Diagnostic Accuracy of Deep Learning for the Prediction of Osteoporosis Using Plain X-rays: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Search Strategy
2.3. Selection Criteria
2.4. Quality Assessment and Risk of Bias
2.5. Data Extraction
2.6. Outcomes
2.7. Data Synthesis and Statistical Analysis
3. Results
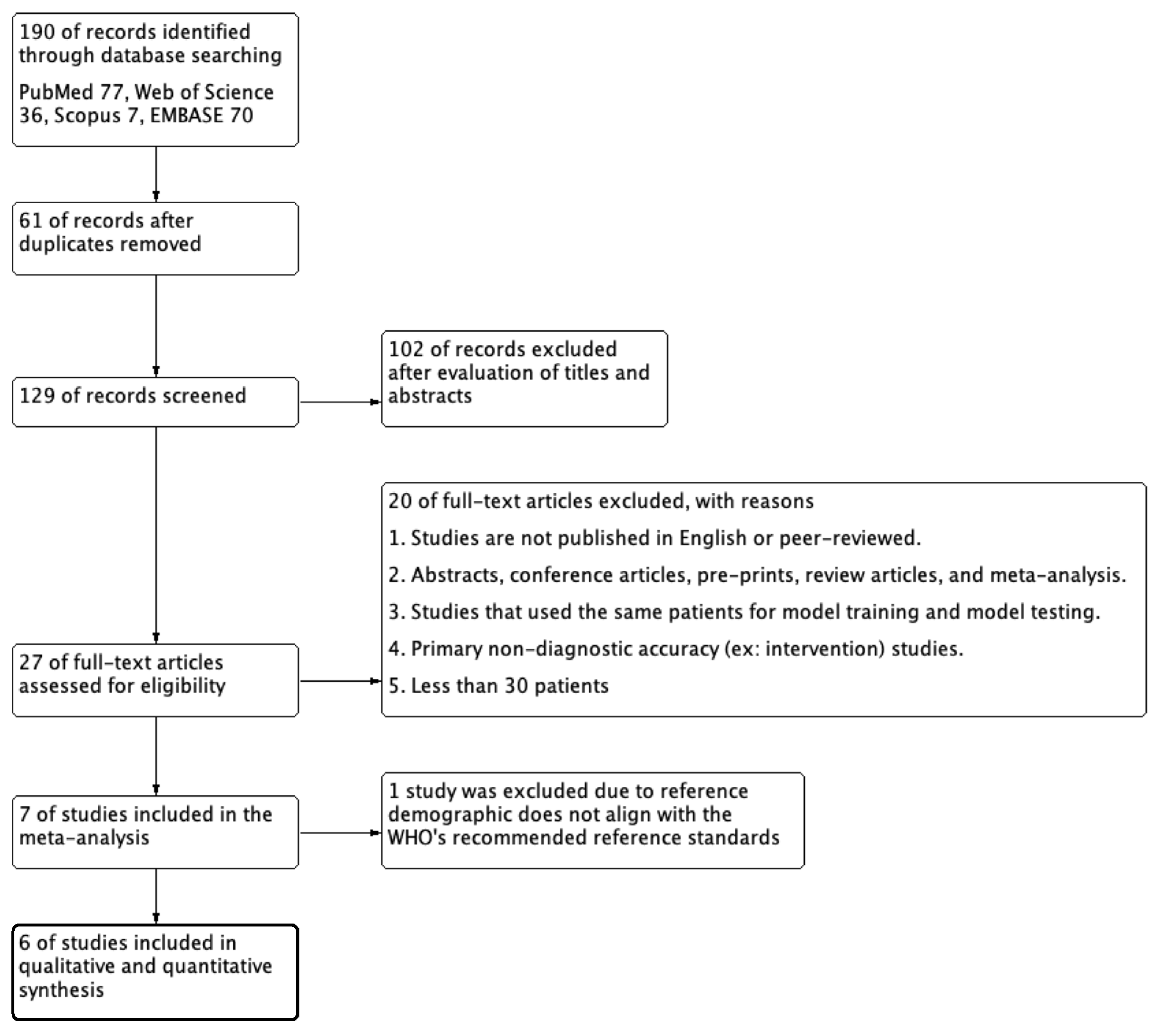
3.1. Literature Search
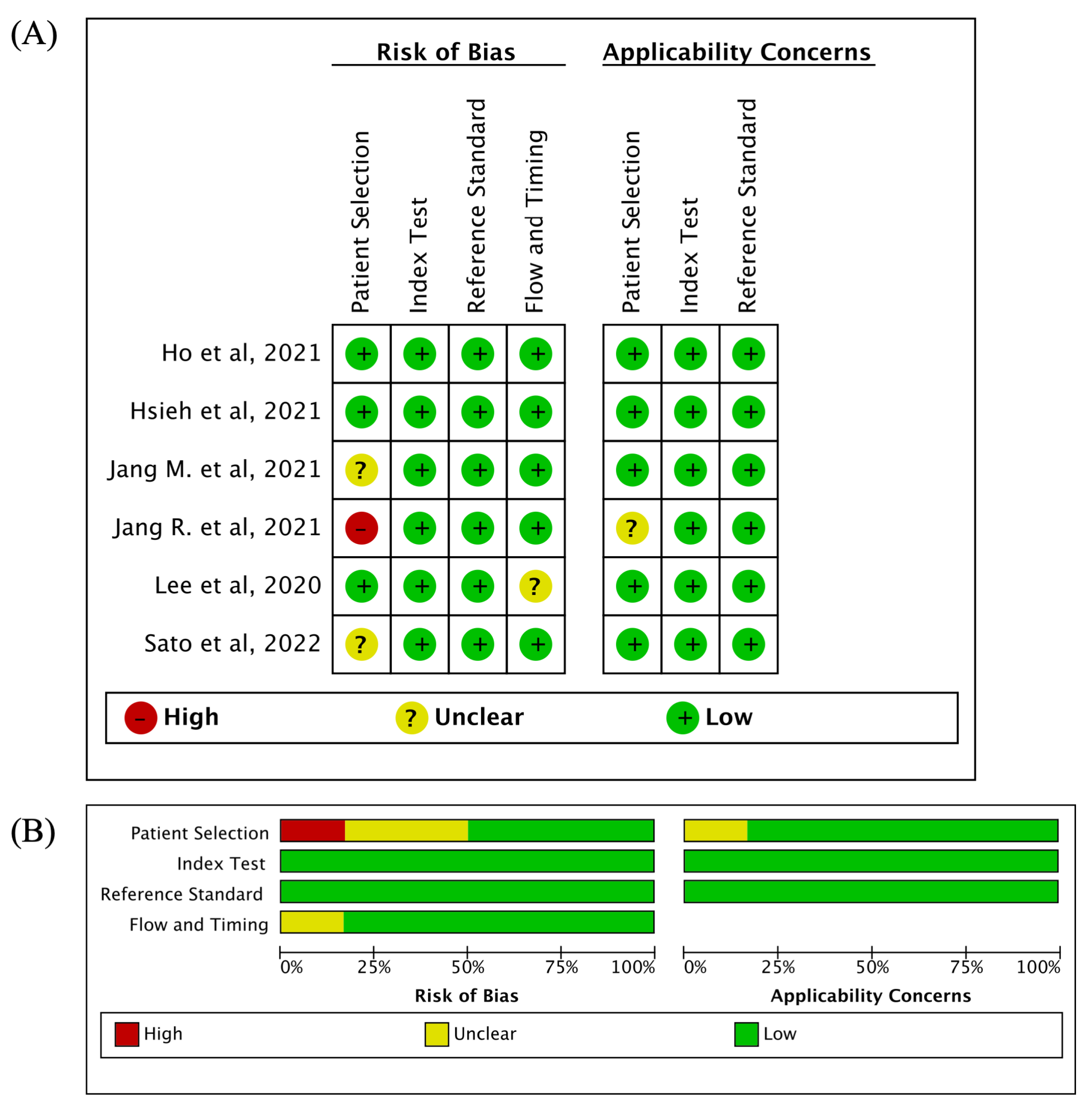
3.2. Quality Assessment
3.3. Characteristics of Included Studies
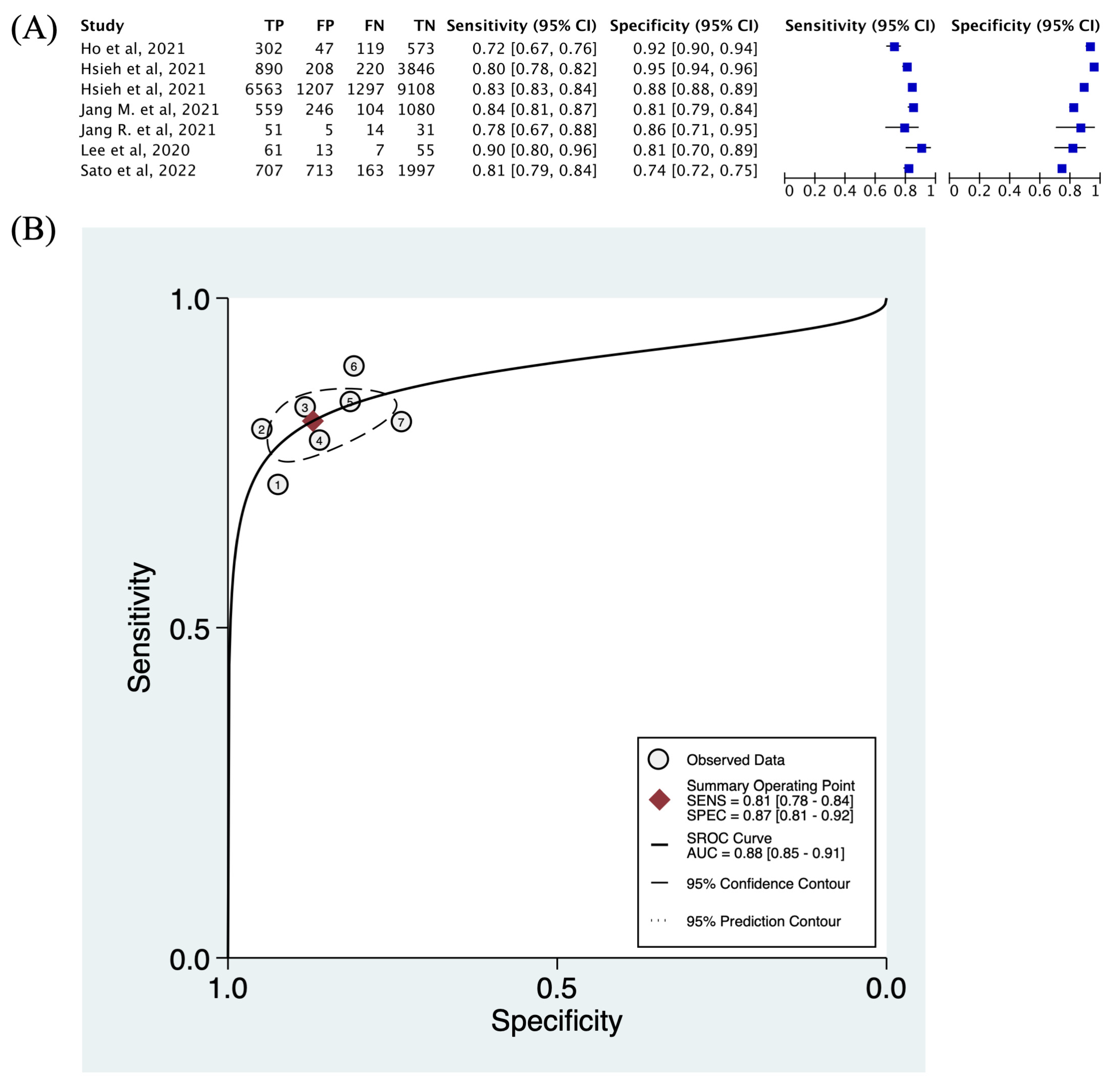
3.4. Descriptive Statistics and Diagnostic Accuracy
3.5. Threshold Effect and Heterogeneity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riggs, B.; Melton, L. The worldwide problem of osteoporosis: Insights afforded by epidemiology. Bone 1995, 17, S505–S511. [Google Scholar]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J., 3rd; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Mithal, A.; Bansal, B.; Kyer, C.S.; Ebeling, P. The Asia-pacific regional audit-epidemiology, costs, and burden of osteoporosis in India 2013: A report of international osteoporosis foundation. Indian J. Endocrinol. Metab. 2014, 18, 449. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Leader, D.; Weiss, R.; Williams, S.A. Challenges in osteoporosis awareness and management: Results from a survey of US postmenopausal women. J. Drug Assess. 2019, 8, 25–31. [Google Scholar] [CrossRef]
- Neuman, M.D.; Kennelly, A.M.; Tosi, L.L. Breakout session: Sex/gender and racial/ethnic disparities in the care of osteoporosis and fragility fractures. Clin. Orthop. Relat. Res. 2011, 469, 1936–1940. [Google Scholar]
- Merle, B.; Haesebaert, J.; Bedouet, A.; Barraud, L.; Flori, M.; Schott, A.-M.; Dupraz, C. Osteoporosis prevention: Where are the barriers to improvement in French general practitioners? A qualitative study. PLoS ONE 2019, 14, e0219681. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; Muñoz del Rio, A.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar]
