical Endometrial Hyperplasia. J. Midlife. Health 2021, 12, 206–210. [Google Scholar] [CrossRef]
- Ianieri, M.M.; Staniscia, T.; Pontrelli, G.; Di Spiezio Sardo, A.; Manzi, F.S.; Recchi, M.; Liberati, M.; Ceccaroni, M. A New Hysteroscopic Risk Scoring System for Diagnosing Endometrial Hyperplasia and Adenocarcinoma. J. Minim. Invasive Gynecol. 2016, 23, 712–718. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Saccone, G.; Carugno, J.; Pacheco, L.A.; Zizolfi, B.; Haimovich, S.; Clark, T.J. Endometrial Biopsy under Direct Hysteroscopic Visualisation versus Blind Endometrial Sampling for the Diagnosis of Endometrial Hyperplasia and Cancer: Systematic Review and Meta-Analysis. Facts Views Vis. ObGyn 2022, 14, 103–110. [Google Scholar] [CrossRef]
- Silverberg, S.G. Problems in the Differential Diagnosis of Endometrial Hyperplasia and Carcinoma. Mod. Pathol. 2000, 13, 309–327. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.D.; Williams, A.R.W.; Arends, M.J.; Saunders, P.T.K. New Concepts for an Old Problem: The Diagnosis of Endometrial Hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Niu, S.; Molberg, K.; Castrillon, D.H.; Lucas, E.; Chen, H. Biomarkers in the Diagnosis of Endometrial Precancers. Molecular Characteristics, Candidate Immunohistochemical Markers, and Promising Results of Three-Marker Panel: Current Status and Future Directions. Cancers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Ferrari, F.; Forte, S.; Arrigoni, G.; Ardighieri, L.; Coppola, M.C.; Salinaro, F.; Barra, F.; Sartori, E.; Odicino, F. Impact of Endometrial Sampling Technique and Biopsy Volume on the Diagnostic Accuracy of Endometrial Cancer. Transl. Cancer Res. 2020, 9, 7697–7705. [Google Scholar] [CrossRef] [PubMed]
- Sone, K.; Eguchi, S.; Asada, K.; Inoue, F.; Miyamoto, Y.; Tanikawa, M.; Tsuruga, T.; Mori-Uchino, M.; Matsumoto, Y.; Hiraike-Wada, O.; et al. Usefulness of Biopsy by Office Hysteroscopy for Endometrial Cancer: A Case Report. Mol. Clin. Oncol. 2020, 13, 141–145. [Google Scholar] [CrossRef]
- Ali, M.; Mumtaz, M.; Naqvi, Z.; Farooqui, R.; Shah, S.A. Assessing Tumor Size by MRI and Pathology in Type I Endometrial Carcinoma to Predict Lymph Node Metastasis. Cureus 2022, 14, e23135. [Google Scholar] [CrossRef] [PubMed]
- Gbelcová, H.; Gergely, L.; Šišovský, V.; Straka, Ľ.; Böhmer, D.; Pastoráková, A.; Sušienková, K.; Repiská, V.; Korbeľ, M.; Danihel, Ľ.; et al. PTEN Mutations as Predictive Marker for the High-Grade Endometrial Cancer Development in Slovak Women. Physiol. Res. 2022, 71, S125–S135. [Google Scholar] [CrossRef]
- Nitecki, R.; Woodard, T.; Rauh-Hain, J.A. Fertility-Sparing Treatment for Early-Stage Cervical, Ovarian, and Endometrial Malignancies. Obstet. Gynecol. 2020, 136, 1157–1169. [Google Scholar] [CrossRef]
- Abu Hashim, H.; Ghayaty, E.; El Rakhawy, M. Levonorgestrel-Releasing Intrauterine System vs Oral Progestins for Non-Atypical Endometrial Hyperplasia: A Systematic Review and Metaanalysis of Randomized Trials. Am. J. Obstet. Gynecol. 2015, 213, 469–478. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, Y.-C.; She, L.-Z.; Wang, T.-J. Comparative Effects of Progestin-Based Combination Therapy for Endometrial Cancer or Atypical Endometrial Hyperplasia: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2024, 14, 1391546. