Differentiation between Parkinson’s Disease and the Parkinsonian Subtype of Multiple System Atrophy Using the Magnetic Resonance T1w/T2w Ratio in the Middle Cerebellar Peduncle
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. MRI Preprocessing
2.4. T1w/T2w and Standardized T1w/T2w Ratios
2.5. Statistical Analyses
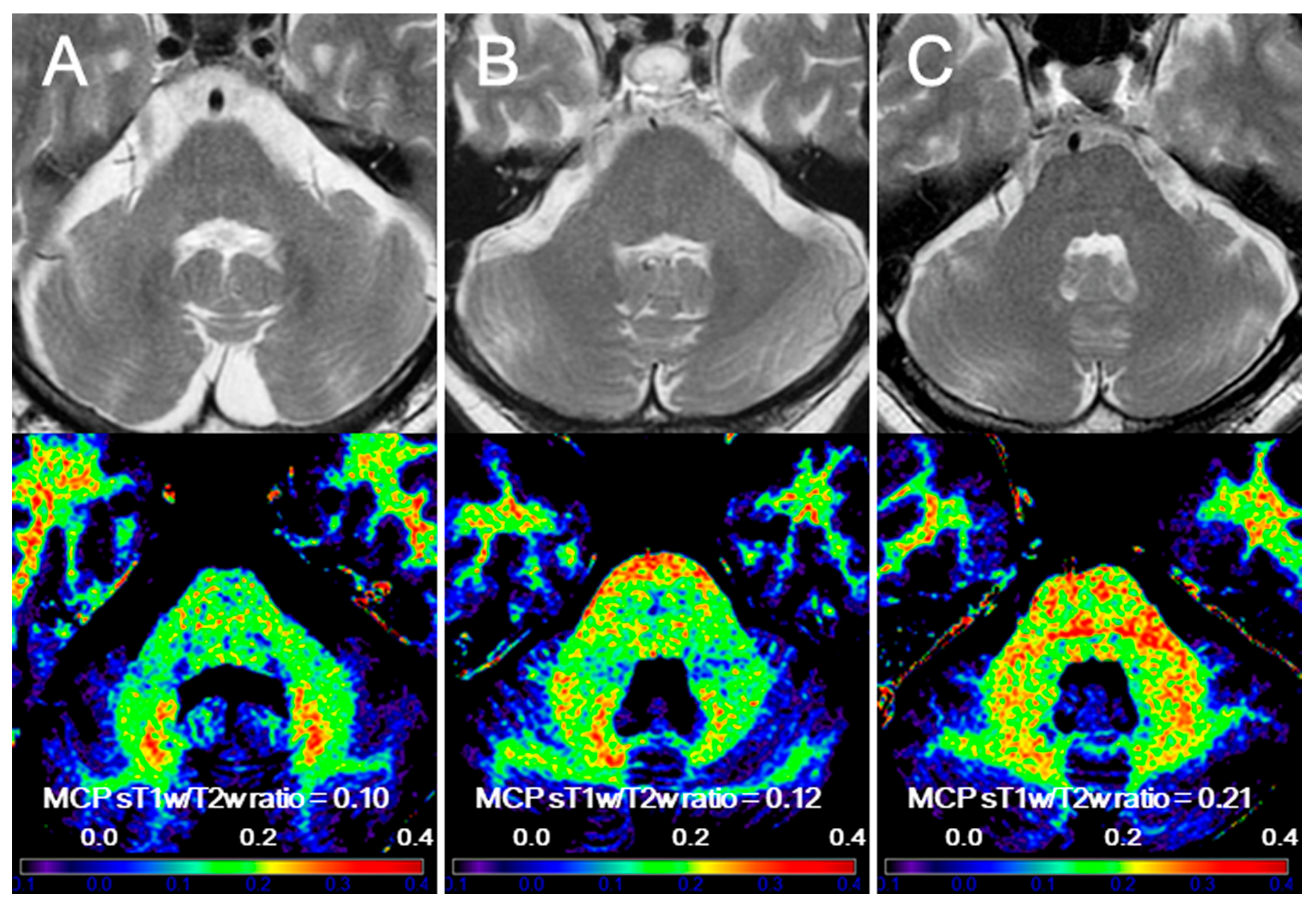
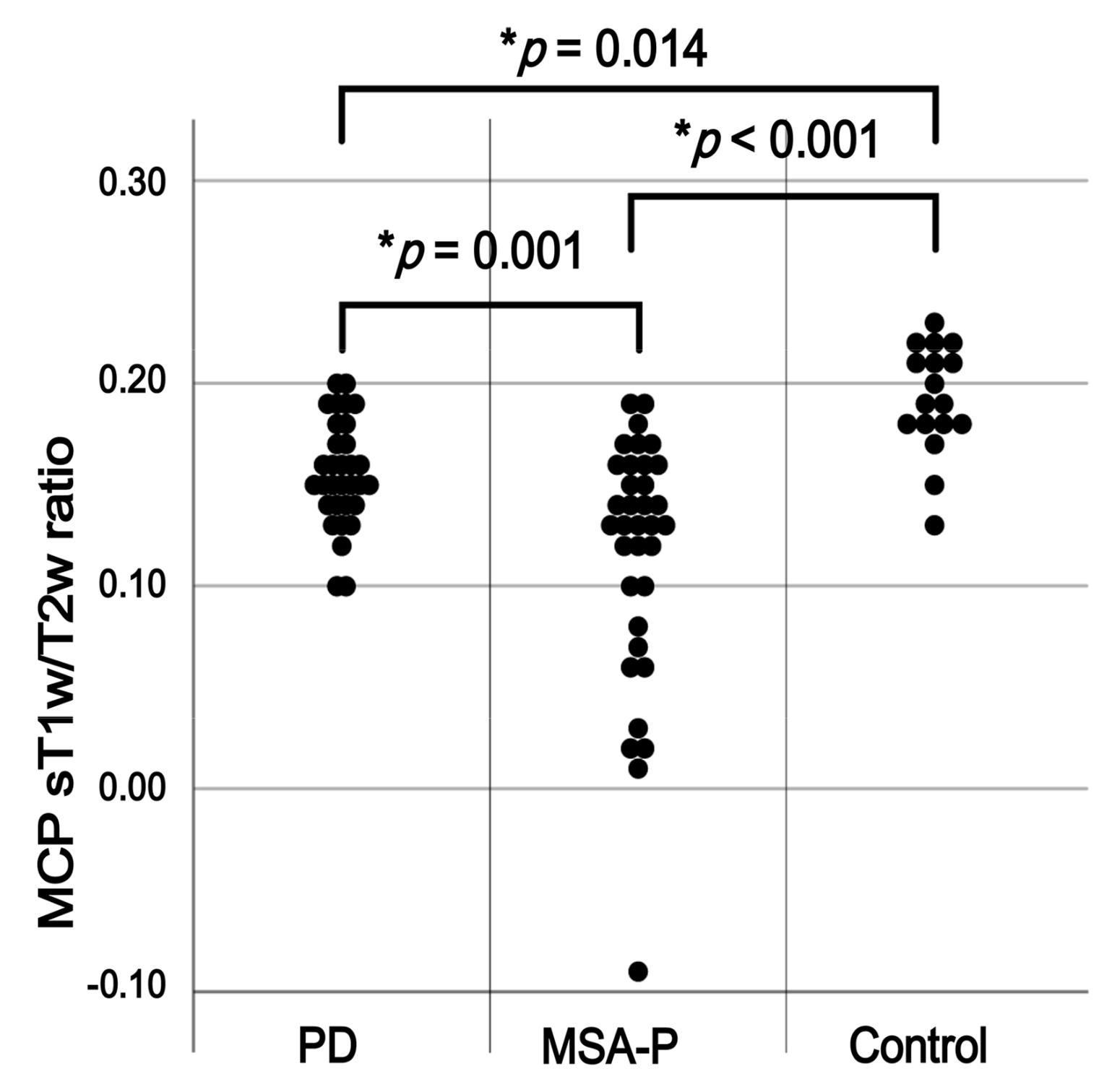
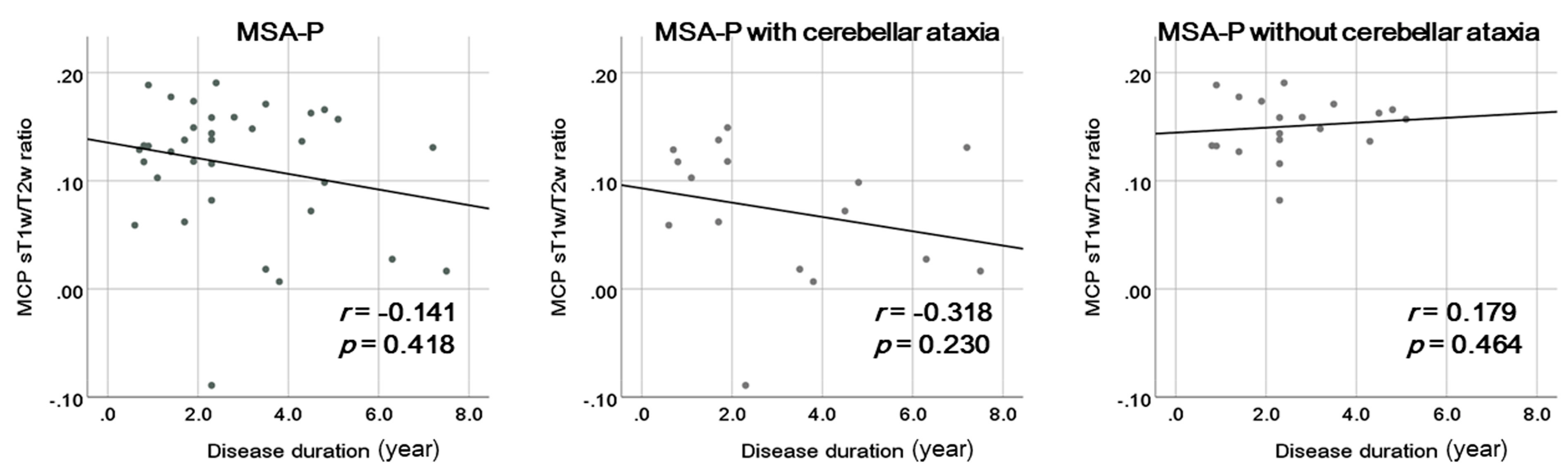
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fanciulli, A.; Wenning, G.K. Multiple-system atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O.A.; Dickson, D.W. Neuropathology and molecular diagnosis of synucleinopathies. Mol. Neurodegener. 2021, 16, 83. [Google Scholar] [CrossRef]
- Watanabe, H.; Saito, Y.; Terao, S.; Ando, T.; Kachi, T.; Mukai, E.; Aiba, I.; Abe, Y.; Tamakoshi, A.; Doyu, M.; et al. Progression and prognosis in multiple system atrophy: An analysis of 230 Japanese patients. Brain 2002, 125, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Geser, F.; Krismer, F.; Seppi, K.; Duerr, S.; Boesch, S.; Köllensperger, M.; Goebel, G.; Pfeiffer, K.P.; Barone, P.; et al. The natural history of multiple system atrophy: A prospective European cohort study. Lancet Neurol. 2013, 12, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Reich, S.G.; Jankovic, J.; Shults, C.W.; Stern, M.B.; Novak, P.; Tanner, C.M.; Gilman, S.; Marshall, F.J.; Wooten, F.; et al. Natural history of multiple system atrophy in the USA: A prospective cohort study. Lancet Neurol. 2015, 14, 710–719. [Google Scholar] [CrossRef]
- Shimohata, T.; Aizawa, N.; Nakayama, H.; Taniguchi, H.; Ohshima, Y.; Okumura, H.; Takahashi, T.; Yokoseki, A.; Inoue, M.; Nishizawa, M. Mechanisms and prevention of sudden death in multiple system atrophy. Parkinsonism Relat. Disord. 2016, 30, 1–6. [Google Scholar] [CrossRef]
- Osaki, Y.; Ben-Shlomo, Y.; Lees, A.J.; Wenning, G.K.; Quinn, N.P. A validation exercise on the new consensus criteria for multiple system atrophy. Mov. Disord. 2009, 24, 2272–2276. [Google Scholar] [CrossRef]