- Alacreu, E.; Moratal, D.; Arana, E. Opportunistic screening for osteoporosis by routine CT in Southern Europe. Osteoporos. Int. 2017, 28, 983–990. [Google Scholar] [CrossRef]
- Kadri, A.; Binkley, N.; Hernando, D.; Anderson, P.A. Opportunistic Use of Lumbar Magnetic Resonance Imaging for Osteoporosis Screening. Osteoporos. Int. 2022, 33, 861–869. [Google Scholar]
- Kim, Y.W.; Kim, J.H.; Yoon, S.H.; Lee, J.H.; Lee, C.-H.; Shin, C.S.; Park, Y.S. Vertebral bone attenuation on low-dose chest CT: Quantitative volumetric analysis for bone fragility assessment. Osteoporos. Int. 2017, 28, 329–338. [Google Scholar] [CrossRef]
- Doi, K. Computer-aided diagnosis in medical imaging: Historical review, current status and future potential. Comput. Med. Imaging Graph. 2007, 31, 198–211. [Google Scholar]
- Avanzo, M.; Wei, L.; Stancanello, J.; Vallières, M.; Rao, A.; Morin, O.; Mattonen, S.A.; El Naqa, I. Machine and deep learning methods for radiomics. Med. Phys. 2020, 47, e185–e202. [Google Scholar] [CrossRef]
- Yasaka, K.; Akai, H.; Kunimatsu, A.; Kiryu, S.; Abe, O. Prediction of bone mineral density from computed tomography: Application of deep learning with a convolutional neural network. Eur. Radiol. 2020, 30, 3549–3557. [Google Scholar]
- Vara, G.; Spinnato, P.; Facchini, G.; Miceli, M.; Ursini, F.; Spinardi, L.; Vornetti, G.; Ratt, S. Assessment of Bone Mineral Density from Lumbosacral MRI: A Retrospective Study with Texture Analysis Radiomics. Appl. Sci. 2023, 13, 6305. [Google Scholar] [CrossRef]
- Lee, K.S.; Jung, S.K.; Ryu, J.J.; Shin, S.W.; Choi, J. Evaluation of Transfer Learning with Deep Convolutional Neural Networks for Screening Osteoporosis in Dental Panoramic Radiographs. J. Clin. Med. 2020, 9, 392. [Google Scholar] [CrossRef]
- Lee, J.-S.; Adhikari, S.; Liu, L.; Jeong, H.-G.; Kim, H.; Yoon, S.-J. Osteoporosis detection in panoramic radiographs using a deep convolutional neural network-based computer-assisted diagnosis system: A preliminary study. Dentomaxillofacial Radiol. 2019, 48, 20170344. [Google Scholar] [CrossRef]