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Kim, J.J.; Benbrook, D.M.; Dwivedi, A.; Rai, R. Therapeutic Options for Management of Endometrial Hyperplasia. J. Gynecol. Oncol. 2016, 27, e8. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L. Group for Chinese Guidelines on The Management of Endometrial Hyperplasia Chinese Guidelines on the Management of Endometrial Hyperplasia. Eur. J. Surg. Oncol. 2024, 50, 108391. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, H.; Yu, M.; Cao, D.; Yang, J. GnRH-a-Based Fertility-Sparing Treatment of Atypical Endometrial Hyperplasia (AEH) and Early Endometrial Carcinoma (EC) Patients: A Multicenter, Open-Label, Randomized Designed Clinical Trial Protocol. Trials 2024, 25, 578. [Google Scholar] [CrossRef]
- Patel, V.; Wilkinson, E.J.; Chamala, S.; Lu, X.; Castagno, J.; Rush, D. Endometrial Thickness as Measured by Transvaginal Ultrasound and the Corresponding Histopathologic Diagnosis in Women with Postmenopausal Bleeding. Int. J. Gynecol. Pathol. 2017, 36, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Schmandt, R.E.; Iglesias, D.A.; Co, N.N.; Lu, K.H. Understanding Obesity and Endometrial Cancer Risk: Opportunities for Prevention. Am. J. Obstet. Gynecol. 2011, 205, 518–525. [Google Scholar] [CrossRef]
- Hüsing, A.; Dossus, L.; Ferrari, P.; Tjønneland, A.; Hansen, L.; Fagherazzi, G.; Baglietto, L.; Schock, H.; Chang-Claude, J.; Boeing, H.; et al. An Epidemiological Model for Prediction of Endometrial Cancer Risk in Europe. Eur. J. Epidemiol. 2016, 31, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bafligil, C.; Thompson, D.J.; Lophatananon, A.; Ryan, N.A.J.; Smith, M.J.; Dennis, J.; Mekli, K.; O’Mara, T.A.; Evans, D.G.; Crosbie, E.J. Development and Evaluation of Polygenic Risk Scores for Prediction of Endometrial Cancer Risk in European Women. Genet. Med. 2022, 24, 1847–1856. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Esnal-Zufiaurre, A.; Arends, M.J.; Herrington, C.S.; Collins, F.; Williams, A.R.W.; Saunders, P.T.K. Improving the Diagnosis of Endometrial Hyperplasia Using Computerized Analysis and Immunohistochemical Biomarkers. Front. Reprod. Health 2022, 4, 896170. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Saccone, G.; Campanino, M.R.; Mollo, A.; De Placido, G.; Insabato, L.; Zullo, F. Loss of PTEN Expression as Diagnostic Marker of Endometrial Precancer: A Systematic Review and Meta-Analysis. Acta Obstet. Gynecol. Scand. 2019, 98, 275–286. [Google Scholar] [CrossRef]
- El Sheikh, S.A.; Elyasergy, D.F. Immunoreactivity of PTEN in Cyclic Endometrium and Endometrial Hyperplasia. World J. Med. Sci. 2016, 13, 126–132. [Google Scholar]
- Travaglino, A.; Raffone, A.; Saccone, G.; Insabato, L.; Mollo, A.; De Placido, G.; Zullo, F. PTEN as a Predictive Marker of Response to Conservative Treatment in Endometrial Hyperplasia and Early Endometrial Cancer. A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 104–110. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Varras, M.; Vasilakaki, T.; Varra, V.-K.; Tsavari, A.; Varra, F.-N.; Nonni, A.; Kavantzas, N.; Lazaris, A.C. Expression of p53 and PTEN in Human Primary Endometrial Carcinomas: Clinicopathological and Immunohistochemical Analysis and Study of Their Concomitant Expression. Oncol. Lett. 2019, 17, 4575–4589. [Google Scholar] [CrossRef]