- Koga, S.; Aoki, N.; Uitti, R.J.; van Gerpen, J.A.; Cheshire, W.P.; Josephs, K.A.; Wszolek, Z.K.; Langston, J.W.; Dickson, D.W. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology 2015, 85, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Foti, S.C.; Asi, Y.T.; Tsushima, E.; Quinn, N.; Ling, H.; Holton, J.L. Improving diagnostic accuracy of multiple system atrophy: A clinicopathological study. Brain 2019, 142, 2813–2827. [Google Scholar] [CrossRef]
- Wenning, G.K.; Tison, F.; Elliott, L.; Quinn, N.P.; Daniel, S.E. Olivopontocerebellar pathology in multiple system atrophy. Mov. Disord. 1996, 11, 157–162. [Google Scholar] [CrossRef]
- Ozawa, T.; Paviour, D.; Quinn, N.P.; Josephs, K.A.; Sangha, H.; Kilford, L.; Healy, D.G.; Wood, N.W.; Lees, A.J.; Holton, J.L.; et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: Clinicopathological correlations. Brain 2004, 127, 2657–2671. [Google Scholar] [CrossRef]
- Brettschneider, J.; Irwin, D.J.; Boluda, S.; Byrne, M.D.; Fang, L.; Lee, E.B.; Robinson, J.L.; Suh, E.; Van Deerlin, V.M.; Toledo, J.B.; et al. Progression of alpha-synuclein pathology in multiple system atrophy of the cerebellar type. Neuropathol. Appl. Neurobiol. 2017, 43, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Fera, F.; Condino, F.; Auteri, W.; Gallo, O.; Pugliese, P.; Arabia, G.; Morgante, L.; Barone, P.; Zappia, M.; et al. MR imaging of middle cerebellar peduncle width: Differentiation of multiple system atrophy from Parkinson disease. Radiology 2006, 239, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.L.; Távora, D.F.; Bomfim, R.C.; Silva, C.E.; Bruin, V.M.; Bruin, P.F. Morphometry MRI in the differential diagnosis of parkinsonian syndromes. Arq. Neuropsiquiatr. 2010, 68, 333–338. [Google Scholar] [CrossRef]
- Nicoletti, G.; Lodi, R.; Condino, F.; Tonon, C.; Fera, F.; Malucelli, E.; Manners, D.; Zappia, M.; Morgante, L.; Barone, P.; et al. Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson’s disease and progressive supranuclear palsy. Brain 2006, 129, 2679–2687. [Google Scholar] [CrossRef]
