Monitoring and Management of Cytomegalovirus Reactivations After Allogeneic Hematopoietic Stem Cell Transplantation in Children: Experience from a Single Pediatric Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Haploidentical transplant;
- Any HSCT subsequent to the first;
- aGvHD;
- cGvHD;
- Steroid dose > 2 mg/kg/day;
- Second-line immunosuppressive drugs for the treatment of GvHD.
2.2. Statistical Analysis
3. Results
3.1. Characteristics of 214 Allogeneic HCTs
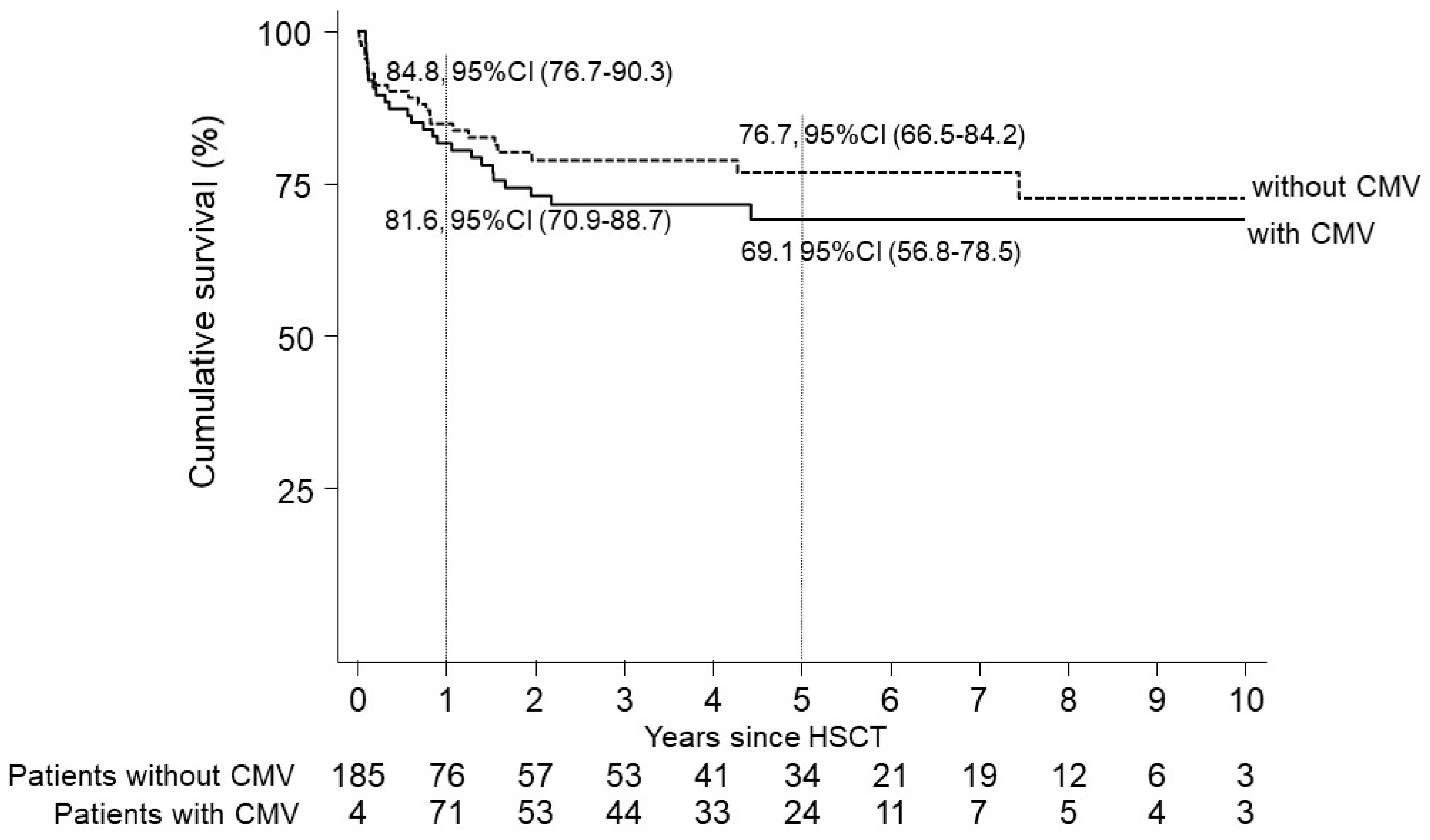
3.2. CMV Reactivations and Risk-Factor Analysis
3.3. Pre-Emptive Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camargo, J.F.; Anderson, A.D.; Rosa, R.; Kimble, E.; Komanduri KV Morris, M.I. Use of maintenance therapy and incidence of recurrent Cytomegalovirus DNAemia among allogeneic hematopoietic cell transplant recipients. Transpl. Infect. Dis. 2019, 21, e13054. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.A.; Jung, V.; Knops, E.; Heger, E.; Wirtz, M.; Steger, G.; Kaiser, R.; Affeldt, P.; Holtick, U.; Klein, F.; et al. CMV-IgG pre-allogeneic hematopoietic stem cell transplantation and the risk for CMV reactivation and mortality. Bone Marrow Transpl. 2023, 58, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Huntley, D.; Giménez, E.; Pascual, M.J.; Hernández-Boluda, J.C.; Gago, B.; Vázquez, L.; Piñana, J.L.; García, M.; Pérez, A.; Serrano, D.; et al. Incidence, features, and outcomes of cytomegalovirus DNAemia in unmanipulated haploidentical allogeneic hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Transpl. Infect. Dis. 2020, 22, e13206. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, S.K.; Ryu, I.H.; Lee, J.W.; Lee, D.G.; Chung, N.G.; Jeong, D.C.; Cho, B. Clinical characteristics and viral load patterns in children with cytomegalovirus gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2021, 56, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.; Leisenring, W.; Xie, H.U.; Mast, T.C.; Cui, Y.; Sandmaier, B.M.; Sorror, M.L.; Goyal, S.; Özkök, S.; Yi, J.; et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: A retrospective cohort study. Lancet Haematol. 2016, 3, e119–e127. [Google Scholar] [CrossRef] [PubMed]
- AdAdmiraal, R.; de Koning, C.C.; Lindemans, C.A.; Bierings, M.B.; Wensing, A.M.; Versluys, A.B.; Wolfs, T.F.; Nierkens, S.; Boelens, J.J. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J. Allergy Clin. Immunol. 2017, 140, 1643–1650.e9. [Google Scholar] [CrossRef] [PubMed]
- Hakki, M.; Aitken, S.L.; Danziger-Isakov, L.; Michaels, M.G.; Carpenter, P.A.; Chemaly, R.F.; Papanicolaou, G.A.; Boeckh, M.; Marty, F.M. American Society for Transplantation and Cellular Therapy Series: #3—Prevention of Cytomegalovirus Infection and Disease After Hematopoietic Cell Transplantation. Transpl. Cell Ther. 2021, 27, 707–719. [Google Scholar]
