Molar Incisor Hypomineralization: Etiology, Correlation with Tooth Number Anomalies and Implications for Comprehensive Management Strategies in Children from Transylvania
Abstract
1. Introduction
Objectives
2. Materials and Methods
Patient Selection Criteria
- Prenatal Factors:
- ○
- Environment (urban, rural)
- ○
- Mother’s educational level and age at conception
- ○
- Socioeconomic status
- ○
- Method of conception (natural or assisted)
- ○
- General health conditions during pregnancy, infections, fever, medication, variations in commonly evaluated health indicators (thyroid hormones, calcium, iron, vitamin D), and other pregnancy complications.
- Perinatal Factors:
- ○
- Term or premature birth
- ○
- Type of delivery (natural or cesarean)
- ○
- Duration of labor (prolonged or normal)
- ○
- Medication administered to facilitate birth
- ○
- Birth weight (low, normal, or high)
- ○
- Presence or absence of hypoxia.
- Postnatal Factors:
- ○
- General conditions developed by the child in the first months of life
- ○
- Type of feeding (breastfeeding, formula)
- ○
- Infections
- ○
- Variations in usual health indicators (Ca, Mg, Fe, vitamin D)
- ○
- Moment of the mother’s return to work.
3. Results
- Female sex was identified as a protective factor (OR = 0.308).
- Prolonged labor increased the risk by 5.25 times.
- Medications administered to facilitate birth increased the risk of MIH tenfold.
- Medications administered in the first 12 months of life increased the risk fourfold.
- ENT/OMF infections increased the risk by 4.632 times.
4. Discussion
5. Conclusions
- The study highlights a significant association between Molar Incisor Hypomineralization (MIH) and specific prenatal, perinatal, and postnatal factors, such as advanced maternal age and complications during labor. The observed correlation between MIH and various dental anomalies underscores the necessity for an interdisciplinary approach in both diagnosis and treatment.
- The study reveals a strong correlation between Molar Incisor Hypomineralization (MIH) and dental agenesis, suggesting that children with MIH are at a higher risk of missing teeth. This significant association emphasizes the need for proactive management strategies in patients with MIH, where early identification of concurrent dental agenesis can inform more effective, individualized treatment plans. Integrating orthodontic and restorative interventions early in the management process is crucial to address both MIH and the related dental anomalies, ultimately improving long-term oral health outcomes for affected children.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olatosi, O.O.; Oyapero, A.; Akinwande, K.O.; Ayedun, O.S.; Aladenika, E.T.; Obe, O.I. Pattern and Prevalence of Dental Anomalies among a Paediatric Population in Lagos, Nigeria. Niger. Postgrad. Med. J. 2022, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Jahanimoghadam, F. Dental Anomalies: An Update. Adv. Hum. Biol. 2016, 6, 112–118. [Google Scholar] [CrossRef]