- Allithy, A.N.; Ammar, I.M.M.; Mohammed, M.H. Diagnostic and Prognostic Values of PTEN Expression in Functional and Pathological Endometrial Biopsies. Asian Pac. J. Canc. Biol. 2022, 7, 21–27. [Google Scholar] [CrossRef]
- Aswathi, R.K.; Arumugam, S.; Muninathan, N.; Baskar, K.; Deepthi, S. P53 Gene as a Promising Biomarker and Potential Target for the Early Diagnosis of Reproductive Cancers. Cureus 2024, 16, e60125. [Google Scholar]
- Schultheis, A.M.; Martelotto, L.G.; De Filippo, M.R.; Piscuglio, S.; Ng, C.K.Y.; Hussein, Y.R.; Reis-Filho, J.S.; Soslow, R.A.; Weigelt, B. TP53 Mutational Spectrum in Endometrioid and Serous Endometrial Cancers. Int. J. Gynecol. Pathol. 2016, 35, 289–300. [Google Scholar] [CrossRef]
- Muller, P.A.J.; Vousden, K.H. Mutant p53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef]
- Edmondson, R.J.; Crosbie, E.J.; Nickkho-Amiry, M.; Kaufmann, A.; Stelloo, E.; Nijman, H.W.; Leary, A.; Auguste, A.; Mileshkin, L.; Pollock, P.; et al. Markers of the p53 Pathway Further Refine Molecular Profiling in High-Risk Endometrial Cancer: A TransPORTEC Initiative. Gynecol. Oncol. 2017, 146, 327–333. [Google Scholar] [CrossRef]
- Zidan, A.; Hassan, A.; Seadah, S.S.; Ibrahim, E.; Attiah, S. Selected Immunohistochemical Prognostic Factors In Endometrial Hyperplasia versus Carcinoma. J. Am. Sci. 2015, 11, 14–22. [Google Scholar]
- Vineetha, P.; John, J.J. A Comparative Study of Expression of PTEN and p53 in Endometrial Hyperplasia and Carcinoma in a Tertiary Care Hospital. Indian J. Forensic Med. Pathol. 2020, 13, 561–567. [Google Scholar] [CrossRef]
- Ray, D.S.; Jha, D.A.; Afreen Islam, D.A.; Sengupta, D.M. Study of Prognostic and Diagnostic Significance of P53 and PTEN Mutation in Proliferative Lesions of Endometrium. J. Curr. Med. Res. Opin. 2020, 3, 563–569. [Google Scholar] [CrossRef]
- Mendiola, M.; Heredia-Soto, V.; Ruz-Caracuel, I.; Baillo, A.; Ramon-Patino, J.L.; Escudero, F.J.; Miguel, M.; Pelaez-Garcia, A.; Hernandez, A.; Feliu, J.; et al. Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 14468. [Google Scholar] [CrossRef]
- Siu, W.Y.S.; Hong, M.-K.; Ding, D.-C. Neuroendocrine Carcinoma of the Endometrium Concomitant with Lynch Syndrome: A Case Report. World J. Clin. Cases 2023, 11, 5160–5166. [Google Scholar] [CrossRef] [PubMed]
- Kazachkov, E.L.; Kazachkova, E.A.; Voropaeva, E.E.; Zatvornitskaya, A.V. Endometrial Hyperplasia and Microsatellite Instability: Possibilities for Predicting Tumor Transformation of the Endometrium. Clin. Exp. Morphol. 2023, 12, 14–22. [Google Scholar] [CrossRef]
- Chapel, D.B.; Patil, S.A.; Plagov, A.; Puranik, R.; Mendybaeva, A.; Steinhardt, G.; Wanjari, P.; Lastra, R.R.; Kadri, S.; Segal, J.P.; et al. Quantitative next-Generation Sequencing-Based Analysis Indicates Progressive Accumulation of Microsatellite Instability between Atypical Hyperplasia/endometrial Intraepithelial Neoplasia and Paired Endometrioid Endometrial Carcinoma. Mod. Pathol. 2019, 32, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.; Pinto, K.; Mutch, D.G.; Herzog, T.J.; Rader, J.S.; Gibb, R.; Bocker-Edmonston, T.; Goodfellow, P.J. Microsatellite Instability, MLH1 Promoter Methylation, and Loss of Mismatch Repair in Endometrial Cancer and Concomitant Atypical Hyperplasia. Gynecol. Oncol. 2002, 86, 62–68. [Google Scholar] [CrossRef]
