IP-10 for the Diagnosis and Treatment Monitoring of Tuberculosis in Children
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical and Laboratory Procedures
2.2.1. TST
2.2.2. QuantiFERON-TB GOLD In-Tube
2.2.3. IP-10 Determination
2.3. Classification of Participants
- ATB diagnosed in symptomatic patients with microbiologically confirmed TB (mATB) (positive microscopy, PCR, or culture result) or in patients fulfilling ≥ two of the following three criteria: (1) clinical symptoms suggestive of ATB, (2) radiological findings consistent with ATB, and (3) a history of contact with PTB, all in conjunction with a positive clinical response to anti-tuberculosis treatment.
- TBI is defined as a positive TST and/or IGRA result in asymptomatic children with a history of contact with PTB and a normal chest X-ray.
- TB contacts—asymptomatic children with a history of contact with PTB, not fulfilling the criteria for ATB or TBI.
- Non-TB—children with clinical suspicion of TB, but with other final diagnoses and a positive response to specific treatment.
- Controls—asymptomatic children after careful exclusion of TB contact and inflammatory comorbidities and with a negative IGRA result.
2.4. Statistical Analysis
3. Results
3.1. Study Participant Characteristics
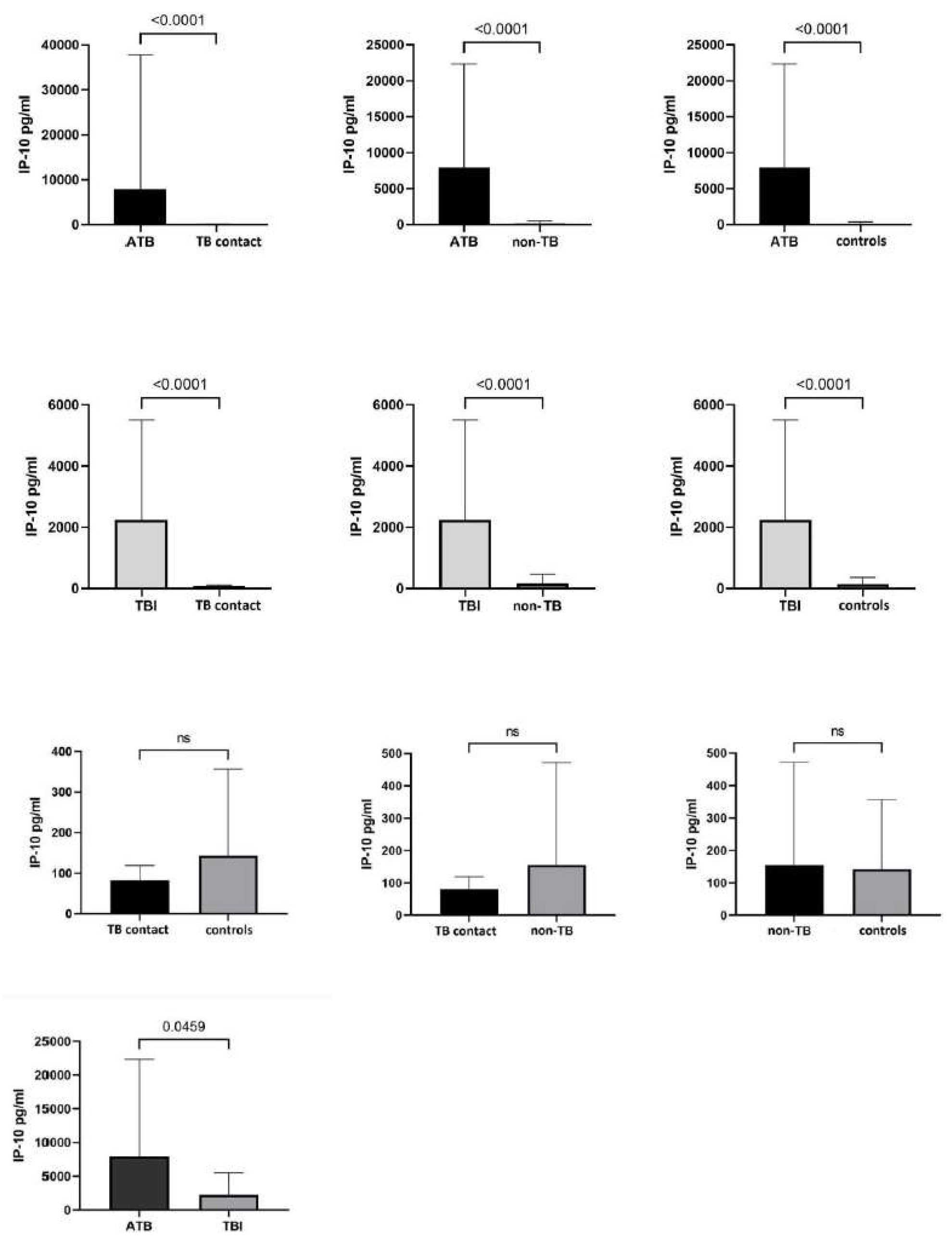
3.2. IP-10 Responses in Mtb.-Infected and Uninfected Children
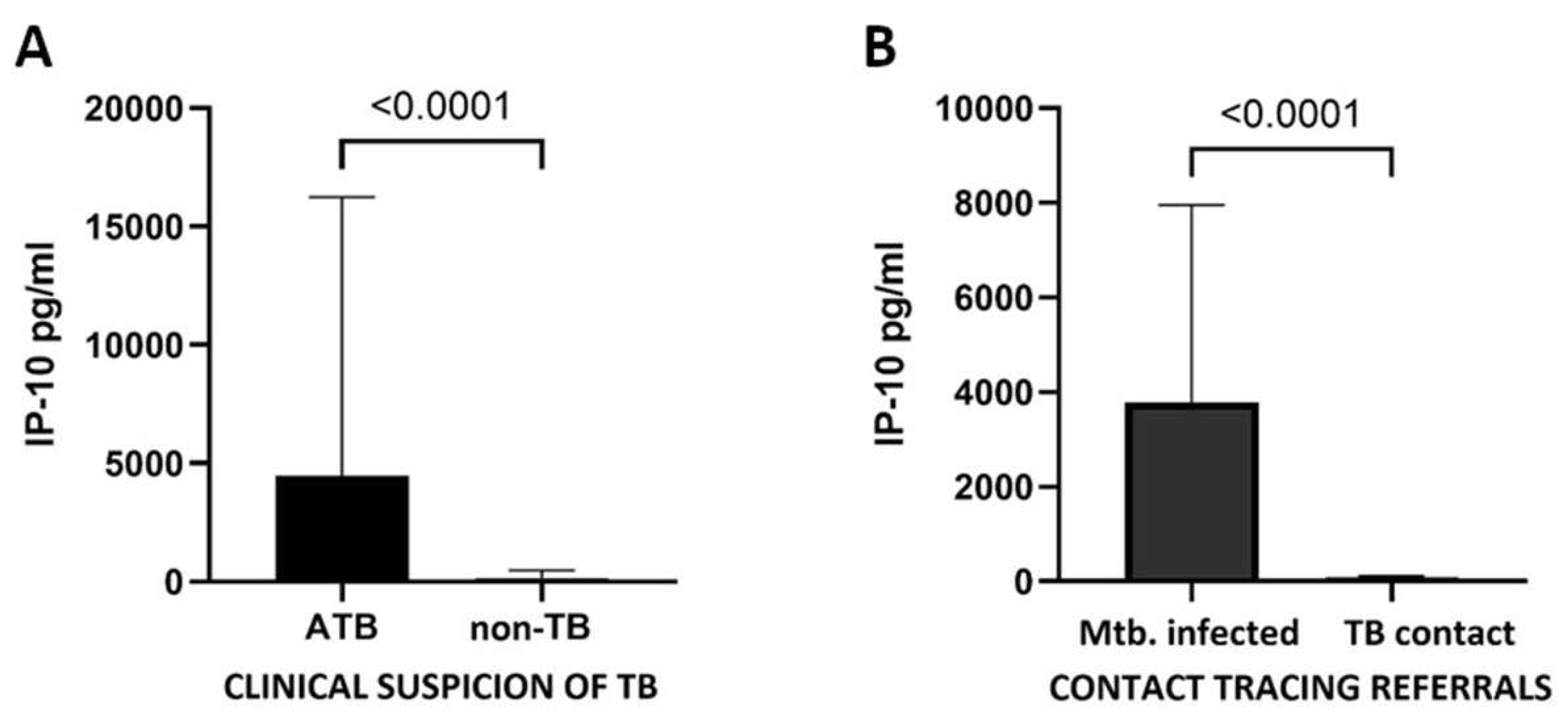
3.3. IP-10 Identifies Mtb. Infection Regardless of the Reason for Admission
3.4. IP-10 Responses According to the TST, QFT, and Microbiological Test Results
3.5. IP-10 and IFN-γ Level Are Not Correlated with Age
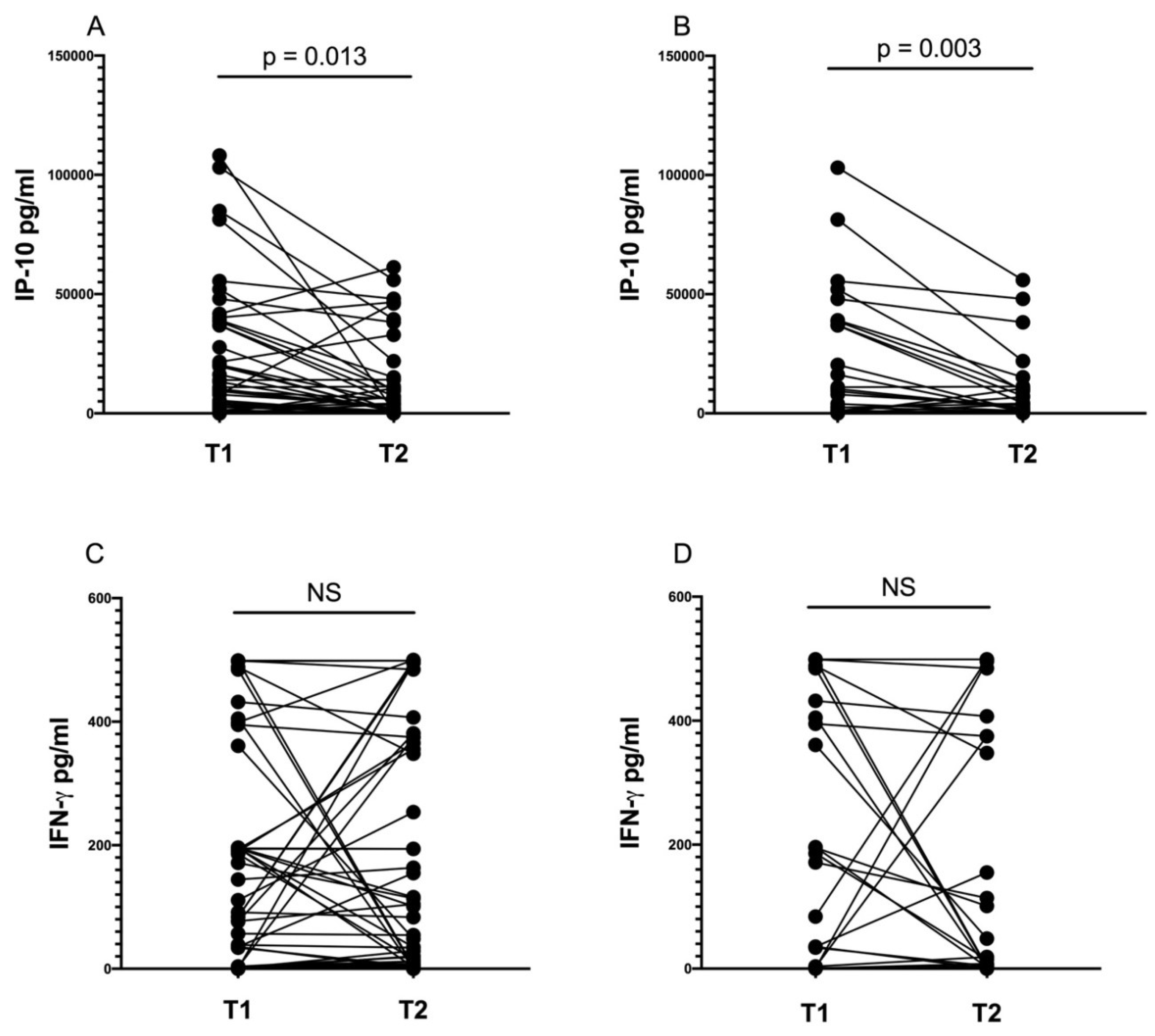
3.6. IP-10 Level Decreases during Anti-tuberculosis Treatment
3.7. Diagnostic Performance of IP-10
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATB | Active tuberculosis |
| IFN-γ | Interferon gamma |
| IGRAs | Interferon-gamma release assays |
| TBI | Tuberculous infection |
| Mtb. | Mycobacterium tuberculosis |
| PTB | Pulmonary tuberculosis |
| TB | Tuberculosis |