- Vorwaller, R.; Kratunova, E.; Da Fonseca, M.A.; Alapati, S.B.; Hill, B.; Stanford, C. Prevalence of Radiographically Identifiable Dental Anomalies in Children and Association with Health Status. Pediatr. Dent. 2021, 43, 451–456. [Google Scholar] [PubMed]
- Amrollahi, N.; Hashemi, S.; Heidari, Z. Impact of molar incisor hypomineralization on oral health-related quality of life in 8–10 years old children: A systematic review and meta-analysis. J. Evid. Based Dent. Pr. 2023, 23, 101889. [Google Scholar] [CrossRef] [PubMed]
- Gevert, M.V.; Wambier, L.M.; Ito, L.Y.; Feltrin de Souza, J.; Rodrigues Chibinski, A.C. Which are the clinical consequences of Molar Incisor hypomineralization (MIH) in children and adolescents? Systematic review and meta-analysis. Clin. Oral Investig. 2024, 28, 415. [Google Scholar] [CrossRef]
- Torlińska-Walkowiak, N.; Majewska, K.A.; Sowińska, A.; Kędzia, A.; Opydo-Szymaczek, J. Developmental enamel defects and dental anomalies of number and size in children with growth hormone deficiency. Sci. Rep. 2023, 13, 14707. [Google Scholar] [CrossRef]
- Altner, S.; Milutinovic, I.; Bekes, K. Possible Etiological Factors for the Development of Molar Incisor Hypomineralization (MIH) in Austrian Children. Dent. J. 2024, 12, 44. [Google Scholar] [CrossRef]
- Ciocan, B.; Săndulescu, M.; Luca, R. Real-World Evidence on the Prevalence of Molar Incisor Hypomineralization in School Children from Bucharest, Romania. Children 2023, 10, 1563. [Google Scholar] [CrossRef]
- Almuallem, Z.; Busuttil-Naudi, A. Molar incisor hypomineralisation (MIH)—An overview. Br. Dent. J. 2018, 225, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Jianu, M.C.; Muntean, A.; Mihălțan, C.I.; Pacurar, M.; Munteanu, A. Molar incisor hypomineralisation: A review of etiology, diagnosis criteria and patterns considering eapd criteria. Rom. J. Oral Rehabil. 2022, 14, 117–124. [Google Scholar]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Barbieri, S.; Bruni, A.; Sinesi, A.; Ricci, M.; Trombini, J.; et al. Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review. Children 2021, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.M.; Vergnes, J.N.; Germa, A.; Azogui, S.; Breinig, S.; Hollande, C.; Bonnet, A.L.; Nabet, C. Factors and Mechanisms Involved in Acquired Developmental Defects of Enamel: A Scoping Review. Front. Pediatr. 2022, 10, 836708. [Google Scholar] [CrossRef] [PubMed]
