From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer
Abstract
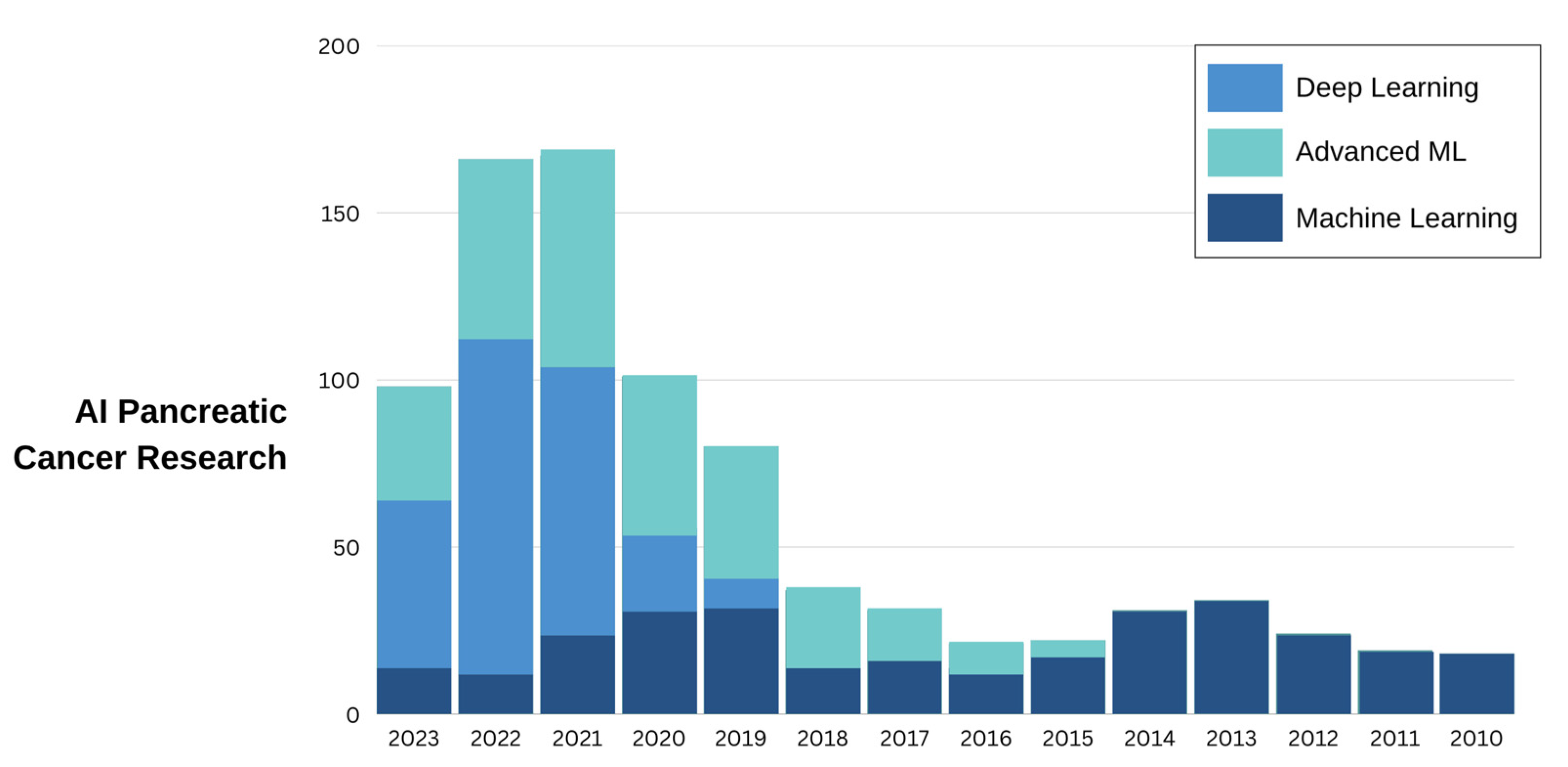
1. Introduction
2. AI Techniques
2.1. Machine Learning
2.2. Deep Learning
2.2.1. Natural Language Processing
2.2.2. Computer Vision
2.3. Transfer Learning
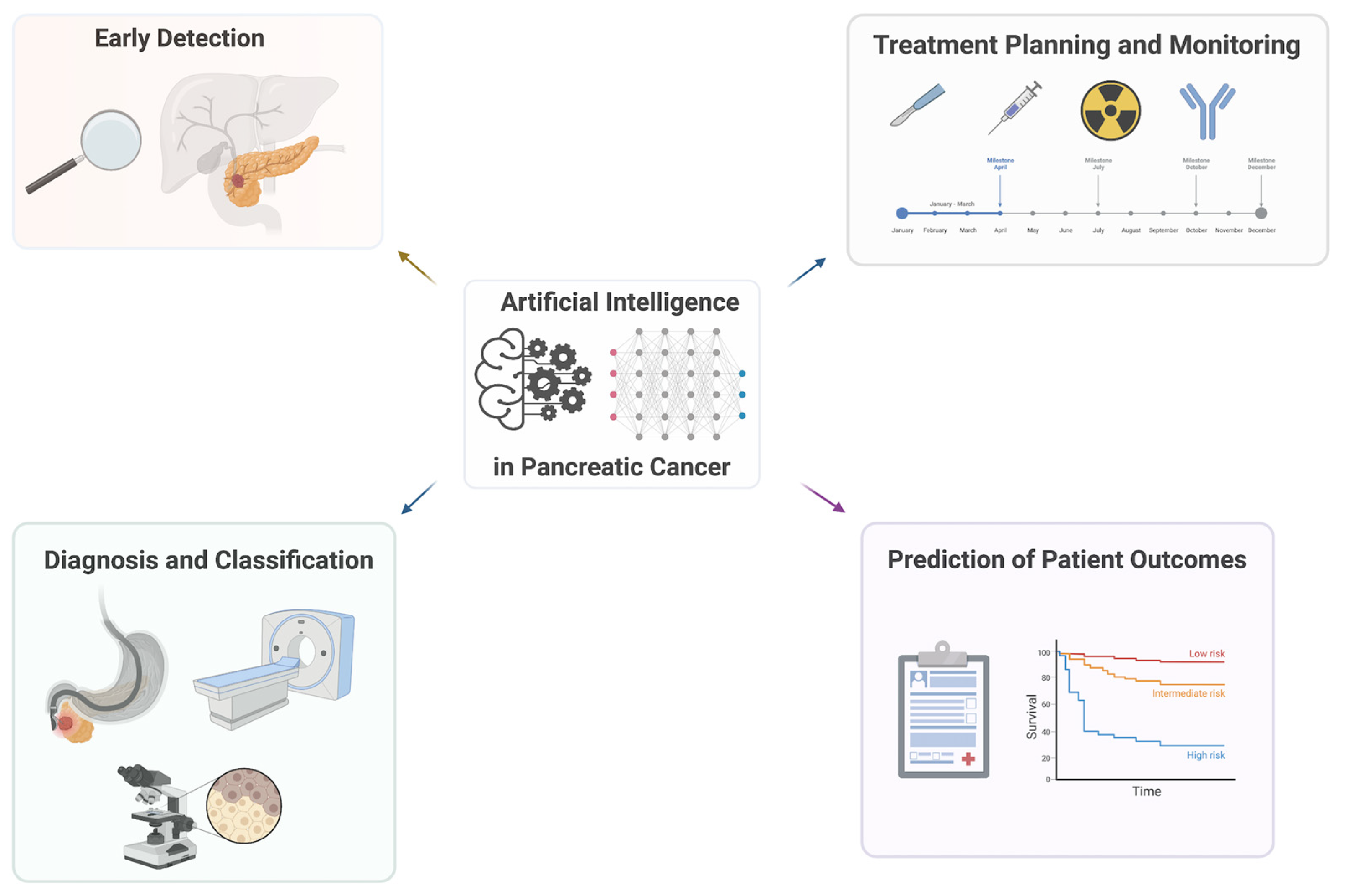
3. Application of AI in Clinical Studies
3.1. Early Detection
3.2. Diagnosis and Classification
3.3. Treatment Planning and Monitoring
3.4. Biomarker Discovery
3.5. Contrast-Enhanced Ultrasound
3.6. Healthcare Workflows
4. Limitations and Challenges
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Hameed, B.S.; Krishnan, U.M. Artificial Intelligence-Driven Diagnosis of Pancreatic Cancer. Cancers 2022, 14, 5382. [Google Scholar] [CrossRef]
- Schuurmans, M.; Alves, N.; Vendittelli, P.; Huisman, H.; Hermans, J. Setting the Research Agenda for Clinical Artificial Intelligence in Pancreatic Adenocarcinoma Imaging. Cancers 2022, 14, 3498. [Google Scholar] [CrossRef]
- Elbanna, K.Y.; Jang, H.-J.; Kim, T.K. Imaging Diagnosis and Staging of Pancreatic Ductal Adenocarcinoma: A Comprehensive Review. Insights Imaging 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital Pathology and Artificial Intelligence in Translational Medicine and Clinical Practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef]
- Sántha, P.; Lenggenhager, D.; Finstadsveen, A.; Dorg, L.; Tøndel, K.; Amrutkar, M.; Gladhaug, I.P.; Verbeke, C. Morphological Heterogeneity in Pancreatic Cancer Reflects Structural and Functional Divergence. Cancers 2021, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Mi, W.; Pan, B.; Guo, Y.; Li, J.; Xu, R.; Zheng, J.; Zou, C.; Zhang, T.; Liang, Z.; et al. Automatic Pancreatic Ductal Adenocarcinoma Detection in Whole Slide Images Using Deep Convolutional Neural Networks. Front. Oncol. 2021, 11, 665929. [Google Scholar] [CrossRef]
- Janssen, B.V.; Tutucu, F.; van Roessel, S.; Adsay, V.; Basturk, O.; Campbell, F.; Doglioni, C.; Esposito, I.; Feakins, R.; Fukushima, N.; et al. Amsterdam International Consensus Meeting: Tumor Response Scoring in the Pathology Assessment of Resected Pancreatic Cancer after Neoadjuvant Therapy. Mod. Pathol. 2021, 34, 4–12. [Google Scholar] [CrossRef]
- Tripathi, S. Artificial Intelligence: A Brief Review. In Analyzing Future Applications of AI, Sensors, and Robotics in Society; IGI Global: Hershey, PA, USA, 2021. [Google Scholar]
- Chen, T.; Carter, J.; Mahmud, M.; Khuman, A.S. Artificial Intelligence in Healthcare: Recent Applications and Developments; Springer Nature: Singapore, 2022; ISBN 9789811952722. [Google Scholar]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A Comparison of Deep Learning Performance against Health-Care Professionals in Detecting Diseases from Medical Imaging: A Systematic Review and Meta-Analysis. Lancet Digit Health 2019, 1, e271–e297. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, L.; Dunn, A.G.; Tong, H.L.; Kocaballi, A.B.; Chen, J.; Bashir, R.; Surian, D.; Gallego, B.; Magrabi, F.; Lau, A.Y.S.; et al. Conversational Agents in Healthcare: A Systematic Review. J. Am. Med. Inform. Assoc. 2018, 25, 1248–1258. [Google Scholar] [CrossRef]
- Karimian, G.; Petelos, E.; Evers, S.M.A.A. The Ethical Issues of the Application of Artificial Intelligence in Healthcare: A Systematic Scoping Review. AI Ethics 2022, 2, 539–551. [Google Scholar] [CrossRef]
- Tripathi, S.; Musiolik, T.H. Fairness and Ethics in Artificial Intelligence-Based Medical Imaging. In Research Anthology on Improving Medical Imaging Techniques for Analysis and Intervention; IGI Global: Hershey, PA, USA, 2023; pp. 79–90. [Google Scholar]
- Huang, B.; Huang, H.; Zhang, S.; Zhang, D.; Shi, Q.; Liu, J.; Guo, J. Artificial Intelligence in Pancreatic Cancer. Theranostics 2022, 12, 6931–6954. [Google Scholar] [CrossRef] [PubMed]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N.; et al. Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas 2021, 50, 251–279. [Google Scholar] [CrossRef]
- Zhu, W.; Xie, L.; Han, J.; Guo, X. The Application of Deep Learning in Cancer Prognosis Prediction. Cancers 2020, 12, 603. [Google Scholar] [CrossRef]
- Zhao, Y.; Kosorok, M.R.; Zeng, D. Reinforcement Learning Design for Cancer Clinical Trials. Stat. Med. 2009, 28, 3294–3315. [Google Scholar] [CrossRef]
- Qureshi, T.A.; Gaddam, S.; Wachsman, A.M.; Wang, L.; Azab, L.; Asadpour, V.; Chen, W.; Xie, Y.; Wu, B.; Pandol, S.J.; et al. Predicting Pancreatic Ductal Adenocarcinoma Using Artificial Intelligence Analysis of Pre-Diagnostic Computed Tomography Images. Cancer Biomark. 2022, 33, 211–217. [Google Scholar] [CrossRef]
- Qiao, Z.; Ge, J.; He, W.; Xu, X.; He, J. Artificial Intelligence Algorithm-Based Computerized Tomography Image Features Combined with Serum Tumor Markers for Diagnosis of Pancreatic Cancer. Comput. Math. Methods Med. 2022, 2022, 8979404. [Google Scholar] [CrossRef]