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Nicol, M.P.; Workman, L.; Prins, M.; Bateman, L.; Ghebrekristos, Y.; Mbhele, S.; Denkinger, C.M.; Zar, H.J. Accuracy of Xpert Mtb/Rif Ultra for the Diagnosis of Pulmonary Tuberculosis in Children. Pediatr. Infect. Dis. J. 2018, 37, e261–e263. [Google Scholar] [CrossRef] [PubMed]
- Marais, B.J.; Hesseling, A.C.; Gie, R.P.; Schaaf, H.S.; Enarson, D.A.; Beyers, N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin. Infect. Dis. 2006, 42, e69–e71. [Google Scholar] [CrossRef]
- Farhat, M.; Greenaway, C.; Pai, M.; Menzies, D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 2006, 10, 1192–1204. [Google Scholar] [PubMed]
- Mancuso, J.D.; Mody, R.M.; Olsen, C.H.; Harrison, L.H.; Santosham, M.; Aronson, N.E. The Long-term Effect of Bacille Calmette-Guerin Vaccination on Tuberculin Skin Testing: A 55-Year Follow-Up Study. Chest 2017, 152, 282–294. [Google Scholar] [CrossRef]
- Mandalakas, A.M.; Detjen, A.K.; Hesseling, A.C.; Benedetti, A.; Menzies, D. Interferon-gamma release assays and childhood tuberculosis: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2011, 15, 1018–1032. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Storelli, F.; Tersigni, C.; Venturini, E.; de Martino, M.; Galli, L. QuantiFERON-TB Gold In-Tube test performance in a large pediatric population investigated for suspected tuberculosis infection. Paediatr. Respir. Rev. 2019, 32, 36–47. [Google Scholar] [CrossRef]
- Overton, K.; Varma, R.; Post, J.J. Comparison of Interferon-gamma Release Assays and the Tuberculin Skin Test for Diagnosis of Tuberculosis in Human Immunodeficiency Virus: A Systematic Review. Tuberc. Respir. Dis. 2018, 81, 59–72. [Google Scholar] [CrossRef]
- Bielecka, T.; Komorowska-Piotrowska, A.; Krenke, K.; Feleszko, W.; Kulus, M. Is secretion of IFN-gamma in response to Mycobacterium tuberculosis antigens in youngest children sufficient to play a role in TB diagnostics? Pediatr. Pulmonol. 2018, 53, 181–188. [Google Scholar] [CrossRef]
- Buonsenso, D.; Noguera-Julian, A.; Moroni, R.; Hernández-Bartolomé, A.; Fritschi, N.; Lancella, L.; Cursi, L.; Soler-Garcia, A.; Krüger, R.; Feiterna-Sperling, C.; et al. Performance of QuantiFERON-TB Gold Plus assays in paediatric tuberculosis: A multicentre PTBNET study. Thorax 2023, 78, 288–296. [Google Scholar] [CrossRef]