- Sako, W.; Abe, T.; Murakami, N.; Miyazaki, Y.; Izumi, Y.; Harada, M.; Kaji, R. Imaging-based differential diagnosis between multiple system atrophy and Parkinson’s disease. J. Neurol. Sci. 2016, 368, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Fan, G.; Sun, W.; Shang, X.; Shi, S.; Wang, S.; Lv, G.; Wu, C. Usefulness of diffusion-tensor MRI in the diagnosis of Parkinson variant of multiple system atrophy and Parkinson’s disease: A valuable tool to differentiate between them? Clin. Radiol. 2017, 72, e9–e610. [Google Scholar] [CrossRef]
- Krismer, F.; Seppi, K.; Göbel, G.; Steiger, R.; Zucal, I.; Boesch, S.; Gizewski, E.R.; Wenning, G.K.; Poewe, W.; Scherfler, C. Morphometric MRI profiles of multiple system atrophy variants and implications for differential diagnosis. Mov. Disord. 2019, 34, 1041–1048. [Google Scholar] [CrossRef]
- Sako, W.; Abe, T.; Haji, S.; Murakami, N.; Osaki, Y.; Izumi, Y.; Harada, M.; Kaji, R. ‘One line’: A method for differential diagnosis of parkinsonian syndromes. Acta Neurol. Scand. 2019, 140, 229–235. [Google Scholar] [CrossRef]
- Beliveau, V.; Krismer, F.; Skalla, E.; Schocke, M.M.; Gizewski, E.R.; Wenning, G.K.; Poewe, W.; Seppi, K.; Scherfler, C. Characterization and diagnostic potential of diffusion tractography in multiple system atrophy. Parkinsonism Relat. Disord. 2021, 85, 30–36. [Google Scholar] [CrossRef]
- Arshad, M.; Stanley, J.A.; Raz, N. Test-retest reliability and concurrent validity of in vivo myelin content indices: Myelin water fraction and calibrated T1 w/T2 w image ratio. Hum. Brain Mapp. 2017, 38, 1780–1790. [Google Scholar] [CrossRef]
- Du, G.; Lewis, M.M.; Sica, C.; Kong, L.; Huang, X. Magnetic resonance T1w/T2w ratio: A parsimonious marker for Parkinson disease. Ann. Neurol. 2019, 85, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ponticorvo, S.; Manara, R.; Russillo, M.C.; Erro, R.; Picillo, M.; Di Salle, G.; Di Salle, F.; Barone, P.; Esposito, F.; Pellecchia, M.T. Magnetic resonance T1w/T2w ratio and voxel-based morphometry in multiple system atrophy. Sci. Rep. 2021, 11, 21683. [Google Scholar] [CrossRef] [PubMed]