- Mohabatpour, F.; Chen, X.; Papagerakis, S.; Papagerakis, P. Novel trends, challenges and new perspectives for enamel repair and regeneration to treat dental defects. Biomater. Sci. 2022, 10, 3062–3087. [Google Scholar] [CrossRef] [PubMed]
- Gachova, D.; Lipovy, B.; Deissova, T.; Izakovicova Holla, L.; Danek, Z.; Borilova Linhartova, P. Polymorphisms in genes expressed during amelogenesis and their association with dental caries: A case-control study. Clin. Oral Investig. 2023, 27, 1681–1695. [Google Scholar] [CrossRef]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and Its Dynamic Protein Matrix. Int. J. Mol. Sci 2020, 21, 4458. [Google Scholar] [CrossRef]
- Şen Yavuz, B.; Sezer, B.; Kaya, R.; Tuğcu, N.; Kargül, B. Is there an association between molar incisor hypomineralization and developmental dental anomalies? A case-control study. BMC Oral Health 2023, 23, 776. [Google Scholar] [CrossRef]
- Rodd, H.D.; Nazzal, H.; Bonifacio, C.C.; Ruth, C.W.; Crombie, F.; El Shahawy, O.; Folayan, M.O.; Gambetta-Tessini, K.; Goyal, A.; Hasmun, N.; et al. An International Investigation of Molar Incisor Hypomineralisation (iMIH) and Its Association with Dental Anomalies: Development of a Protocol. Dent. J. 2023, 11, 117. [Google Scholar] [CrossRef]
- Hanan, S.A.; De Farias, A.L.; Santos-Pinto, L. Molar Incisor Hypomineralization in adolescents and adults and its association with facial profile and occlusion. Clin. Oral Investig. 2023, 27, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Houari, S.; Loiodice, S.; Jedeon, K.; Berdal, A.; Babajko, S. Expression of Steroid Receptors in Ameloblasts during Amelogenesis in Rat Incisors. Front. Physiol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Walshaw, E.G.; Noble, F.; Conville, R.; Anne Lawson, J.; Hasmun, N.; Rodd, H. Molar incisor hypomineralisation and dental anomalies: A random or real association? Int. J. Paediatr. Dent. 2020, 30, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Bekes, K.; Weerheijm, K.L. Diagnosis, Classifications and Treatment Strategies of MIH-Affected Teeth. In Molar Incisor Hypomineralization; Bekes, K., Ed.; Springer: Cham, Switzerland, 2020; pp. 47–59. [Google Scholar] [CrossRef]