- Jang, M.; Kim, M.; Bae, S.J.; Lee, S.H.; Koh, J.-M.; Kim, N. Opportunistic osteoporosis screening using chest radiographs with deep learning: Development and external validation with a cohort dataset. J. Bone Miner. Res. 2022, 37, 369–377. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, K.; Wang, Y.; Zhou, X.; Lu, L.; Xiao, J.; Wu, M.; Kuo, C.-F.; Miao, S. Opportunistic screening of osteoporosis using plain film chest X-ray. In International Workshop on Predictive Intelligence in Medicine; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Ho, C.-S.; Chen, Y.-P.; Fan, T.-Y.; Kuo, C.-F.; Yen, T.-Y.; Liu, Y.-C.; Pei, Y.-C. Application of deep learning neural network in predicting bone mineral density from plain X-ray radiography. Arch. Osteoporos. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-I.; Zheng, K.; Lin, C.; Mei, L.; Lu, L.; Li, W.; Chen, F.-P.; Wang, Y.; Zhou, X.; Wang, F.; et al. Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nat. Commun. 2021, 12, 5472. [Google Scholar] [PubMed]
- Yamamoto, N.; Sukegawa, S.; Kitamura, A.; Goto, R.; Noda, T.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H.; Kawasaki, K.; et al. Deep learning for osteoporosis classification using hip radiographs and patient clinical covariates. Biomolecules 2020, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Sukegawa, S.; Yamashita, K.; Manabe, M.; Nakano, K.; Takabatake, K.; Kawai, H.; Ozaki, T.; Kawasaki, K.; Nagatsuka, H.; et al. Effect of patient clinical variables in osteoporosis classification using hip x-rays in deep learning analysis. Medicina 2021, 57, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yu, K.; Ning, Z.; Wang, K.; Dong, Y.; Liu, X.; Liu, S.; Wang, J.; Zhu, C.; Yu, Q.; et al. Deep learning of lumbar spine X-ray for osteopenia and osteoporosis screening: A multicenter retrospective cohort study. Bone 2020, 140, 115561. [Google Scholar] [PubMed]