- van der Heiden, P.; Marijt, E.; Falkenburg, F.; Jedema, I. Control of Cytomegalovirus Viremia after Allogeneic Stem Cell Transplantation: A Review on CMV-Specific T Cell Reconstitution. Biol. Blood Marrow Transplant. 2018, 24, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Kerridge, I.H.; Gilroy, N.; Huang, G.; Hertzberg, M.S.; Bradstock, K.F.; Gottlieb, D.J. A risk score for early cytomegalovirus reactivation after allogeneic stem cell transplantation identifies low-, intermediate-, and high-risk groups: Reactivation risk is increased by graft-versus-host disease only in the intermediate-risk group. Transpl. Infect. Dis. 2012, 14, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Servais, S.; Dumontier, N.; Biard, L.; Schnepf, N.; Resche-Rigon, M.; de Latour, R.P.; Scieux, C.; Robin, M.; Meunier, M.; Xhaard, A.; et al. Response to antiviral therapy in haematopoietic stem cell transplant recipients with cytomegalovirus (CMV) reactivation according to the donor CMV serological status. Clin. Microbiol. Infect. 2016, 22, 289.e1–289.e7. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Shulman, H.M.; Cardona, D.M.; Greenson, J.K.; Hingorani, S.; Horn, T.; Huber, E.; Kreft, A.; Longerich, T.; Morton, T.; Myerson, D.; et al. NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. The 2014 Pathology Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 589–603. [Google Scholar] [CrossRef]
- Ogonek, J.; Juric, M.K.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; de la Camara, R.; Robin, C.; Crocchiolo, R.; Einsele, H.; Hill, J.A.; Hubacek, P.; Navarro, D.; Cordonnier, C.; Ward, K.N. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e260–e272. [Google Scholar] [CrossRef] [PubMed]
- Annaloro, C.; Serpenti, F.; Saporiti, G.; Galassi, G.; Cavallaro, F.; Grifoni, F.; Goldaniga, M.; Baldini, L.; Onida, F. Viral infections in HSCT: Detection, monitoring, clinical management, and immunologic implications. Front. Immunol. 2021, 11, 569381. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Nishida, T.; Asano-Mori, Y.; Oshima, K.; Ohashi, K.; Mori, T.; Kanamori, H.; Miyamura, K.; Kato, C.; Kobayashi, N.; et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol. Blood Marrow Transplant. 2015, 21, 2008–2016. [Google Scholar] [PubMed]
- Lonnqvist, B.; Ringdén, O.; Wahren, B.; Gahrton, G.; Ludgren, G. Cytomegalovirus infection associated with and preceding Chronic Graft-versus Host Disease. Transplantation 1984, 38, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Lazzarotto, T.; Bonifazi, F.; Patriarca, F.; Irrera, G.; Ciceri, F.; Aversa, F.; Citterio, F.; Cillo, U.; Cozzi, E.; et al. Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies. Clin. Transplant. 2019, 33, e13666. [Google Scholar] [CrossRef] [PubMed]