- Yamunadevi, A.; Pratibha, R.; Rajmohan, M.; Mahendraperumal, S.; Ganapathy, N.; Srivandhana, R. First Molars in Permanent Dentition and their Malformations in Various Pathologies: A Review. J. Pharm. Bioallied. Sci. 2021, 1, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Tesi, C.; Ricci, S.; Levrini, L.; Giorgetti, G.; Campagnolo, M.; Ciliberti, R.; Fusco, R.; Larentis, O.; Licata, M. Defects-related early childhood caries as hints of possible maternal–fetal health issues: Evidence from medieval northern Italy. Int. J. Osteoarchaeol. 2023, 33, 251–270. [Google Scholar] [CrossRef]
- Glasier, I.; Hunt, E.E. The permanent mandibular first molar: Its calcification, eruption and decay. Am. J. Phys. Anthropol. 1955, 13, 253–283. [Google Scholar] [CrossRef]
- Liversidge, H.M.; Molleson, T. Variation in crown and root formation and eruption of human deciduous teeth. Am. J. Phys. Anthr. 2004, 123, 172–180. [Google Scholar] [CrossRef]
- Reid, D.J.; Dean, M.C. Variation in modern human enamel formation times. J. Hum. Evol. 2006, 50, 29–46. [Google Scholar] [CrossRef]
- Noor Mohamed, R.; Basha, S.; Virupaxi, S.G.; Idawara, E.N.; Parameshwarappa, P. Hypomineralized Primary Teeth in Preterm Low Birth Weight Children and Its Association with Molar Incisor Hypomineralization—A 3-Year-Prospective Study. Children 2021, 8, 1111. [Google Scholar] [CrossRef]
- Brejawi, M.S.; Venkiteswaran, A.; Ergieg, S.M.O.; Sabri, B.M. Correlation between Molar-Incisor Hypomineralization, Stress, and Family Functioning. J. Int. Soc. Prev. Community Dent. 2022, 12, 547–553. [Google Scholar] [CrossRef]
- Ghanim, A.; Manton, D.; Bailey, D.; Mariño, R.; Morgan, M. Risk factors in the occurrence of molar-incisor hypomineralization amongst a group of Iraqi children. Int. J. Paediatr. Dent. 2013, 3, 197–206. [Google Scholar] [CrossRef]
- Schwartze, J.T.; Becker, S.; Sakkas, E.; Wujak, Ł.A.; Niess, G.; Usemann, J.; Reichenberger, F.; Herold, S.; Vadász, I.; Mayer, K.; et al. Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts. J. Biol. Chem. 2014, 6, 3262–3275. [Google Scholar] [CrossRef]
- Georgina-Pérez, L.; Ribas-Pérez, D.; Dehesa-Santos, A.; Mendoza, M.A. Relationship between the TGFBR1 Gene and Molar Incisor Hypomineralization. J. Pers. Med. 2023, 13, 777. [Google Scholar] [CrossRef] [PubMed]
- Neelon, B.S.E.; Stroo, M.; Mayhew, M.; Maselko, J.; Hoyo, C. Correlation between maternal and infant cortisol varies by breastfeeding status. Infant Behav. Dev. 2015, 40, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Juárez-López, M.L.A.; Salazar-Treto, L.V.; Hernández-Monjaraz, B.; Molina-Frechero, N. Etiological Factors of Molar Incisor Hypomineralization: A Systematic Review and Meta-Analysis. Dent. J. 2023, 11, 111. [Google Scholar] [CrossRef]