- Chegou, N.N.; Heyckendorf, J.; Walzl, G.; Lange, C.; Ruhwald, M. Beyond the IFN-gamma horizon: Biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur. Respir. J. 2014, 43, 1472–1486. [Google Scholar] [CrossRef]
- Daniel, E.A.; Thiruvengadam, K.; Rajamanickam, A.; Chandrasekaran, P.; Pattabiraman, S.; Bhanu, B.; Sivaprakasam, A.; Paradkar, M.; Kulkarni, V.; Karyakarte, R.; et al. QuantiFERON Supernatant-Based Host Biomarkers Predicting Progression to Active Tuberculosis Disease Among Household Contacts of Tuberculosis Patients. Clin. Infect. Dis. 2023, 76, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Ruhwald, M.; Aabye, M.G.; Ravn, P. IP-10 release assays in the diagnosis of tuberculosis infection: Current status and future directions. Expert Rev. Mol. Diagn. 2012, 12, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Suárez, I.; Rohr, S.; Stecher, M.; Lehmann, C.; Winter, S.; Jung, N.; Priesner, V.; Berger, M.; Wyen, C.; Augustin, M.; et al. Plasma interferon-γ-inducible protein 10 (IP-10) levels correlate with disease severity and paradoxical reactions in extrapulmonary tuberculosis. Infection 2021, 49, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, Y.; Wang, Q.; Wen, Z.; Song, R.; Li, Y.; Zhou, Y.; Ma, R.; Jia, H.; Bai, S.; et al. Evaluation of the IP-10 mRNA release assay for diagnosis of TB in HIV-infected individuals. Front. Cell. Infect. Microbiol. 2023, 13, 1152665. [Google Scholar] [CrossRef] [PubMed]
- Ruhwald, M.; Petersen, J.; Kofoed, K.; Nakaoka, H.; Cuevas, L.E.; Lawson, L.; Squire, S.B.; Eugen-Olsen, J.; Ravn, P. Improving T-cell assays for the diagnosis of latent TB infection: Potential of a diagnostic test based on IP-10. PLoS ONE 2008, 3, e2858. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.; Abu Amer, N.; Gurgel, R.Q.; Sherchand, J.B.; Doria, L.; Lama, C.; Ravn, P.; Ruhwald, M.; Yassin, M.; Harper, G.; et al. Interferon gamma, interferon-gamma-induced-protein 10, and tuberculin responses of children at high risk of tuberculosis infection. Pediatr. Infect. Dis. J. 2008, 27, 1073–1077. [Google Scholar] [CrossRef]