- Keogan, M.T.; Lo, J.Y.; Freed, K.S.; Raptopoulos, V.; Blake, S.; Kamel, I.R.; Weisinger, K.; Rosen, M.P.; Nelson, R.C. Outcome Analysis of Patients with Acute Pancreatitis by Using an Artificial Neural Network. Acad. Radiol. 2002, 9, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, F.; Yang, X.; Meng, X.; Miao, Y.; Noor Hussain, M.S.; Yang, L.; Li, Z. Research Trends of Artificial Intelligence in Pancreatic Cancer: A Bibliometric Analysis. Front. Oncol. 2022, 12, 973999. [Google Scholar] [CrossRef]
- Ramesh, A.N.; Kambhampati, C.; Monson, J.R.T.; Drew, P.J. Artificial Intelligence in Medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334–338. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in Health and Medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Qu, S.; Zhang, H. Support Vector Machine Combined with Magnetic Resonance Imaging for Accurate Diagnosis of Paediatric Pancreatic Cancer. IET Image Proc. 2020, 14, 1233–1239. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Jonckheere, N.; Auwercx, J.; Hadj Bachir, E.; Coppin, L.; Boukrout, N.; Vincent, A.; Neve, B.; Gautier, M.; Treviño, V.; Van Seuningen, I. Unsupervised Hierarchical Clustering of Pancreatic Adenocarcinoma Dataset from TCGA Defines a Mucin Expression Profile That Impacts Overall Survival. Cancers 2020, 12, 3309. [Google Scholar] [CrossRef]
- Moffitt, R.A.; Marayati, R.; Flate, E.L.; Volmar, K.E.; Loeza, S.G.H.; Hoadley, K.A.; Rashid, N.U.; Williams, L.A.; Eaton, S.C.; Chung, A.H.; et al. Virtual Microdissection Identifies Distinct Tumor- and Stroma-Specific Subtypes of Pancreatic Ductal Adenocarcinoma. Nat. Genet. 2015, 47, 1168–1178. [Google Scholar] [CrossRef]
- Yang, C.-H.; Lin, Y.-D.; Yang, C.-S.; Chuang, L.-Y. An Efficiency Analysis of High-Order Combinations of Gene–gene Interactions Using Multifactor-Dimensionality Reduction. BMC Genom. 2015, 16, 489. [Google Scholar] [CrossRef]
- Brough, D.B.; Kannan, A.; Haaland, B.; Bucknall, D.G.; Kalidindi, S.R. Extraction of Process-Structure Evolution Linkages from X-ray Scattering Measurements Using Dimensionality Reduction and Time Series Analysis. Integr. Mater. Manuf. Innov. 2017, 6, 147–159. [Google Scholar] [CrossRef]
- Cavallo, M.; Demiralp, Ç. A Visual Interaction Framework for Dimensionality Reduction Based Data Exploration. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal, QC, Canada, 21 April 2018; Association for Computing Machinery: New York, NY, USA, 2018; pp. 1–13. [Google Scholar]
- Yang, K.; Yang, T.; Yu, J.; Li, F.; Zhao, X. Integrated Transcriptional Analysis Reveals Macrophage Heterogeneity and Macrophage-Tumor Cell Interactions in the Progression of Pancreatic Ductal Adenocarcinoma. BMC Cancer 2023, 23, 199. [Google Scholar] [CrossRef]
- Fan, X.; Lu, P.; Wang, H.; Bian, S.; Wu, X.; Zhang, Y.; Liu, Y.; Fu, D.; Wen, L.; Hao, J.; et al. Integrated Single-Cell Multiomics Analysis Reveals Novel Candidate Markers for Prognosis in Human Pancreatic Ductal Adenocarcinoma. Cell Discov. 2022, 8, 13. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Pan, S.; Wang, M.; Wang, Z.; He, Z.; Zhang, G.; Cui, F.; Song, Y.; Li, W.; et al. Single-Cell Sequencing Reveals Heterogeneity between Pancreatic Adenosquamous Carcinoma and Pancreatic Ductal Adenocarcinoma with Prognostic Value. Front. Immunol. 2022, 13, 972298. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic Analyses Identify Molecular Subtypes of Pancreatic Cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Ke, M. Systematic Analysis of Molecular Subtypes Based on the Expression Profile of Immune-Related Genes in Pancreatic Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 3124122. [Google Scholar] [CrossRef]
- Abel, L.; Wasserthal, J.; Weikert, T.; Sauter, A.W.; Nesic, I.; Obradovic, M.; Yang, S.; Manneck, S.; Glessgen, C.; Ospel, J.M.; et al. Automated Detection of Pancreatic Cystic Lesions on CT Using Deep Learning. Diagnostics 2021, 11, 901. [Google Scholar] [CrossRef]
- Dinesh, M.G.; Bacanin, N.; Askar, S.S.; Abouhawwash, M. Diagnostic Ability of Deep Learning in Detection of Pancreatic Tumour. Sci. Rep. 2023, 13, 9725. [Google Scholar] [CrossRef]
- Viriyasaranon, T.; Chun, J.W.; Koh, Y.H.; Cho, J.H.; Jung, M.K.; Kim, S.-H.; Kim, H.J.; Lee, W.J.; Choi, J.-H.; Woo, S.M. Annotation-Efficient Deep Learning Model for Pancreatic Cancer Diagnosis and Classification Using CT Images: A Retrospective Diagnostic Study. Cancers 2023, 15, 3392. [Google Scholar] [CrossRef]
- Chen, P.-T.; Wu, T.; Wang, P.; Chang, D.; Liu, K.-L.; Wu, M.-S.; Roth, H.R.; Lee, P.-C.; Liao, W.-C.; Wang, W. Pancreatic Cancer Detection on CT Scans with Deep Learning: A Nationwide Population-Based Study. Radiology 2023, 306, 172–182. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.; Zhang, H.; Wu, Y. A Neural Network Model to Screen Feature Genes for Pancreatic Cancer. BMC Bioinform. 2023, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Placido, D.; Yuan, B.; Hjaltelin, J.X.; Zheng, C.; Haue, A.D.; Chmura, P.J.; Yuan, C.; Kim, J.; Umeton, R.; Antell, G.; et al. A Deep Learning Algorithm to Predict Risk of Pancreatic Cancer from Disease Trajectories. Nat. Med. 2023, 29, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Locke, S.; Bashall, A.; Al-Adely, S.; Moore, J.; Wilson, A.; Kitchen, G.B. Natural Language Processing in Medicine: A Review. Trends Anaesth. Crit. Care 2021, 38, 4–9. [Google Scholar] [CrossRef]