- Misaki, M.; Savitz, J.; Zotev, V.; Phillips, R.; Yuan, H.; Young, K.D.; Drevets, W.C.; Bodurka, J. Contrast enhancement by combining T1- and T2-weighted structural brain MR images. Magn. Reson. Med. 2015, 74, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Yokota, H.; Hirano, S.; Cooper, G.; Mukai, H.; Koide, K.; Wang, J.; Ito, S.; Finke, C.; Brandt, A.U.; et al. Magnetic resonance T1w/T2w ratio in the middle cerebellar peduncle might be a sensitive biomarker for multiple system atrophy. Eur. Radiol. 2021, 31, 4277–4284. [Google Scholar] [CrossRef]
- Wang, J.; Sugiyama, A.; Yokota, H.; Hirano, S.; Cooper, G.; Mukai, H.; Ohira, K.; Koide, K.; Ito, S.; Finke, C.; et al. Diagnostic efficacy of the magnetic resonance T1w/T2w ratio for the middle cerebellar peduncle in multiple system atrophy and spinocerebellar ataxia: A preliminary study. PLoS ONE 2022, 17, e0267024. [Google Scholar] [CrossRef]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L.; Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011, 54, 313–327. [Google Scholar] [CrossRef]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Van Baarsen, K.M.; Kleinnijenhuis, M.; Jbabdi, S.; Sotiropoulos, S.N.; Grotenhuis, J.A.; van Cappellen van Walsum, A.M. A probabilistic atlas of the cerebellar white matter. Neuroimage 2016, 124, 724–732. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Itoh, K.; Kasai, T.; Tsuji, Y.; Saito, K.; Mizuta, I.; Harada, Y.; Sudoh, S.; Mizuno, T.; Nakagawa, M.; Fushiki, S. Definite familial multiple system atrophy with unknown genetics. Neuropathology 2014, 34, 309–313. [Google Scholar] [CrossRef]