- Whittaker, E.; Gordon, A.; Kampmann, B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS ONE 2008, 3, e3901. [Google Scholar] [CrossRef]
- Latorre, I.; Diaz, J.; Mialdea, I.; Serra-Vidal, M.; Altet, N.; Prat, C.; Diez, N.; Escribano, A.; Casas, I.; Rodrigo, C.; et al. IP-10 is an accurate biomarker for the diagnosis of tuberculosis in children. J. Infect. 2014, 69, 590–599. [Google Scholar] [CrossRef]
- Tebruegge, M.; Dutta, B.; Donath, S.; Ritz, N.; Forbes, B.; Camacho-Badilla, K.; Clifford, V.; Zufferey, C.; Robins-Browne, R.; Hanekom, W.; et al. Mycobacteria-Specific Cytokine Responses Detect Tuberculosis Infection and Distinguish Latent from Active Tuberculosis. Am. J. Respir. Crit. Care Med. 2015, 192, 485–499. [Google Scholar] [CrossRef]
- Wergeland, I.; Pullar, N.; Assmus, J.; Ueland, T.; Tonby, K.; Feruglio, S.; Kvale, D.; Damas, J.K.; Aukrust, P.; Mollnes, T.E.; et al. IP-10 differentiates between active and latent tuberculosis irrespective of HIV status and declines during therapy. J. Infect. 2015, 70, 381–391. [Google Scholar] [CrossRef]
- Nausch, N.; Lundtoft, C.; Schulz, G.; Henckel, H.; Mayatepek, E.; Fleischer, B.; Marx, F.M.; Jacobsen, M. Multiple cytokines for the detection of Mycobacterium tuberculosis infection in children with tuberculosis. Int. J. Tuberc. Lung Dis. 2017, 21, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, G.H.; Jereb, J.; Vernon, A.; LoBue, P.; Goldberg, S.; Castro, K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2010, 59, 1–25. [Google Scholar]
- Lighter, J.; Rigaud, M.; Huie, M.; Peng, C.H.; Pollack, H. Chemokine IP-10: An adjunct marker for latent tuberculosis infection in children. Int. J. Tuberc. Lung Dis. 2009, 13, 731–736. [Google Scholar] [PubMed]
- Armand, M.; Chhor, V.; de Lauzanne, A.; Guerin-El Khourouj, V.; Pedron, B.; Jeljeli, M.; Gressens, P.; Faye, A.; Sterkers, G. Cytokine responses to quantiferon peptides in pediatric tuberculosis: A pilot study. J. Infect. 