- Cohen, K.B.; Demner-Fushman, D. Biomedical Natural Language Processing; John Benjamins Publishing Company: Amsterdam, The Netherlands, 2014; ISBN 9789027271068. [Google Scholar]
- Bohr, A.; Memarzadeh, K. Artificial Intelligence in Healthcare; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128184394. [Google Scholar]
- Jensen, L.J.; Saric, J.; Bork, P. Literature Mining for the Biologist: From Information Retrieval to Biological Discovery. Nat. Rev. Genet. 2006, 7, 119–129. [Google Scholar] [CrossRef]
- Torii, M.; Hu, Z.; Wu, C.H.; Liu, H. BioTagger-GM: A Gene/protein Name Recognition System. J. Am. Med. Inform. Assoc. 2009, 16, 247–255. [Google Scholar] [CrossRef]
- Wei, C.-H.; Kao, H.-Y.; Lu, Z. PubTator: A Web-Based Text Mining Tool for Assisting Biocuration. Nucleic Acids Res. 2013, 41, W518–W522. [Google Scholar] [CrossRef]
- Lu, Z.; Kao, H.-Y.; Wei, C.-H.; Huang, M.; Liu, J.; Kuo, C.-J.; Hsu, C.-N.; Tsai, R.T.-H.; Dai, H.-J.; Okazaki, N.; et al. The Gene Normalization Task in BioCreative III. BMC Bioinform. 2011, 12 (Suppl. S8), S2. [Google Scholar] [CrossRef]
- Juhn, Y.; Liu, H. Artificial Intelligence Approaches Using Natural Language Processing to Advance EHR-Based Clinical Research. J. Allergy Clin. Immunol. 2020, 145, 463–469. [Google Scholar] [CrossRef]
- Kenner, B.J.; Abrams, N.D.; Chari, S.T.; Field, B.F.; Goldberg, A.E.; Hoos, W.A.; Klimstra, D.S.; Rothschild, L.J.; Srivastava, S.; Young, M.R.; et al. Early Detection of Pancreatic Cancer: Applying Artificial Intelligence to Electronic Health Records. Pancreas 2021, 50, 916–922. [Google Scholar] [CrossRef]
- Roch, A.M.; Mehrabi, S.; Krishnan, A.; Schmidt, H.E.; Kesterson, J.; Beesley, C.; Dexter, P.R.; Palakal, M.; Schmidt, C.M. Automated Pancreatic Cyst Screening Using Natural Language Processing: A New Tool in the Early Detection of Pancreatic Cancer. HPB 2015, 17, 447–453. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Weng, Y.; Wang, B.; Li, Z. Natural Language Processing Applications for Computer-Aided Diagnosis in Oncology. Diagnostics 2023, 13, 286. [Google Scholar] [CrossRef]
- Data, M.C. Secondary Analysis of Electronic Health Records; Springer: Cham, Switzerland, 2016; ISBN 9783319437422. [Google Scholar]
- Jain, V.; Chatterjee, J.M. Machine Learning with Health Care Perspective: Machine Learning and Healthcare; Springer Nature: Cham, Switzerland, 2020; ISBN 9783030408503. [Google Scholar]
- Datta, S.; Bernstam, E.V.; Roberts, K. A Frame Semantic Overview of NLP-Based Information Extraction for Cancer-Related EHR Notes. J. Biomed. Inform. 2019, 100, 103301. [Google Scholar] [CrossRef]
- Savova, G.K.; Danciu, I.; Alamudun, F.; Miller, T.; Lin, C.; Bitterman, D.S.; Tourassi, G.; Warner, J.L. Use of Natural Language Processing to Extract Clinical Cancer Phenotypes from Electronic Medical Records. Cancer Res. 2019, 79, 5463–5470. [Google Scholar] [CrossRef]
- Sohn, S.; Wang, Y.; Wi, C.-I.; Krusemark, E.A.; Ryu, E.; Ali, M.H.; Juhn, Y.J.; Liu, H. Clinical Documentation Variations and NLP System Portability: A Case Study in Asthma Birth Cohorts across Institutions. J. Am. Med. Inform. Assoc. 2018, 25, 353–359. [Google Scholar] [CrossRef]
- Dernoncourt, F.; Lee, J.Y.; Szolovits, P. Neural Networks for Joint Sentence Classification in Medical Paper Abstracts. arXiv 2016, arXiv:1612.05251. [Google Scholar]
- Voulodimos, A.; Doulamis, N.; Doulamis, A.; Protopapadakis, E. Deep Learning for Computer Vision: A Brief Review. Comput. Intell. Neurosci. 2018, 2018, 7068349. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Zhou, S.K.; Greenspan, H.; Davatzikos, C.; Duncan, J.S.; Van Ginneken, B.; Madabhushi, A.; Prince, J.L.; Rueckert, D.; Summers, R.M. A Review of Deep Learning in Medical Imaging: Imaging Traits, Technology Trends, Case Studies With Progress Highlights, and Future Promises. Proc. IEEE 2021, 109, 820–838. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial Intelligence in Radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Kouzahkanan, Z.M.; Radmard, A.R.; Kafieh, R.; Salehnia, A.; Davarpanah, A.H.; Arabalibeik, H.; Ahmadian, A. Segmentation of Pancreatic Ductal Adenocarcinoma (PDAC) and Surrounding Vessels in CT Images Using Deep Convolutional Neural Networks and Texture Descriptors. Sci. Rep. 2022, 12, 3092. [Google Scholar] [CrossRef]
- Li, J.; Qi, L.; Chen, Q.; Zhang, Y.-D.; Qian, X. A Dual Meta-Learning Framework Based on Idle Data for Enhancing Segmentation of Pancreatic Cancer. Med. Image Anal. 2022, 78, 102342. [Google Scholar] [CrossRef]
- Wong, J.; Baine, M.; Wisnoskie, S.; Bennion, N.; Zheng, D.; Yu, L.; Dalal, V.; Hollingsworth, M.A.; Lin, C.; Zheng, D. Effects of Interobserver and Interdisciplinary Segmentation Variabilities on CT-Based Radiomics for Pancreatic Cancer. Sci. Rep. 2021, 11, 16328. [Google Scholar] [CrossRef]