- Aine, L.; Backström, M.C.; Mäki, R.; Kuusela, A.; Koivisto, A.M.; Ikonen, R.S.; Mäki, M. Enamel defects in primary and permanent teeth of children born prematurely. J. Oral Pathol. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Enamel: Composition, Formation, and Structure. In Ten Cate’s Oral Histology Development, Structure, and Function, 8th ed.; Elsevier Mosby: Saint Louis, MO, USA, 2018; pp. 122–164. [Google Scholar]
- Foster, B.L.; Nociti, F.H., Jr.; Somerman, M.J. The rachitic tooth. Endocr. Rev. 2014, 35, 1–34. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Chaussain, C.; Osdoby, P.; Brandi, M.L.; Clarke, B.; Thakker, R.V. The role of biomineralization in disorders of skeletal development and tooth formation. Nat. Rev. Endocrinol. 2021, 17, 336–349. [Google Scholar] [CrossRef]
- Bailleul-Forestier, I.; Berdal, A.; Vinckier, F.; De Ravel, T.; Fryns, J.P.; Verloes, A. The genetic basis of inherited anomalies of the teeth. Part 2: Syndromes with significant dental involvement. Eur. J. Med. Genet. 2008, 51, 383–408. [Google Scholar] [CrossRef] [PubMed]
- Ngangom, A.; Jain, M.; Verma, S. Need of Early Dental Intervention in Vitamin D Deficiency Rickets. Indian J. Dent. Sci. 2018, 10, 229–232. [Google Scholar] [CrossRef]
- Al-Ani, A.H.; Antoun, J.S.; Thomson, W.M.; Merriman, T.R.; Farella, M. Hypodontia: An Update on Its Etiology, Classification, and Clinical Management. BioMed Res. Int. 2017, 2017, 9378325. [Google Scholar] [CrossRef]
- Khazaei, Y.; Harris, C.P.; Heinrich, J.; Standl, M.; Kühnisch, J. Association Study on Nutrition in the First Year of Life and Molar-Incisor Hypomineralization (MIH)—Results from the GINIplus and LISA Birth Cohort Studies. Int. J. Environ. Res. Public Health 2021, 18, 11411. [Google Scholar] [CrossRef]
- Garot, E.; Rouas, P.; Somani, C.; Taylor, G.D.; Wong, F.; Lygidakis, N.A. An update of the aetiological factors involved in molar incisor hypomineralisation (MIH): A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2022, 23, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Bezamat, M.; Souza, J.F.; Silva, F.M.F.; Corrêa, E.G.; Fatturi, A.L.; Brancher, J.A.; Carvalho, F.M.; Cavallari, T.; Bertolazo, L.; Machado-Souza, C.; et al. Gene-environment interaction in molar-incisor hypomineralization. PLoS ONE 2021, 16, 0241898. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.P.S.; Pereira, P.S.A.; Carvalho, F.A.R.; Soviero, V.M. Influence of genetics on the occurrence of enamel hypomineralization affecting permanent and primary teeth: A scoping review. Int. J. Paediatr. Dent. 2024, 34, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.C.; Santos, P.B. Association between molar-incisor hypomineralisation and dental anomalies. Int. J. Paediatr. Dent. 2024. [Google Scholar] [CrossRef]