- Tison, F.; Yekhlef, F.; Balestre, E.; Chrysostome, V.; Quinn, N.; Wenning, G.K.; Poewe, W. Application of the international cooperative ataxia scale rating in multiple system atrophy. Mov. Disord. 2002, 17, 1248–1254. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. The cerebellum in Parkinson’s disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef]
- Sako, W.; Murakami, N.; Miyazaki, Y.; Abe, T.; Harada, M.; Izumi, Y.; Kaji, R. The effect of tremor onset on middle cerebellar peduncle of Parkinson’s disease. J. Neurol. Sci. 2015, 358, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, S.; Leunissen, I.; Vervoort, G.; Vandenberghe, W.; Swinnen, S.; Nieuwboer, A. Microstructural changes in white matter associated with freezing of gait in Parkinson’s disease. Mov. Disord. 2015, 30, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Sherbaf, F.; Rahmani, F.; Jooyandeh, S.M.; Aarabi, M.H. Microstructural changes in patients with Parkinson disease and REM sleep behavior disorder: Depressive symptoms versus non-depressed. Acta Neurol. Belg. 2018, 118, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bharti, K.; Suppa, A.; Pietracupa, S.; Upadhyay, N.; Giannì, C.; Leodori, G.; Di Biasio, F.; Modugno, N.; Petsas, N.; Grillea, G.; et al. Abnormal cerebellar connectivity patterns in patients with Parkinson’s disease and freezing of gait. Cerebellum 2019, 18, 298–308. [Google Scholar] [CrossRef]
- Hett, K.; Lyu, I.; Trujillo, P.; Lopez, A.M.; Aumann, M.; Larson, K.E.; Hedera, P.; Dawant, B.; Landman, B.A.; Claassen, D.O.; et al. Anatomical texture patterns identify cerebellar distinctions between essential tremor and Parkinson’s disease. Hum. Brain Mapp. 2021, 42, 2322–2331. [Google Scholar] [CrossRef] [PubMed]


| Group (n) | p-Value for Group Comparisons | p-Value for Post Hoc Group Comparisons | |||||
|---|---|---|---|---|---|---|---|
| PD (n = 32) | MSA-P (n = 35) | Control (n = 17) | PD vs. MSA-P | PD vs. Control | MSA-P vs. Control | ||
| Sex distribution (male/female) 1 | 12/20 | 13/22 | 9/8 | 0.503 | 0.976 | 0.299 | 0.279 |
| Age at MRI (years, median, range) 2 | 68.5 (49–88) | 68.0 (46–79) | 63.0 (44–80) | 0.321 | 0.806 | 0.115 | 0.173 |