- Bronstein, Y.L.; Loyer, E.M.; Kaur, H.; Choi, H.; David, C.; DuBrow, R.A.; Broemeling, L.D.; Cleary, K.R.; Charnsangavej, C. Detection of Small Pancreatic Tumors with Multiphasic Helical CT. AJR Am. J. Roentgenol. 2004, 182, 619–623. [Google Scholar] [CrossRef]
- Shah, J.; Surve, S.; Turkar, V. Pancreatic Tumor Detection Using Image Processing. Procedia Comput. Sci. 2015, 49, 11–16. [Google Scholar] [CrossRef]
- Marin, D.; Nelson, R.C.; Barnhart, H.; Schindera, S.T.; Ho, L.M.; Jaffe, T.A.; Yoshizumi, T.T.; Youngblood, R.; Samei, E. Detection of Pancreatic Tumors, Image Quality, and Radiation Dose during the Pancreatic Parenchymal Phase: Effect of a Low-Tube-Voltage, High-Tube-Current CT Technique--Preliminary Results. Radiology 2010, 256, 450–459. [Google Scholar] [CrossRef]
- Althobaiti, M.M.; Almulihi, A.; Ashour, A.A.; Mansour, R.F.; Gupta, D. Design of Optimal Deep Learning-Based Pancreatic Tumor and Nontumor Classification Model Using Computed Tomography Scans. J. Healthc. Eng. 2022, 2022, 2872461. [Google Scholar] [CrossRef]
- Li, J.; Yin, W.; Wang, Y. CT Classification Model of Pancreatic Serous Cystic Neoplasm and Mucinous Cystic Neoplasm Based on Deep Transfer Learning. J. X-ray Sci. Technol. 2023, 31, 167–180. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Y.; Dai, W.; Pan, S.J. Transfer Learning; Cambridge University Press: Cambridge, UK, 2020; ISBN 9781107016903. [Google Scholar]
- Zhuang, F.; Qi, Z.; Duan, K.; Xi, D.; Zhu, Y.; Zhu, H.; Xiong, H.; He, Q. A Comprehensive Survey on Transfer Learning. Proc. IEEE 2021, 109, 43–76. [Google Scholar] [CrossRef]
- Volpp, M.; Fröhlich, L.P.; Fischer, K.; Doerr, A.; Falkner, S.; Hutter, F.; Daniel, C. Meta-Learning Acquisition Functions for Transfer Learning in Bayesian Optimization. arXiv 2019, arXiv:1904.02642. [Google Scholar]
- Tan, C.; Sun, F.; Kong, T.; Zhang, W.; Yang, C.; Liu, C. A Survey on Deep Transfer Learning. In Proceedings of the Artificial Neural Networks and Machine Learning—ICANN 2018, Rhodes, Greece, 4–7 October 2018; Springer International Publishing: Cham, Switzerland, 2018; pp. 270–279. [Google Scholar]
- Wang, K.; Gao, X.; Zhao, Y.; Li, X.; Dou, D.; Xu, C.-Z. Pay Attention to Features, Transfer Learn Faster CNNs. In Proceedings of the International Conference on Learning Representations, New Orleans, LA, USA, 6–9 May 2019. [Google Scholar]
- Rezaei, S.; Liu, X. A Target-Agnostic Attack on Deep Models: Exploiting Security Vulnerabilities of Transfer Learning. arXiv 2019, arXiv:1904.04334. [Google Scholar]
- Shin, H.-C.; Roth, H.R.; Gao, M.; Lu, L.; Xu, Z.; Nogues, I.; Yao, J.; Mollura, D.; Summers, R.M. Deep Convolutional Neural Networks for Computer-Aided Detection: CNN Architectures, Dataset Characteristics and Transfer Learning. IEEE Trans. Med. Imaging 2016, 35, 1285–1298. [Google Scholar] [CrossRef]
- Hussain, M.; Bird, J.J.; Faria, D.R. A Study on CNN Transfer Learning for Image Classification. In Proceedings of the Advances in Computational Intelligence Systems: Contributions Presented at the 18th UK Workshop on Computational Intelligence, Nottingham, UK, 5–7 September 2018; Springer International Publishing: Cham, Switzerland, 2019; pp. 191–202. [Google Scholar]
- Shafahi, A.; Saadatpanah, P.; Zhu, C.; Ghiasi, A.; Studer, C.; Jacobs, D.; Goldstein, T. Adversarially Robust Transfer Learning. arXiv 2019, arXiv:1905.08232. [Google Scholar]
- Passalis, N.; Tefas, A. Learning Deep Representations with Probabilistic Knowledge Transfer. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 268–284. [Google Scholar]
- Glatt, R.; Da Silva, F.L.; Costa, A.H.R. Towards Knowledge Transfer in Deep Reinforcement Learning. In Proceedings of the 2016 5th Brazilian Conference on Intelligent Systems (BRACIS), Recife, Brazil, 9–12 October 2016; pp. 91–96. [Google Scholar]
- Akbarian, S.; Seyyed-Kalantari, L.; Khalvati, F.; Dolatabadi, E. Evaluating Knowledge Transfer in the Neural Network for Medical Images. IEEE Access 2023, 11, 85812–85821. [Google Scholar] [CrossRef]
- Venkateswara, H.; Panchanathan, S. Domain Adaptation in Computer Vision with Deep Learning; Springer Nature: Cham, Switzerland, 2020; ISBN 9783030455293. [Google Scholar]
- Venkataramani, R.; Ravishankar, H.; Anamandra, S. Towards Continuous Domain Adaptation For Medical Imaging. In Proceedings of the 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), Venice, Italy, 8–11 April 2019; pp. 443–446. [Google Scholar]
- Morra, L.; Delsanto, S.; Correale, L. Artificial Intelligence in Medical Imaging: From Theory to Clinical Practice; CRC Press: Boca raton, FL, USA, 2019; ISBN 9781000753080. [Google Scholar]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’Amico, N.C.; Sardanelli, F. AI Applications to Medical Images: From Machine Learning to Deep Learning. Phys. Med. 2021, 83, 9–24. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic Fairness in Artificial Intelligence for Medicine and Healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef]