- Moulis, E.; Barthélemi, S.; Delsol, L. Orthodontic treatment of children with class II division 1 with severe MIH involving first permanent molars extractions: A case report. Int. Orthod. 2020, 18, 885–894. [Google Scholar] [CrossRef]
- Cobourne, M.T.; Williams, A.; Harrison, M. National clinical guidelines for the extraction of first permanent molars in children. Br. Dent. J. 2014, 217, 643–648. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Mahler, M.E. Major depressive disorder. Psychopathology, medical management and dental implications. J. Am. Dent. Assoc. 2001, 132, 629–638. [Google Scholar] [CrossRef]
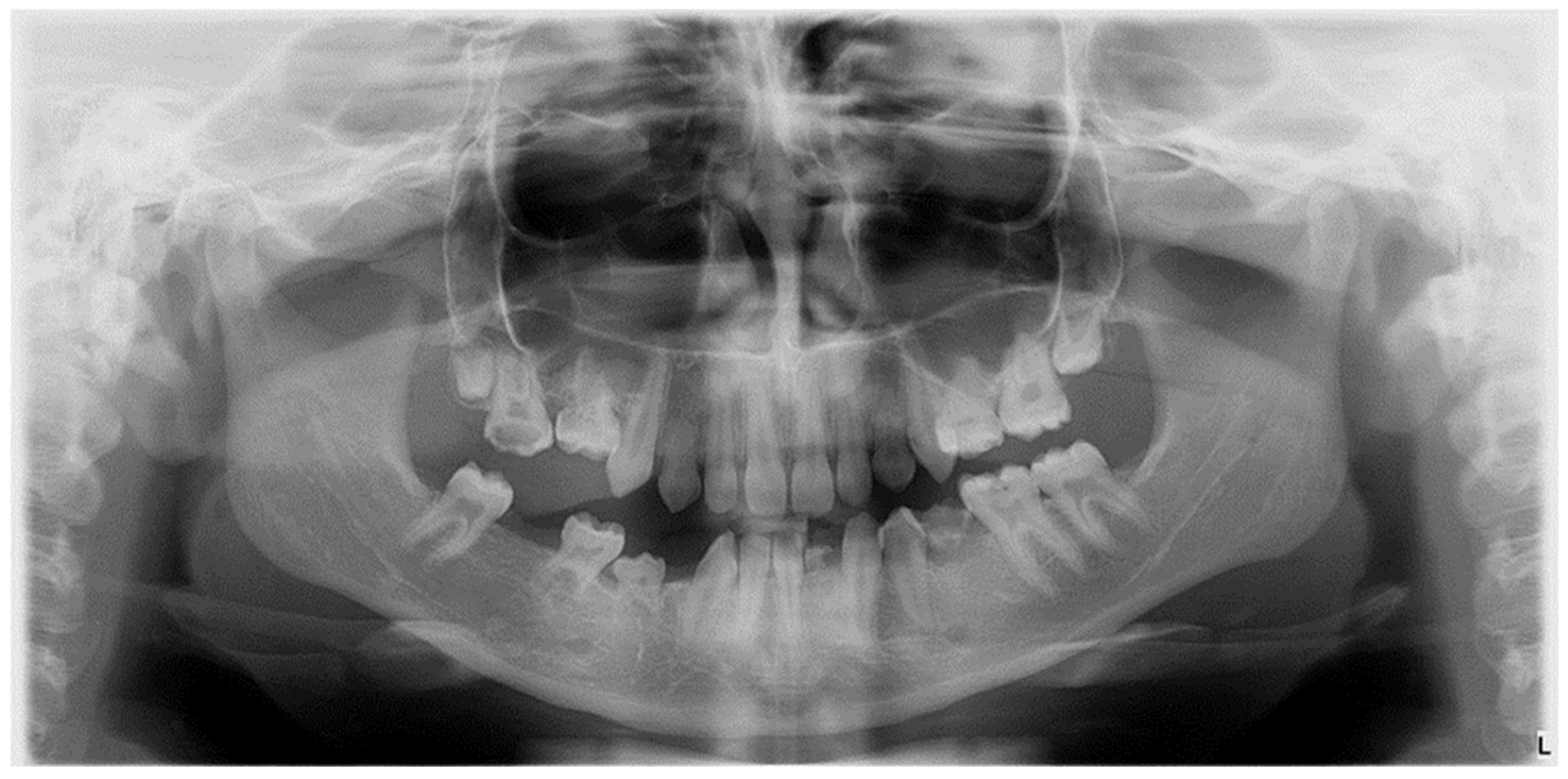
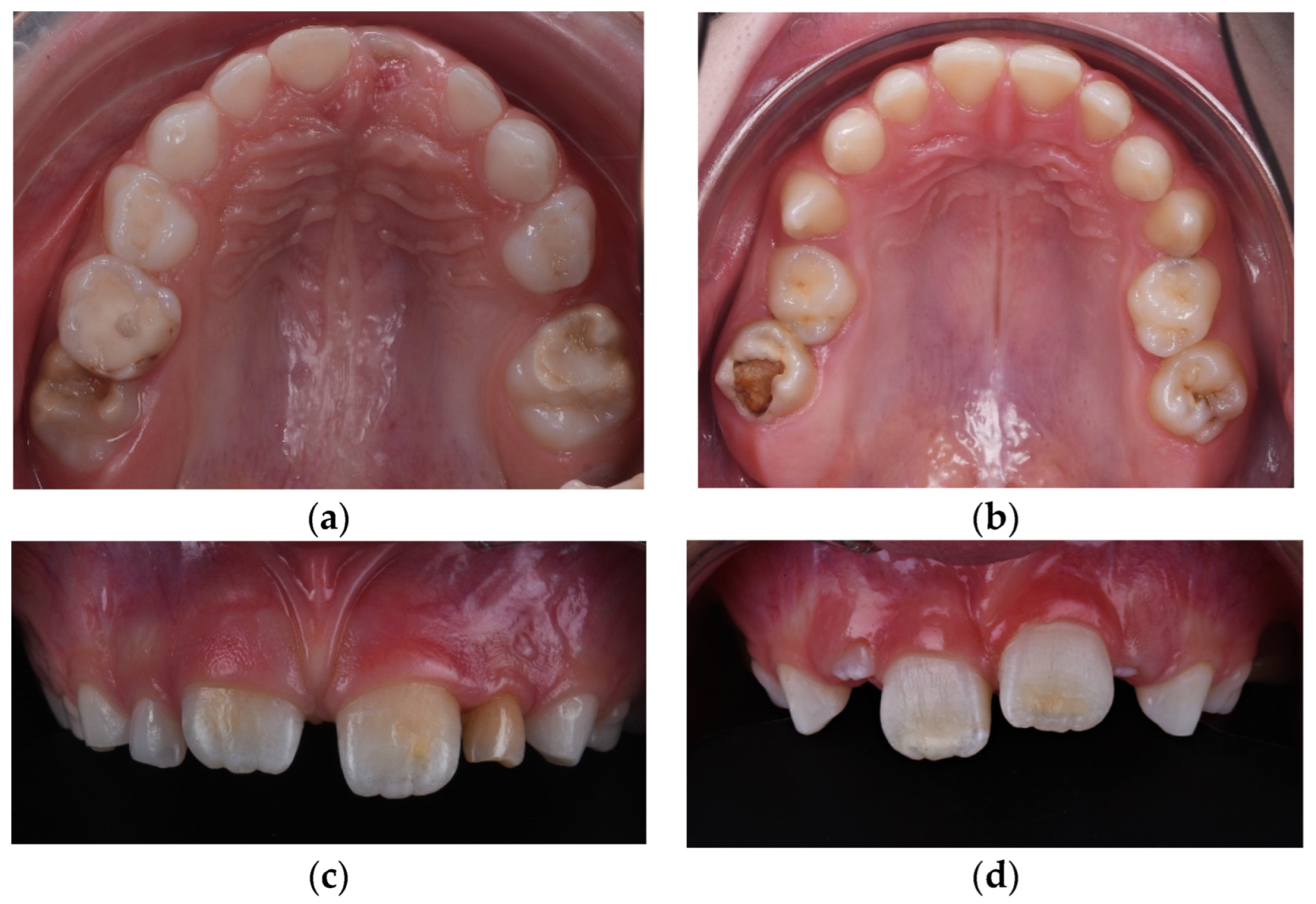




| Type of Dental Developmental Anomaly (DDA) | Number | Size | Shape | Structure |
|---|---|---|---|---|
| Clinical Manifestation | Anodontia Oligodontia Hypodontia Supranumerary teeth Pleiodontia | Macrodontia Microdontia | Fusion Gemination Peg-shape Dens in dente Dens invaginatus Enamel pearl Taurodntism | Hypoplasia Hypomieralization Molar-Incisor Hypomineralization Fluorosis Amelogenesis imperfecta Dentinogenesis imperfecta |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Children with mixed dentition | Biological mother unable to complete questionnaire (adopted, orphans, foster care) |
| Age between 6–11 years old | Mothers with chronic medication during pregnancy |
| Urban environment | No previous tooth extractions |
| No general diseases or syndromes | Patients with genetic syndromes with dental manifestation (Down Syndrome, ectodermal dysplasia) Cleft lip/palate |
| No history of chronic medication | Orthodontic fixed appliances on the first permanent molars/incisors |
| Patient’s Mean Age (Years) with SD | Mother’s Mean Age (Years) with SD | |
|---|---|---|
| Case | 7.50 ± 1.36 | 35.56 ± 5.74 |
| Control | 7.06 ± 1.09 | 29.36 ± 3.18 |
| p-value | 0.17 | 0.0001 |
| No Crt | Prenatal Factor | p-Value |
|---|---|---|
| 1 | Natural conception or in vitro fertilization | 0.37 |
| 2 | Number of pregnancies | 0.60 |
| 3 | General diseases during pregnancy | 0.22 |
| 4 | Medication during pregnancy | 0.14 |
| 5 | Variation of thyroid hormones | 0.95 |
| 6 | Variation of Vitamin D serum level | 0.85 |
| 7 | Variation of Calcium serum level | 0.38 |
| 8 | Infections during pregnancy | 0.28 |
| 9 | Fever during pregnancy | 0.26 |
| 10 | Gestational complications | 0.58 |
| 11 | Term or preterm birth | 0.94 |
| Labor Induction Medication | Prolonged Labor | |
|---|---|---|
| Cases (%) | 93.8% | 50% |
| Control (%) | 40% | 16% |