- Sutter, C.; Dallenbach-Hellweg, G.; Schmidt, D.; Baehring, J.; Bielau, S.; von Knebel Doeberitz, M.; Gebert, J. Molecular Analysis of Endometrial Hyperplasia in HNPCC-Suspicious Patients May Predict Progression to Endometrial Carcinoma. Int. J. Gynecol. Pathol. 2004, 23, 18–25. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y. The Advance and Correlation of KRAS Mutation with the Fertility-Preservation Treatment of Endometrial Cancer in the Background of Molecular Classification Application. Pathol. Oncol. Res. 2021, 27, 1609906. [Google Scholar] [CrossRef] [PubMed]
- Maru, Y.; Tanaka, N.; Tatsumi, Y.; Nakamura, Y.; Itami, M.; Hippo, Y. Kras Activation in Endometrial Organoids Drives Cellular Transformation and Epithelial-Mesenchymal Transition. Oncogenesis 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Lupini, L.; Scutiero, G.; Iannone, P.; Martinello, R.; Bassi, C.; Ravaioli, N.; Soave, I.; Bonaccorsi, G.; Lanza, G.; Gafà, R.; et al. Molecular Biomarkers Predicting Early Development of Endometrial Carcinoma: A Pilot Study. Eur. J. Cancer Care 2019, 28, e13137. [Google Scholar] [CrossRef] [PubMed]
- Sideris, M.; Darwish, A.; Rallis, K.; Emin, E.I.; Mould, T. 548 Prognostic Biomarkers for Atypical Endometrial Hyperplasia: A Mini Review. Int. J. Gynecol. Cancer 2021, 31, A337. [Google Scholar]
- Corr, B.; Cosgrove, C.; Spinosa, D.; Guntupalli, S. Endometrial Cancer: Molecular Classification and Future Treatments. BMJ Med. 2022, 1, e000152. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular Subtypes of Endometrial Cancer: Implications for Adjuvant Treatment Strategies. Int. J. Gynaecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef]
- Wei, H.; Pan, N.; Zhang, W.; Xiong, G.; Guo, W.; Dong, Z.; Ma, C. Levonorgestrel-Releasing Intrauterine System-Based Therapies for Early-Stage Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Gynecol. Oncol. 2023, 34, e36. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.-X. Fertility-Preserving Treatment in Women with Early Endometrial Cancer: The Chinese Experience. Cancer Manag. Res. 2018, 10, 6803–6813. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Nam, J.-H. Progestins in the Fertility-Sparing Treatment and Retreatment of Patients with Primary and Recurrent Endometrial Cancer. Oncologist 2015, 20, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Bidhendi-Yarandi, R.; Fallahzadeh, A.; Marzban, Z.; Ramezani Tehrani, F. Risk of Endometrial, Ovarian, and Breast Cancers in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Int. J. Reprod. Biomed. 2022, 20, 893–914. [Google Scholar] [CrossRef]
- Lashen, H. Role of Metformin in the Management of Polycystic Ovary Syndrome. Ther. Adv. Endocrinol. Metab. 2010, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Melin, J.; Forslund, M.; Alesi, S.; Piltonen, T.; Romualdi, D.; Spritzer, P.M.; Tay, C.T.; Pena, A.; Witchel, S.F.; Mousa, A.; et al. The Impact of Metformin with or without Lifestyle Modification versus Placebo on Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Endocrinol. 2023, 189, S37–S63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nisenblat, V.; Tao, L.; Zhang, X.; Li, H.; Ma, C. Combined Estrogen-Progestin Pill Is a Safe and Effective Option for Endometrial Hyperplasia without Atypia: A Three-Year Single Center Experience. J. Gynecol. Oncol. 