| Factors | Total, n = 214 | No Reactivation, n = 114 | Reactivation, n = 100 | p-Value |
|---|---|---|---|---|
| Age at HSCT, years, median (1st–3rd quartiles) | 7.9 (3.3–13.6) | 7.8 (2.8–13.6) | 8.0 (3.4–13.7) | 0.536 |
| Sex, Males, n (%) | 131 (61.2) | 70 (61.4) | 61 (61.0) | 0.952 |
| Non-malignant disease, n (%) | 111 (51.9) | 60 (52.6) | 51 (51.0) | 0.812 |
| High serological risk, n (%) | 46 (21.5) | 18 (15.8) | 28 (28.0) | 0.030 |
| Subsequent HSCT, n (%) | 29 (13.5) | 14 (12.3) | 15 (15.0) | 0.562 |
| Donor type, n (%) | 0.169 | |||
| Haplo | 85 (39.7) | 52 (45.61) | 33 (33.0) | 0.069 |
| TCR-αβ+/CD19+-depleted | 46 (54.1) | 30 (57.7) | 16 (48.5) | 0.406 |
| PT-Cy | 39 (45.9) | 22 (42.3) | 17 (51.5) | |
| AD | 86 (40.2) | 41 (36.0) | 45 (45.0) | |
| MRD | 43 (20.1) | 21 (18.4) | 22 (22.0) | |
| Second-line immunosuppressive therapy, n (%) | 41 (19.2) | 19 (16.7) | 22 (22.0) | 0.385 |
| Methylprednisolone > 2 mg/kg, n (%) | 43 (20.1) | 15 (13.2) | 28 (28.0) | 0.007 |
| Acute GvHD, n (%) | 96/195 (49.2) | 48/100 * (48.0) | 48/95 * (50.5) | 0.724 |
| Acute GvHD grade III-IV, n (%) | 55/195 (28.2) | 23/100 * (23.0) | 32/95 * (33.7) | 0.063 |
| Chronic GvHD, n (%) | 46/182 (25.3) | 18/91 * (19.8) | 28/91 * (30.8) | 0.088 |
| Chronic GvHD extensive, n (%) | 20/182 (11) | 7/91 * (7.7) | 13/91 * (14.3) | 0.615 |
| Presence of at least one predefined condition at risk for CMV ** | 174 (81.3) | 93 (81.6) | 81 (81) | 0.914 |
| Deaths, n (%) | 48/189 *** (25.4) | 25/95 *** (26.3) | 23/94 *** (24.5) | 0.770 |
| TRM | 35 | 16 | 19 | |
| Graft failure | 2 | - | ||
| Infection | 6 | 6 | ||
| GvHD | 3 | 7 | ||
| Toxicity | 3 | 6 | ||
| Other | 2 | - | ||
| Relapse/Progression | 13 | 9 | 4 |
| Pre-Emptive Antiviral Therapy | ||||
|---|---|---|---|---|
| Factors | Total, n = 69 | Yes, n = 59 (85.5) | No, n = 10 (14.5) | p-Value |
| Sex, Males, n (%) | 37 (53.6) | 33 (55.9) | 4 (40) | 0.350 |
| Age at HCT, years, median (1st–3rd quartiles) | 8.5 (4.7–13.6) | 7.9 (4.6–13.6) | 10.4 (6.3–17.5) | 0.200 |
| Malignant diagnosis, n (%) | 35 (50.7) | 29 (49.1) | 6 (60) | 0.526 |
| High serological risk, n (%) | 21 (30.4) | 20 (33.9) | 1 (10) | 0.263 |
| Donor type, n (%) | 0.082 | |||
| Haplo | 18 (26.1) | 17 (28.8) | 1 (10) | |
| AD | 36 (52.2) | 32 (54.2) | 4 (40) | |
| MRD | 15 (21.7) | 10 (17) | 5 (50) | |
| Source of stem cell graft, n (%) | 0.248 | |||
| Bone marrow | 54 (78.3) | 44 (74.6) | 10 (100) | |
| Peripheral blood | 13 (18.8) | 13 (22) | 0 | |
| Cord blood | 2 (2.9) | 2 (3.4) | 0 | |