2014, 68, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Amanatidou, V.; Critselis, E.; Trochoutsou, A.; Soldatou, A.; Benetatou, K.; Spyridis, N.; Papadopoulos, N.G.; Tsolia, M.N. Interferon gamma inducible protein-10 in the diagnosis of paediatric tuberculosis infection in a low TB incidence country. Int. J. Tuberc. Lung Dis. 2015, 19, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Petrucci, R.; Garie, K.T.; Harper, G.; Teshome, A.; Arbide, I.; Asnake, G.; Ahmed, H.J.; Mammo, T.; Yesuf, K.; et al. Use of tuberculin skin test, IFN-gamma release assays and IFN-gamma-induced protein-10 to identify children with TB infection. Eur. Respir. J. 2013, 41, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Cannas, A.; Aloi, F.; Nsubuga, M.; Sserumkuma, J.; Nazziwa, R.A.; Jugheli, L.; Lukindo, T.; Girardi, E.; Reither, K.; et al. Blood or Urine IP-10 Cannot Discriminate between Active Tuberculosis and Respiratory Diseases Different from Tuberculosis in Children. BioMed Res. Int. 2015, 2015, 589471. [Google Scholar] [CrossRef] [PubMed]
- Sudbury, E.L.; Otero, L.; Tebruegge, M.; Messina, N.L.; Seas, C.; Montes, M.; Rìos, J.; Germano, S.; Gardiner, K.; Clifford, V.; et al. Mycobacterium tuberculosis-specific cytokine biomarkers for the diagnosis of childhood TB in a TB-endemic setting. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 16, 100102. [Google Scholar] [CrossRef]
- Chegou, N.N.; Detjen, A.K.; Thiart, L.; Walters, E.; Mandalakas, A.M.; Hesseling, A.C.; Walzl, G. Utility of host markers detected in Quantiferon supernatants for the diagnosis of tuberculosis in children in a high-burden setting. PLoS ONE 2013, 8, e64226. [Google Scholar] [CrossRef]
- Alsleben, N.; Ruhwald, M.; Russmann, H.; Marx, F.M.; Wahn, U.; Magdorf, K. Interferon-gamma inducible protein 10 as a biomarker for active tuberculosis and latent tuberculosis infection in children: A case-control study. Scand. J. Infect. Dis. 2012, 44, 256–262. [Google Scholar] [CrossRef]
- Holm, L.L.; Rose, M.V.; Kimaro, G.; Bygbjerg, I.C.; Mfinanga, S.G.; Ravn, P.; Ruhwald, M. A comparison of interferon-gamma and IP-10 for the diagnosis of tuberculosis. Pediatrics 2014, 134, e1568–e1575. [Google Scholar] [CrossRef]