- Salameh, J.P.; McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar]
- Chu, H.; Cole, S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006, 59, 1331–1332. [Google Scholar]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar]
- Trikalinos, T.A.; Balion, C.M.; Coleman, C.I.; Griffith, L.; Santaguida, P.L.; Vandermeer, B.; Fu, R. Meta-analysis of test performance when there is a “gold standard”. J. Gen. Intern. Med. 2012, 27, 56–66. [Google Scholar] [CrossRef]
- Macaskill, P. Empirical Bayes estimates generated in a hierarchical summary ROC analysis agreed closely with those of a full Bayesian analysis. J. Clin. Epidemiol. 2004, 57, 925–932. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.S.; Scholten, R.J.P.M.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar]
- Macaskill, P.; Gatsonis, C.; Deeks, J.J.; Harbord, R.M.; Takwoingi, Y. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; Version 1.0; The Cochrane Collaboration: London, UK, 2010. [Google Scholar]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: A practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.-J.; Lee, J. Diagnostic test accuracy: Application and practice using R software. Epidemiol. Health 2019, 41, e2019007. [Google Scholar]
- Jang, R.; Choi, J.H.; Kim, N.; Chang, J.S.; Yoon, P.W.; Kim, C.H. Prediction of osteoporosis from simple hip radiography using deep learning algorithm. Sci. Rep. 2021, 11, 1997. [Google Scholar] [CrossRef]
- Sato, Y.; Yamamoto, N.; Inagaki, N.; Iesaki, Y.; Asamoto, T.; Suzuki, T.; Takahara, S. Deep learning for bone mineral density and T-score prediction from chest X-rays: A multicenter study. Biomedicines 2022, 10, 2323. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Gao, L.; Jiao, T.; Feng, Q.; Wang, W. Application of artificial intelligence in diagnosis of osteoporosis using medical images: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 1279–1286. [Google Scholar] [CrossRef]