2019, 30, e49. [Google Scholar] [CrossRef]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiz, M.; Pavlakis, K.; Vermeulen, N.; et al. ESGO/ESHRE/ESGE Guidelines for the Fertility-Sparing Treatment of Patients with Endometrial Carcinoma. Hum. Reprod. Open 2023, 2023, hoac057. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.A.; Brinton, L.A.; Wentzensen, N.; Pan, K.; Chen, C.; Anderson, G.L.; Pfeiffer, R.M.; Xu, X.; Rohan, T.E.; Trabert, B. Postmenopausal Androgen Metabolism and Endometrial Cancer Risk in the Women’s Health Initiative Observational Study. JNCI Cancer Spectr. 2019, 3, kz029. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, H.; Qu, L.; Liu, H. Association between Hormone Replacement Therapy and Development of Endometrial Cancer: Results from a Prospective US Cohort Study. Front. Med. 2021, 8, 802959. [Google Scholar] [CrossRef]
- Kamii, M.; Nagayoshi, Y.; Ueda, K.; Saito, M.; Takano, H.; Okamoto, A. Laparoscopic Surgery for Atypical Endometrial Hyperplasia with Awareness Regarding the Possibility of Endometrial Cancer. Gynecol. Minim. Invasive Ther. 2023, 12, 32–37. [Google Scholar] [CrossRef]
- Depypere, H.; Inki, P. The Levonorgestrel-Releasing Intrauterine System for Endometrial Protection during Estrogen Replacement Therapy: A Clinical Review. Climacteric 2015, 18, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Method, M.; Leitao, M.; Lu, K.; Ioffe, O.; Hampton, M.; Higgins, R.; Zaino, R.; Mutter, G.L. Society of Gynecologic Oncology Clinical Practice Committee Management of Endometrial Precancers. Obstet. Gynecol. 2012, 120, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, R.S.; Ciccone, M.A.; Nusbaum, D.J.; Khoshchehreh, M.; Purswani, H.; Morocco, E.B.; Smith, M.B.; Matsuzaki, S.; Dancz, C.E.; Ozel, B.; et al. Progestin Therapy for Obese Women with Complex Atypical Hyperplasia: Levonorgestrel-Releasing Intrauterine Device vs Systemic Therapy. Am. J. Obstet. Gynecol. 2020, 223, 103.e1–103.e13. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Catena, U.; Saccone, G.; Di Spiezio Sardo, A. Conservative Surgery in Endometrial Cancer. J. Clin. Med. Res. 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
| Items | Specification |
|---|---|
| Timeframe | To 31 August 2024 |
| Database | PubMed |
| Search terms used | “endometrial atypical hyperplasia”, “endometrial cancer” |
| Inclusion and exclusion criteria | All references were SCI-indexed articles The language is English |
| Selection process | Two independent reviewers evaluated the titles and abstracts to determine eligibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, A.-J.; Bing, R.-S.; Ding, D.-C. Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer. Diagnostics 2024, 14, 2471. https://doi.org/10.3390/diagnostics14222471
Chou A-J, Bing R-S, Ding D-C. Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer. Diagnostics. 2024; 14(22):2471. https://doi.org/10.3390/diagnostics14222471
Chicago/Turabian StyleChou, An-Ju, Ruo-Shi Bing, and Dah-Ching Ding. 2024. "Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer" Diagnostics 14, no. 22: 2471. https://doi.org/10.3390/diagnostics14222471
APA StyleChou, A.-J., Bing, R.-S., & Ding, D.-C. (2024). Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer. Diagnostics, 14(22), 2471. https://doi.org/10.3390/diagnostics14222471