- Ruhwald, M.; Dominguez, J.; Latorre, I.; Losi, M.; Richeldi, L.; Pasticci, M.B.; Mazzolla, R.; Goletti, D.; Butera, O.; Bruchfeld, J.; et al. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis 2011, 91, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Lee, H.J.; Kim, S.Y.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; et al. Efficacy of IP-10 as a biomarker for monitoring tuberculosis treatment. J. Infect. 2014, 68, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.; Zhang, X.; Yu, Q.; Zhao, P.; Wang, J.; Duan, C.; Li, J.; Johnson, H.; Feng, X.; et al. IP-10 and RANTES as biomarkers for pulmonary tuberculosis diagnosis and monitoring. Tuberculosis 2018, 111, 45–53. [Google Scholar] [CrossRef] [PubMed]
- García-Basteiro, A.L.; Mambuque, E.; den Hertog, A.; Saavedra, B.; Cuamba, I.; Oliveras, L.; Blanco, S.; Bulo, H.; Brew, J.; Cuevas, L.E.; et al. IP-10 Kinetics in the First Week of Therapy are Strongly Associated with Bacteriological Confirmation of Tuberculosis Diagnosis in HIV-Infected Patients. Sci. Rep. 2017, 7, 14302. [Google Scholar] [CrossRef]
- Clifford, V.; Tebruegge, M.; Zufferey, C.; Germano, S.; Forbes, B.; Cosentino, L.; McBryde, E.; Eisen, D.; Robins-Browne, R.; Street, A.; et al. Mycobacteria-specific cytokine responses as correlates of treatment response in active and latent tuberculosis. J. Infect. 2017, 75, 132–145. [Google Scholar] [CrossRef]
- Petrone, L.; Chiacchio, T.; Vanini, V.; Petruccioli, E.; Cuzzi, G.; Di Giacomo, C.; Pucci, L.; Montalbano, M.; Lionetti, R.; Testa, A.; et al. High urine IP-10 levels associate with chronic HCV infection. J. Infect. 2014, 68, 591–600. [Google Scholar] [CrossRef]
- Puapatanakul, P.; Chansritrakul, S.; Susantitaphong, P.; Ueaphongsukkit, T.; Eiam-Ong, S.; Praditpornsilpa, K.; Kittanamongkolchai, W.; Avihingsanon, Y. Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4954. [Google Scholar] [CrossRef]
- Zar, H.J.; Hanslo, D.; Apolles, P.; Swingler, G.; Hussey, G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: A prospective study. Lancet 2005, 365, 130–134. [Google Scholar] [CrossRef]
- Available online: http://wwwold.pzh.gov.pl/oldpage/epimeld/hiv_aids/index.htm (accessed on 3 October 2023).