| Disease duration (years, median, range) 3 | 5.9 (0.3–23.8) | 2.3 (0.7–7.5) | NA | 0.001 | NA | NA | NA |
| Age at onset (years, median, range) 3 | 61.0 (43–81) | 65.0 (43–76) | NA | 0.081 | NA | NA | NA |
| Hoehn and Yahr stage (median, range) 4 | 3 (1–4) | 3 (2–4) | NA | 0.161 | NA | NA | NA |
| LEDD (mg, median, range) 3 | 300.0 (0–2038.9) | 0 (0–1472.0) | NA | 0.007 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sugiyama, A.; Yokota, H.; Hirano, S.; Yamamoto, T.; Yamanaka, Y.; Araki, N.; Ito, S.; Paul, F.; Kuwabara, S. Differentiation between Parkinson’s Disease and the Parkinsonian Subtype of Multiple System Atrophy Using the Magnetic Resonance T1w/T2w Ratio in the Middle Cerebellar Peduncle. Diagnostics 2024, 14, 201. https://doi.org/10.3390/diagnostics14020201
Wang J, Sugiyama A, Yokota H, Hirano S, Yamamoto T, Yamanaka Y, Araki N, Ito S, Paul F, Kuwabara S. Differentiation between Parkinson’s Disease and the Parkinsonian Subtype of Multiple System Atrophy Using the Magnetic Resonance T1w/T2w Ratio in the Middle Cerebellar Peduncle. Diagnostics. 2024; 14(2):201. https://doi.org/10.3390/diagnostics14020201
Chicago/Turabian StyleWang, Jiaqi, Atsuhiko Sugiyama, Hajime Yokota, Shigeki Hirano, Tatsuya Yamamoto, Yoshitaka Yamanaka, Nobuyuki Araki, Shoichi Ito, Friedemann Paul, and Satoshi Kuwabara. 2024. "Differentiation between Parkinson’s Disease and the Parkinsonian Subtype of Multiple System Atrophy Using the Magnetic Resonance T1w/T2w Ratio in the Middle Cerebellar Peduncle" Diagnostics 14, no. 2: 201. https://doi.org/10.3390/diagnostics14020201
APA StyleWang, J., Sugiyama, A., Yokota, H., Hirano, S., Yamamoto, T., Yamanaka, Y., Araki, N., Ito, S., Paul, F., & Kuwabara, S. (2024). Differentiation between Parkinson’s Disease and the Parkinsonian Subtype of Multiple System Atrophy Using the Magnetic Resonance T1w/T2w Ratio in the Middle Cerebellar Peduncle. Diagnostics, 14(2), 201. https://doi.org/10.3390/diagnostics14020201