| p-value | 0.003 | 0.011 |
| Postnatal Factor | Percentage in Case Group | Percentage in Control Group | p-Value | Statistically Significance |
|---|---|---|---|---|
| Hypoxia at birth | 18.8% | 12.4% | 0.122 | No |
| General diseases in first months | 56.1% | 43.9% | 0.57 | No |
| Medication in first months after birth | 50% | 20% | 0.028 | Yes |
| Low levels of Vitamin D in serum | 9.4% | 4% | 0.62 | No |
| Low levels of Calcium in serum | 21.9% | 0% | 0.015 | Yes |
| Low levels of Magnesium in serum | 0% | 0% | - | - |
| Low levels of Iron in serum | 28% | 16% | 0.35 | No |
| Breastfeeding less than four months | 51.6% | 48.4% | 0.59 | No |
| Formula before six months | 60.9% | 39.1% | 0.59 | No |
| ENT/OMF infections in first year | 16.9% | 16% | 0.023 | Yes |
| Mother’s return to work before six months | 40.6% | 36% | 0.72 | No |
| B (Regression Coefficient) | SE (Standard Error) | Wald | p-Value | O.R. (Odd Ratio) | |
|---|---|---|---|---|---|
| Female sex | −1.179 | 0.572 | 4.249 | 0.039 | 0.308 |
| Prolonged labor | 1.658 | 0.650 | 6.506 | 0.011 | 5.250 |
| Medication to facilitate birth | 2.303 | 0.837 | 7.544 | 0.006 | 10.000 |
| Medication in first twelve months | 1.386 | 0.612 | 5.125 | 0.024 | 4.000 |
| ENT/OMF infections | 1.533 | 0.650 | 5.555 | 0.180 | 4.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contac, L.-R.; Pop, S.I.; Voidazan, S.; Bica, C.I. Molar Incisor Hypomineralization: Etiology, Correlation with Tooth Number Anomalies and Implications for Comprehensive Management Strategies in Children from Transylvania. Diagnostics 2024, 14, 2370. https://doi.org/10.3390/diagnostics14212370
Contac L-R, Pop SI, Voidazan S, Bica CI. Molar Incisor Hypomineralization: Etiology, Correlation with Tooth Number Anomalies and Implications for Comprehensive Management Strategies in Children from Transylvania. Diagnostics. 2024; 14(21):2370. https://doi.org/10.3390/diagnostics14212370
Chicago/Turabian StyleContac, Laura-Roxana, Silvia Izabella Pop, Septimiu Voidazan, and Cristina Ioana Bica. 2024. "Molar Incisor Hypomineralization: Etiology, Correlation with Tooth Number Anomalies and Implications for Comprehensive Management Strategies in Children from Transylvania" Diagnostics 14, no. 21: 2370. https://doi.org/10.3390/diagnostics14212370
APA StyleContac, L.-R., Pop, S. I., Voidazan, S., & Bica, C. I. (2024). Molar Incisor Hypomineralization: Etiology, Correlation with Tooth Number Anomalies and Implications for Comprehensive Management Strategies in Children from Transylvania. Diagnostics, 14(21), 2370. https://doi.org/10.3390/diagnostics14212370