| Total n = 225 (%) | ATB n = 33 (%) | TBI n = 48 (%) | TB Contact n = 83 (%) | non-TB n = 20 (%) | Controls n = 41 (%) | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| female | 110 (48.9) | 18 (54.5) | 23 (48) | 36 (43.4) | 12 (60) | 21 (51.2) |
| male | 115 (51.1) | 15 (45.5) | 25 (52) | 47(56.6) | 8 (40) | 20 (48.8) |
| age (yrs), median (IQR) | 6 (3–11) | 13 (7–16) | 7 (4.5–11) | 4 (2–8) | 7 (5–10.5) | 6 (1–11) |
| ≤5 y | 84 (37.3) | 6 (18.2) | 12 (25) | 45 (54.2) | 4 (20) | 17 (41.5) |
| 0–2 y | 44 (19.6) | 3 (9.1) | 7 (14.6) | 20 (24.1) | 2 (10) | 12 (29.3) |
| 2–5 y | 40 (17.8) | 3 (9.1) | 5 (10.4) | 25 (30.1) | 2 (10) | 5 (12.2) |
| >5 y | 141 (62.7) | 27 (81.8) | 36 (75) | 38 (45.8) | 16 (80) | 24 (58.5) |
| 5–10 y | 71 (31.6) | 4 (12.1) | 20 (41.7) | 24 (28.9) | 10 (50) | 13 (31.7) |
| >10 y | 70 (31.1) | 23 (69.7) | 16 (33.3) | 14 (16.9) | 6 (30) | 11 (26.8) |
| BCG-vaccinated | ||||||
| yes | 217 (96.4) | 30 (91) | 48 (100) | 80 (96.4) | 19 (95) | 40 (97.6) |
| no | 3 (1.3) | 1 (3) | - | - | 1 (5) | 1 (2.4) |
| not recorded | 5 (2.2) | 2 (6) | - | 3(3.6) | - | - |
| Confirmed TB | 15 (6.7) | 15 (45.4) | - | - | - | - |
| microscopy | 7 (3.1) | 7 (21.1) | - | - | - | - |
| PCR | 12 (5.3) | 12 (36.4) | - | - | - | - |
| culture | 9 (4) | 9 (27.3) | - | - | - | - |
| TST results | ||||||
| positive | 72 (32) | 28 (84.8) | 41 (85.4) | - | 3 (15) | - |
| negative | 99 (44) | 2 (6) | 5 (10.4) | 77 (92.8) | 15 (75) | - |
| not performed | 54 (24) | 3 (9.1) | 2 (4.2) | 6 (7.2) | 2 (10) | 41 (100) |
| QFT results | ||||||
| positive | 56 (24.9) | 23 (69.7) | 33 (68.6) | - | - | - |
| negative | 167 (74.2) | 9 (27.3) | 15 (31.3) | 82 (98.8) | 20 (100) | 41 (100) |
| indeterminate | 2 (0.9) | 1 (3) | - | 1 (1.2) | - | - |
| TST+/QFT− | 45 (20) | 19 (57.6) | 26 (54.2) | - | - | - |
| TST+/QFT− | 23 (10.2) | 8 (24.2) | 15 (31.3) | - | 3 (15) | - |
| TST−/QFT+ | 6 (2.7) | 1 (3) | 5 (10.4) | - | - | - |
| TST−/QFT− | 1 (0.4) | 1 (3) | - | - | - | - |
| IP-10 Unstimulated Median (IQR) pg/mL | IP-10 Mitogen Stimulated Median (IQR) pg/mL | IP-10 Mtb. Antigen-Stimulated Median (IQR) pg/mL | p a-Value | |
|---|---|---|---|---|
| ATB | 257 (158–637) | 18,963 (10,857–26,266) | 7930 (1194–36,968) | <0.001 |