- Kumar, Y.; Gupta, S.; Singla, R.; Hu, Y.-C. A Systematic Review of Artificial Intelligence Techniques in Cancer Prediction and Diagnosis. Arch. Comput. Methods Eng. 2022, 29, 2043–2070. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Zhang, X.-Y.; Cheng, Y.-T.; Li, B.; Teng, X.-Z.; Zhang, J.; Lam, S.; Zhou, T.; Ma, Z.-R.; Sheng, J.-B.; et al. Artificial Intelligence-Driven Radiomics Study in Cancer: The Role of Feature Engineering and Modeling. Mil. Med. Res. 2023, 10, 22. [Google Scholar] [CrossRef]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Qureshi, T.A.; Lynch, C.; Azab, L.; Xie, Y.; Gaddam, S.; Pandol, S.J.; Li, D. Morphology-Guided Deep Learning Framework for Segmentation of Pancreas in Computed Tomography Images. J. Med. Imaging 2022, 9, 024002. [Google Scholar] [CrossRef]
- Babic, A.; Rosenthal, M.H.; Sundaresan, T.K.; Khalaf, N.; Lee, V.; Brais, L.K.; Loftus, M.; Caplan, L.; Denning, S.; Gurung, A.; et al. Adipose Tissue and Skeletal Muscle Wasting Precede Clinical Diagnosis of Pancreatic Cancer. Nat. Commun. 2023, 14, 4317. [Google Scholar] [CrossRef]
- Chen, W.; Butler, R.K.; Zhou, Y.; Parker, R.A.; Jeon, C.Y.; Wu, B.U. Prediction of Pancreatic Cancer Based on Imaging Features in Patients With Duct Abnormalities. Pancreas 2020, 49, 413–419. [Google Scholar] [CrossRef]
- Kang, M.; Ko, E.; Mersha, T.B. A Roadmap for Multi-Omics Data Integration Using Deep Learning. Brief. Bioinform. 2022, 23, bbab454. [Google Scholar] [CrossRef]
- Gillies, R.J.; Schabath, M.B. Radiomics Improves Cancer Screening and Early Detection. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2556–2567. [Google Scholar] [CrossRef]
- Walsh, T. Android Dreams: The Past, Present and Future of Artificial Intelligence; Oxford University Press: Oxford, UK, 2017; ISBN 9781849048712. [Google Scholar]
- Aier, I.; Semwal, R.; Sharma, A.; Varadwaj, P.K. A Systematic Assessment of Statistics, Risk Factors, and Underlying Features Involved in Pancreatic Cancer. Cancer Epidemiol. 2019, 58, 104–110. [Google Scholar] [CrossRef]
- Juiz, N.A.; Iovanna, J.; Dusetti, N. Pancreatic Cancer Heterogeneity Can Be Explained Beyond the Genome. Front. Oncol. 2019, 9, 246. [Google Scholar] [CrossRef]
- Yin, J.; Ngiam, K.Y.; Teo, H.H. Role of Artificial Intelligence Applications in Real-Life Clinical Practice: Systematic Review. J. Med. Internet Res. 2021, 23, e25759. [Google Scholar] [CrossRef]
- Lin, D.; Xiong, J.; Liu, C.; Zhao, L.; Li, Z.; Yu, S.; Wu, X.; Ge, Z.; Hu, X.; Wang, B.; et al. Application of Comprehensive Artificial Intelligence Retinal Expert (CARE) System: A National Real-World Evidence Study. Lancet Digit Health 2021, 3, e486–e495. [Google Scholar] [CrossRef]
- Choudhury, A.; Asan, O. Role of Artificial Intelligence in Patient Safety Outcomes: Systematic Literature Review. JMIR Med. Inform. 2020, 8, e18599. [Google Scholar] [CrossRef]
- Zech, J.R.; Santomartino, S.M.; Yi, P.H. Artificial Intelligence (AI) for Fracture Diagnosis: An Overview of Current Products and Considerations for Clinical Adoption, From the AJR Special Series on AI Applications. AJR Am. J. Roentgenol. 2022, 219, 869–878. [Google Scholar] [CrossRef]
- Sheikhalishahi, S.; Bhattacharyya, A.; Celi, L.A.; Osmani, V. An Interpretable Deep Learning Model for Time-Series Electronic Health Records: Case Study of Delirium Prediction in Critical Care. Artif. Intell. Med. 2023, 144, 102659. [Google Scholar] [CrossRef]
- Kim, C.; Yang, Z.; Park, S.H.; Hwang, S.H.; Oh, Y.-W.; Kang, E.-Y.; Yong, H.S. Multicentre External Validation of a Commercial Artificial Intelligence Software to Analyse Chest Radiographs in Health Screening Environments with Low Disease Prevalence. Eur. Radiol. 2023, 33, 3501–3509. [Google Scholar] [CrossRef]
- Kearney, V.; Chan, J.W.; Valdes, G.; Solberg, T.D.; Yom, S.S. The Application of Artificial Intelligence in the IMRT Planning Process for Head and Neck Cancer. Oral Oncol. 2018, 87, 111–116. [Google Scholar] [CrossRef]
- Chan, H.-P.; Samala, R.K.; Hadjiiski, L.M. CAD and AI for Breast Cancer—Recent Development and Challenges. BJR Suppl. 2020, 93, 20190580. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Hong, J.C.; Zheng, D. Artificial Intelligence in Radiotherapy Treatment Planning: Present and Future. Technol. Cancer Res. Treat. 2019, 18, 1533033819873922. [Google Scholar] [CrossRef]
- Tenenbaum, J.M.; Shrager, J. Cancer: A Computational Disease That AI Can Cure. AI Mag. 2011, 32, 14–26. [Google Scholar] [CrossRef][Green Version]
- Quero, G.; Mascagni, P.; Kolbinger, F.R.; Fiorillo, C.; De Sio, D.; Longo, F.; Schena, C.A.; Laterza, V.; Rosa, F.; Menghi, R.; et al. Artificial Intelligence in Colorectal Cancer Surgery: Present and Future Perspectives. Cancers 2022, 14, 3803. [Google Scholar] [CrossRef]