| Conditioning regimen, n (%) | 1.000 | |||
| MAC | 41 (59.4) | 35 (59.3) | 6 (60) | |
| RIC | 28 (40.6) | 24 (40.7) | 4 (40) | |
| Methylprednisolone > 2 mg/kg, n (%) | 28 (40.6) | 26 (44.1) | 2 (20) | 0.184 |
| Second-line immunosuppressive therapy, n (%) | 17 (24.6) | 14 (23.7) | 3 (30) | 0.699 |
| Acute GvHD, n (%) | 32/65 * (49.2) | 30/56 * (53.6) | 2/9 * (22.2) | 0.149 |
| Chronic GvHD, n (%) | 18/62 * (29.0) | 18/54 * (33.3) | 0/8 * | 0.092 |
| Presence of at least one predefined condition at risk for CMV **, n (%) | 56 (81.2) | 52 (88.1) | 4 (40) | 0.002 |
| CMV-DNA copies, median (1st–3rd quartiles) at the beginning of the therapy | - | 2200 (800–4500) | - | - |
| CMV-DNA copies, median (1st–3rd quartiles) at maximum monitoring value | 5300 (2000–17050) | 6600 (3150–19700) | 1100 (300–1650) | <0.001 |
| Days from HCT to reactivation, median (1st–3rd quartiles) | 23 (15–43) | 24 (15–43) | 19.5 (15–40) | 0.547 |
| CMV reactivation after HCT subsequent to the first, n (%) | 9 (13) | 8 (13.6) | 1 (190) | 0.594 |
| Deaths, n (%) | 17/65 *** (26.1) | 16/55 *** (29.1) | 1/10 *** (10) | 0.270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrando, G.; Bagnasco, F.; Giardino, S.; Pierri, F.; Pestarino, S.; Di Marco, E.; Santaniello, M.; Castagnola, E.; Faraci, M. Monitoring and Management of Cytomegalovirus Reactivations After Allogeneic Hematopoietic Stem Cell Transplantation in Children: Experience from a Single Pediatric Center. Diagnostics 2024, 14, 2461. https://doi.org/10.3390/diagnostics14212461
Ferrando G, Bagnasco F, Giardino S, Pierri F, Pestarino S, Di Marco E, Santaniello M, Castagnola E, Faraci M. Monitoring and Management of Cytomegalovirus Reactivations After Allogeneic Hematopoietic Stem Cell Transplantation in Children: Experience from a Single Pediatric Center. Diagnostics. 2024; 14(21):2461. https://doi.org/10.3390/diagnostics14212461
Chicago/Turabian StyleFerrando, Giulia, Francesca Bagnasco, Stefano Giardino, Filomena Pierri, Sara Pestarino, Eddi Di Marco, Maria Santaniello, Elio Castagnola, and Maura Faraci. 2024. "Monitoring and Management of Cytomegalovirus Reactivations After Allogeneic Hematopoietic Stem Cell Transplantation in Children: Experience from a Single Pediatric Center" Diagnostics 14, no. 21: 2461. https://doi.org/10.3390/diagnostics14212461
APA StyleFerrando, G., Bagnasco, F., Giardino, S., Pierri, F., Pestarino, S., Di Marco, E., Santaniello, M., Castagnola, E., & Faraci, M. (2024). Monitoring and Management of Cytomegalovirus Reactivations After Allogeneic Hematopoietic Stem Cell Transplantation in Children: Experience from a Single Pediatric Center. Diagnostics, 14(21), 2461. https://doi.org/10.3390/diagnostics14212461