| TBI | 335 (198–538) | 25,456 (11,330–43,298) | 2250 (282–9168) | <0.001 |
| TB contact | 208 (133–328) | 4874 (4056–20,811) | 82 (21–188) | >0.05 |
| Non-TB | 433 (266–721) | 20,026 (1329–38,490) | 155 (16–484) | >0.05 |
| Control | 387 (244–610) | 788 (424–28,501) | 143 (12–582) | >0.05 |
| Cut-Off pg/mL | AUC (95% CI) | p-Value | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|
| Total cohort: | |||||
| ATB vs. control | >1084.5 | 0.935 (0.836–0.983) | <0.0001 | 93.3 (68.1–99.8) | 87.8 (73.8–95.9) |
| TBI vs. control | >1498.1 | 0.977 (0.906–0.998) | <0.0001 | 88.5 (69.8–97) | 97.6 (87.1–99.9) |
| ATB+TBI vs. control | >1084.5 | 0.961 (0.894–0.991) | <0.0001 | 92.68 (80.1–98.5) | 87.8 (73.8–95.9) |
| ATB vs. TBI | ≤2593.3 | 0.556 (0.393–0.711) | 0.5692 | 44.4 (13.7–78.8) | 88.5 (69.8–97.6) |
| Clinical suspicion of TB: | |||||
| ATB vs. RTI | >626.8 | 0.921 (0.746–0.990) | <0.0001 | 90.9 (58.7–99.8) | 93.3 (68.1–99.8) |
| Contact tracing referrals: | |||||
| Mtb.-infected vs. TB contact | >761 | 0.999 (0.963–1.000) | <0.0001 | 100 (88.1–100) | 98.7 (92.9–100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelak, A.; Komorowska-Piotrowska, A.; Borowa, A.; Krasińska, M.; Feleszko, W.; Kulus, M. IP-10 for the Diagnosis and Treatment Monitoring of Tuberculosis in Children. Diagnostics 2024, 14, 177. https://doi.org/10.3390/diagnostics14020177
Strzelak A, Komorowska-Piotrowska A, Borowa A, Krasińska M, Feleszko W, Kulus M. IP-10 for the Diagnosis and Treatment Monitoring of Tuberculosis in Children. Diagnostics. 2024; 14(2):177. https://doi.org/10.3390/diagnostics14020177
Chicago/Turabian StyleStrzelak, Agnieszka, Anna Komorowska-Piotrowska, Agnieszka Borowa, Maria Krasińska, Wojciech Feleszko, and Marek Kulus. 2024. "IP-10 for the Diagnosis and Treatment Monitoring of Tuberculosis in Children" Diagnostics 14, no. 2: 177. https://doi.org/10.3390/diagnostics14020177
APA StyleStrzelak, A., Komorowska-Piotrowska, A., Borowa, A., Krasińska, M., Feleszko, W., & Kulus, M. (2024). IP-10 for the Diagnosis and Treatment Monitoring of Tuberculosis in Children. Diagnostics, 14(2), 177. https://doi.org/10.3390/diagnostics14020177