- Stanzione, A.; Verde, F.; Romeo, V.; Boccadifuoco, F.; Mainenti, P.P.; Maurea, S. Radiomics and Machine Learning Applications in Rectal Cancer: Current Update and Future Perspectives. World J. Gastroenterol. 2021, 27, 5306–5321. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, H.; Morris, E.D.; Glide-Hurst, C.K.; Pai, S.; Traverso, A.; Wee, L.; Hadzic, I.; Lønne, P.-I.; Shen, C.; et al. Artificial Intelligence in Radiation Therapy. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 158–181. [Google Scholar] [CrossRef]
- Huynh, E.; Hosny, A.; Guthier, C.; Bitterman, D.S.; Petit, S.F.; Haas-Kogan, D.A.; Kann, B.; Aerts, H.J.W.L.; Mak, R.H. Artificial Intelligence in Radiation Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 771–781. [Google Scholar] [CrossRef]
- Klein-Brill, A.; Amar-Farkash, S.; Lawrence, G.; Collisson, E.A.; Aran, D. Comparison of FOLFIRINOX vs Gemcitabine Plus Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma. JAMA Netw. Open 2022, 5, e2216199. [Google Scholar] [CrossRef]
- Li, C.; Qin, Y.; Zhang, W.-H.; Jiang, H.; Song, B.; Bashir, M.R.; Xu, H.; Duan, T.; Fang, M.; Zhong, L.; et al. Deep Learning-Based AI Model for Signet-Ring Cell Carcinoma Diagnosis and Chemotherapy Response Prediction in Gastric Cancer. Med. Phys. 2022, 49, 1535–1546. [Google Scholar] [CrossRef]
- Dercle, L.; McGale, J.; Sun, S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Mokrane, F.-Z.; Farwell, M.; Ammari, S.; Schoder, H.; et al. Artificial Intelligence and Radiomics: Fundamentals, Applications, and Challenges in Immunotherapy. J. Immunother. Cancer 2022, 10, e005292. [Google Scholar] [CrossRef]
- Kesharwani, R.K.; Misra, K. Biotechnology in the Modern Medicinal System: Advances in Gene Therapy, Immunotherapy, and Targeted Drug Delivery; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781000290592. [Google Scholar]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, F.; Xu, L.; Yue, L.; Kon, O.L.; Zhu, Y.; Guo, T. High-Throughput Proteomics and AI for Cancer Biomarker Discovery. Adv. Drug Deliv. Rev. 2021, 176, 113844. [Google Scholar] [CrossRef]
- Ledesma, D.; Symes, S.; Richards, S. Advancements within Modern Machine Learning Methodology: Impacts and Prospects in Biomarker Discovery. Curr. Med. Chem. 2021, 28, 6512–6531. [Google Scholar] [CrossRef]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar]
- Chen, R.J.; Lu, M.Y.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Noor, Z.; Shaban, M.; Shady, M.; Williams, M.; Joo, B.; et al. Pan-Cancer Integrative Histology-Genomic Analysis via Multimodal Deep Learning. Cancer Cell 2022, 40, 865–878.e6. [Google Scholar] [CrossRef]
- Karar, M.E.; El-Fishawy, N.; Radad, M. Automated Classification of Urine Biomarkers to Diagnose Pancreatic Cancer Using 1-D Convolutional Neural Networks. J. Biol. Eng. 2023, 17, 28. [Google Scholar] [CrossRef]
- Mikdadi, D.; O’Connell, K.A.; Meacham, P.J.; Dugan, M.A.; Ojiere, M.O.; Carlson, T.B.; Klenk, J.A. Applications of Artificial Intelligence (AI) in Ovarian Cancer, Pancreatic Cancer, and Image Biomarker Discovery. Cancer Biomark. 2022, 33, 173–184. [Google Scholar] [CrossRef]
- Singhi, A.D.; Wood, L.D. Early Detection of Pancreatic Cancer Using DNA-Based Molecular Approaches. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 457–468. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early Detection of Pancreatic Cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Yao, J.; Cao, K.; Hou, Y.; Zhou, J.; Xia, Y.; Nogues, I.; Song, Q.; Jiang, H.; Ye, X.; Lu, J.; et al. Deep Learning for Fully Automated Prediction of Overall Survival in Patients Undergoing Resection for Pancreatic Cancer: A Retrospective Multicenter Study. Ann. Surg. 2023, 278, e68–e79. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Z.; Keles, E.; Yazici, C.; Tirkes, T.; Bagci, U. A Review of Deep Learning and Radiomics Approaches for Pancreatic Cancer Diagnosis from Medical Imaging. Curr. Opin. Gastroenterol. 2023, 39, 436–447. [Google Scholar] [CrossRef]
- Cheerla, A.; Gevaert, O. Deep Learning with Multimodal Representation for Pancancer Prognosis Prediction. Bioinformatics 2019, 35, i446–i454. [Google Scholar] [CrossRef]
- Tong, T.; Gu, J.; Xu, D.; Song, L.; Zhao, Q.; Cheng, F.; Yuan, Z.; Tian, S.; Yang, X.; Tian, J.; et al. Deep Learning Radiomics Based on Contrast-Enhanced Ultrasound Images for Assisted Diagnosis of Pancreatic Ductal Adenocarcinoma and Chronic Pancreatitis. BMC Med. 2022, 20, 74. [Google Scholar] [CrossRef]
- Shao, Y.; Dang, Y.; Cheng, Y.; Gui, Y.; Chen, X.; Chen, T.; Zeng, Y.; Tan, L.; Zhang, J.; Xiao, M.; et al. Predicting the Efficacy of Neoadjuvant Chemotherapy for Pancreatic Cancer Using Deep Learning of Contrast-Enhanced Ultrasound Videos. Diagnostics 2023, 13, 2183. [Google Scholar] [CrossRef]
- Iwasa, Y.; Iwashita, T.; Takeuchi, Y.; Ichikawa, H.; Mita, N.; Uemura, S.; Shimizu, M.; Kuo, Y.-T.; Wang, H.-P.; Hara, T. Automatic Segmentation of Pancreatic Tumors Using Deep Learning on a Video Image of Contrast-Enhanced Endoscopic Ultrasound. J. Clin. Med. Res. 2021, 10, 3589. [Google Scholar] [CrossRef]