- Maurício, J.; Domingues, I.; Bernardino, J. Comparing Vision Transformers and Convolutional Neural Networks for Image Classification: A Literature Review. Appl. Sci. 2023, 13, 5521. [Google Scholar]
- Kanis, J.; McCloskey, E.V.; Johansson, H.; Oden, A.; Ström, O.; Borgström, F. Development and use of FRAX® in osteoporosis. Osteoporos. Int. 2010, 21, 407–413. [Google Scholar]
- Gaget, V.; Inacio, M.C.; Tivey, D.R.; Jorissen, R.N.; Babidge, W.; Visvanathan, R.; Maddern, G.J. Trends in utilisation of plain X-rays by older Australians (2010–2019). BMC Geriatr. 2022, 22, 100. [Google Scholar]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar]

| Author | Publication Year | Study Design/Center | Country | Medical Images | Reference Diagnosis | Reference Standard | Deep Learning Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ho et al. [21] | 2021 | Retrospective/Single-center | Taiwan | Pelvic X-ray | DXA | Osteoporosis and non-osteoporosis (cutoff T-score = −2.5 SD) | ResNet18 | |||||
| Hsieh et al. [22] | 2021 | Retrospective/Single-center | Taiwan | Pelvic X-ray | DXA | Osteoporosis and non-osteoporosis (cutoff T-score = −2.5 SD) | VGG16 | |||||
| 2021 | Retrospective/Single-center | Taiwan | Lumbar X-ray | DXA | Osteoporosis and non-osteoporosis (cutoff T-score = −2.5 SD) | VGG16 | ||||||
| Jang, R. et al. [38] | 2021 | Retrospective/Single-center | Korea | Pelvic X-ray | DXA | Osteoporosis (T-score ≤ −2.5) and osteopenia (T-score between −1.0 and −2.5) | VGG16 | |||||
| Jang, M. et al. [19] | 2022 | Retrospective/Single-center | Korea | Chest X-ray | DXA | Normal (T-score ≥ −1.0), osteopenia (−2.5 < T-score < −1.0), and osteoporosis (T-score ≤ −2.5) | OsPor-screen model | |||||
| Lee et al. [17] | 2020 | Retrospective/Single-center | Korea | Dental X-ray | DXA | Osteoporosis and non-osteoporosis (cutoff T-score = −2.5 SD) | VGG16 | |||||
| Sato et al. [39] | 2022 | Retrospective/ Multi-center | Japan | Chest X-ray | DXA | Normal (T-score above −1.0), osteopenia (T-score between −1.0 and −2.5), and osteoporosis (T-score below −2.5) | ResNet50 | |||||