- Tang, A.; Tian, L.; Gao, K.; Liu, R.; Hu, S.; Liu, J.; Xu, J.; Fu, T.; Zhang, Z.; Wang, W.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasound (CH-EUS) MASTER: A Novel Deep Learning-Based System in Pancreatic Mass Diagnosis. Cancer Med. 2023, 12, 7962–7973. [Google Scholar] [CrossRef]
- Ali, O.; Abdelbaki, W.; Shrestha, A.; Elbasi, E.; Alryalat, M.A.A.; Dwivedi, Y.K. A Systematic Literature Review of Artificial Intelligence in the Healthcare Sector: Benefits, Challenges, Methodologies, and Functionalities. J. Innov. Knowl. 2023, 8, 100333. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key Challenges for Delivering Clinical Impact with Artificial Intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Koh, D.-M.; Papanikolaou, N.; Bick, U.; Illing, R.; Kahn, C.E., Jr.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S.K.; et al. Artificial Intelligence and Machine Learning in Cancer Imaging. Commun. Med. 2022, 2, 133. [Google Scholar] [CrossRef]
- Petersson, L.; Larsson, I.; Nygren, J.M.; Nilsen, P.; Neher, M.; Reed, J.E.; Tyskbo, D.; Svedberg, P. Challenges to Implementing Artificial Intelligence in Healthcare: A Qualitative Interview Study with Healthcare Leaders in Sweden. BMC Health Serv. Res. 2022, 22, 850. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic Cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of Adjuvant Gemcitabine and Capecitabine with Gemcitabine Monotherapy in Patients with Resected Pancreatic Cancer (ESPAC-4): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Tempero, M.A.; Pelzer, U.; O’Reilly, E.M.; Winter, J.; Oh, D.-Y.; Li, C.-P.; Tortora, G.; Chang, H.-M.; Lopez, C.D.; Bekaii-Saab, T.; et al. Adjuvant Nab-Paclitaxel + Gemcitabine in Resected Pancreatic Ductal Adenocarcinoma: Results From a Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 2007–2019. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Wang, H.; Egger, M.E.; Tzeng, C.-W.D.; Prakash, L.R.; Maitra, A.; Varadhachary, G.R.; Shroff, R.; Javle, M.; Fogelman, D.; et al. Association of Clinical Factors With a Major Pathologic Response Following Preoperative Therapy for Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2017, 152, 1048–1056. [Google Scholar] [CrossRef]
- Petersen, G.M. Familial Pancreatic Cancer. Semin. Oncol. 2016, 43, 548–553. [Google Scholar] [CrossRef]
- Overbeek, K.A.; Cahen, D.L.; Canto, M.I.; Bruno, M.J. Surveillance for Neoplasia in the Pancreas. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 971–986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matthaei, H.; Schulick, R.D.; Hruban, R.H.; Maitra, A. Cystic Precursors to Invasive Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Falcomatà, C.; Bärthel, S.; Schneider, G.; Rad, R.; Schmidt-Supprian, M.; Saur, D. Context-Specific Determinants of the Immunosuppressive Tumor Microenvironment in Pancreatic Cancer. Cancer Discov. 2023, 13, 278–297. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Dougan, S.K.; Aguirre, A.J.; Maitra, A. Translational Advances in Pancreatic Ductal Adenocarcinoma Therapy. Nat. Cancer 2022, 3, 272–286. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Gori, S.S.; Tsukamoto, T.; Rais, R.; Slusher, B.S. Clinical Development of Metabolic Inhibitors for Oncology. J. Clin. Investig. 2022, 132, e148550. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic Cancer: Advances and Challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Suman, G.; Patra, A.; Korfiatis, P.; Majumder, S.; Chari, S.T.; Truty, M.J.; Fletcher, J.G.; Goenka, A.H. Quality Gaps in Public Pancreas Imaging Datasets: Implications & Challenges for AI Applications. Pancreatology 2021, 21, 1001–1008. [Google Scholar]
- Parikh, R.B.; Teeple, S.; Navathe, A.S. Addressing Bias in Artificial Intelligence in Health Care. JAMA 2019, 322, 2377–2378. [Google Scholar] [CrossRef]
- Gichoya, J.W.; Banerjee, I.; Bhimireddy, A.R.; Burns, J.L.; Celi, L.A.; Chen, L.-C.; Correa, R.; Dullerud, N.; Ghassemi, M.; Huang, S.-C.; et al. AI Recognition of Patient Race in Medical Imaging: A Modelling Study. Lancet Digit. Health 2022, 4, e406–e414. [Google Scholar] [CrossRef]
- Seyyed-Kalantari, L.; Zhang, H.; McDermott, M.B.A.; Chen, I.Y.; Ghassemi, M. Underdiagnosis Bias of Artificial Intelligence Algorithms Applied to Chest Radiographs in under-Served Patient Populations. Nat. Med. 2021, 27, 2176–2182. [Google Scholar] [CrossRef]
- Haibe-Kains, B.; Adam, G.A.; Hosny, A.; Khodakarami, F.; Massive Analysis Quality Control (MAQC) Society Board of Directors; Waldron, L.; Wang, B.; McIntosh, C.; Goldenberg, A.; Kundaje, A.; et al. Transparency and Reproducibility in Artificial Intelligence. Nature 2020, 586, E14–E16. [Google Scholar] [CrossRef]
- Pianykh, O.S.; Langs, G.; Dewey, M.; Enzmann, D.R.; Herold, C.J.; Schoenberg, S.O.; Brink, J.A. Continuous Learning AI in Radiology: Implementation Principles and Early Applications. Radiology 2020, 297, 6–14. [Google Scholar] [CrossRef]