| Study ID | Image Number | Training/Validation/Test/External Dataset | TP | FP | FN | TN | Sensitivity | Specificity | AUROC | |||
| Ho et al. [21] | 5027 | 3972/1041/0/0 | 302 | 47 | 119 | 573 | 0.717 | 0.924 | NA | |||
| Hsieh et al. [22] | 10,797 (hip) | 5633/0/5164/2060 | 890 | 208 | 220 | 3846 | 0.802 | 0.949 | 0.97 | |||
| 25,482 (spine) | 7307/0/18175/3346 | 6563 | 1207 | 1297 | 9108 | 0.835 | 0.883 | 0.92 | ||||
| Jang, R. et al. [38] | 1001 | 800/100/101/117 | 51 | 5 | 14 | 31 | 0.785 | 0.861 | 0.7 | |||
| Jang, M. et al. [19] | 14,115 | 9825/1212/1989/1089 | 559 | 246 | 104 | 1080 | 0.843 | 0.815 | 0.91 | |||
| Lee et al. [17] | 680 | 544 (split to 4:1 for training and validation)/136/0 | 61 | 13 | 7 | 55 | 0.897 | 0.809 | 0.858 | |||
| Sato et al. [39] | 17,899 | 12529/1790/3580/0 | 707 | 713 | 163 | 1997 | 0.813 | 0.737 | 0.84 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, T.-Y.; Ho, C.-S.; Chen, Y.-P.; Pei, Y.-C. Diagnostic Accuracy of Deep Learning for the Prediction of Osteoporosis Using Plain X-rays: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 207. https://doi.org/10.3390/diagnostics14020207
Yen T-Y, Ho C-S, Chen Y-P, Pei Y-C. Diagnostic Accuracy of Deep Learning for the Prediction of Osteoporosis Using Plain X-rays: A Systematic Review and Meta-Analysis. Diagnostics. 2024; 14(2):207. https://doi.org/10.3390/diagnostics14020207
Chicago/Turabian StyleYen, Tzu-Yun, Chan-Shien Ho, Yueh-Peng Chen, and Yu-Cheng Pei. 2024. "Diagnostic Accuracy of Deep Learning for the Prediction of Osteoporosis Using Plain X-rays: A Systematic Review and Meta-Analysis" Diagnostics 14, no. 2: 207. https://doi.org/10.3390/diagnostics14020207
APA StyleYen, T.-Y., Ho, C.-S., Chen, Y.-P., & Pei, Y.-C. (2024). Diagnostic Accuracy of Deep Learning for the Prediction of Osteoporosis Using Plain X-rays: A Systematic Review and Meta-Analysis. Diagnostics, 14(2), 207. https://doi.org/10.3390/diagnostics14020207