- Tripathi, S.; Augustin, A.; Dako, F.; Kim, E. Turing Test-Inspired Method for Analysis of Biases Prevalent in Artificial Intelligence-Based Medical Imaging. AI Ethics 2022, 3, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.P.; Neri, E. Artificial Intelligence in Radiology-Ethical Considerations. Diagnostics 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Markus, A.F.; Kors, J.A.; Rijnbeek, P.R. The Role of Explainability in Creating Trustworthy Artificial Intelligence for Health Care: A Comprehensive Survey of the Terminology, Design Choices, and Evaluation Strategies. J. Biomed. Inform. 2021, 113, 103655. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Abuhmed, T.; El-Sappagh, S.; Muhammad, K.; Alonso-Moral, J.M.; Confalonieri, R.; Guidotti, R.; Del Ser, J.; Díaz-Rodríguez, N.; Herrera, F. Explainable Artificial Intelligence (XAI): What We Know and What Is Left to Attain Trustworthy Artificial Intelligence. Inf. Fusion 2023, 99, 101805. [Google Scholar] [CrossRef]
- Chaddad, A.; Peng, J.; Xu, J.; Bouridane, A. Survey of Explainable AI Techniques in Healthcare. Sensors 2023, 23, 634. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Chakraborty, P.; Kwon, B.C.; Dhurandhar, A.; Ghalwash, M.; Suarez Saiz, F.J.; Ng, K.; Sow, D.; Varshney, K.R.; Meyer, P. Human-Centered Explainability for Life Sciences, Healthcare, and Medical Informatics. Patterns 2022, 3, 100493. [Google Scholar] [CrossRef]
- Rajkomar, A.; Hardt, M.; Howell, M.D.; Corrado, G.; Chin, M.H. Ensuring Fairness in Machine Learning to Advance Health Equity. Ann. Intern. Med. 2018, 169, 866–872. [Google Scholar] [CrossRef]
- Cirillo, D.; Catuara-Solarz, S.; Morey, C.; Guney, E.; Subirats, L.; Mellino, S.; Gigante, A.; Valencia, A.; Rementeria, M.J.; Chadha, A.S.; et al. Sex and Gender Differences and Biases in Artificial Intelligence for Biomedicine and Healthcare. NPJ Digit. Med. 2020, 3, 81. [Google Scholar] [CrossRef]
- Tripathi, S.; Gabriel, K.; Dheer, S.; Parajuli, A.; Augustin, A.I.; Elahi, A.; Awan, O.; Dako, F. Dataset Development Review. J. Am. Coll. Radiol. 2023, 20, 836–841. [Google Scholar] [CrossRef]
- Murdoch, B. Privacy and Artificial Intelligence: Challenges for Protecting Health Information in a New Era. BMC Med. Ethics 2021, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Allan, S.; Coghlan, S.; Cooper, P. A Governance Model for the Application of AI in Health Care. J. Am. Med. Inform. Assoc. 2019, 27, 491–497. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.-M. Ethics and Governance of Trustworthy Medical Artificial Intelligence. BMC Med. Inform. Decis. Mak. 2023, 23, 7. [Google Scholar] [CrossRef]
- Rigby, M.J. Ethical Dimensions of Using Artificial Intelligence in Health Care. AMA J. Ethics 2019, 21, 121–124. [Google Scholar]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef] [PubMed]
- DeCamp, M.; Lindvall, C. Latent Bias and the Implementation of Artificial Intelligence in Medicine. J. Am. Med. Inform. Assoc. 2020, 27, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Topol, E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again; Basic Books: New York, NY, USA, 2019; ISBN 9781541644649. [Google Scholar]
- Straw, I. The Automation of Bias in Medical Artificial Intelligence (AI): Decoding the Past to Create a Better Future. Artif. Intell. Med. 2020, 110, 101965. [Google Scholar] [CrossRef]
- Holmes, W.; Porayska-Pomsta, K. The Ethics of Artificial Intelligence in Education: Practices, Challenges, and Debates; Taylor & Francis: Abingdon, UK, 2022; ISBN 9781000620702. [Google Scholar]
- Jobin, A.; Ienca, M.; Vayena, E. The Global Landscape of AI Ethics Guidelines. Nat. Mach. Intell. 2019, 1, 389–399. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, A.; Khasawneh, H.; Korfiatis, P.; Rajamohan, N.; Suman, G.; Majumder, S.; Panda, A.; Johnson, M.P.; Larson, N.B.; et al. Radiomics-Based Machine-Learning Models Can Detect Pancreatic Cancer on Prediagnostic Computed Tomography Scans at a Substantial Lead Time Before Clinical Diagnosis. Gastroenterology 2022, 163, 1435–1446.e3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, S.; Tabari, A.; Mansur, A.; Dabbara, H.; Bridge, C.P.; Daye, D. From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer. Diagnostics 2024, 14, 174. https://doi.org/10.3390/diagnostics14020174
Tripathi S, Tabari A, Mansur A, Dabbara H, Bridge CP, Daye D. From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer. Diagnostics. 2024; 14(2):174. https://doi.org/10.3390/diagnostics14020174
Chicago/Turabian StyleTripathi, Satvik, Azadeh Tabari, Arian Mansur, Harika Dabbara, Christopher P. Bridge, and Dania Daye. 2024. "From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer" Diagnostics 14, no. 2: 174. https://doi.org/10.3390/diagnostics14020174
APA StyleTripathi, S., Tabari, A., Mansur, A., Dabbara, H., Bridge, C. P., & Daye, D. (2024). From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer. Diagnostics, 14(2), 174. https://doi.org/10.3390/diagnostics14020174







