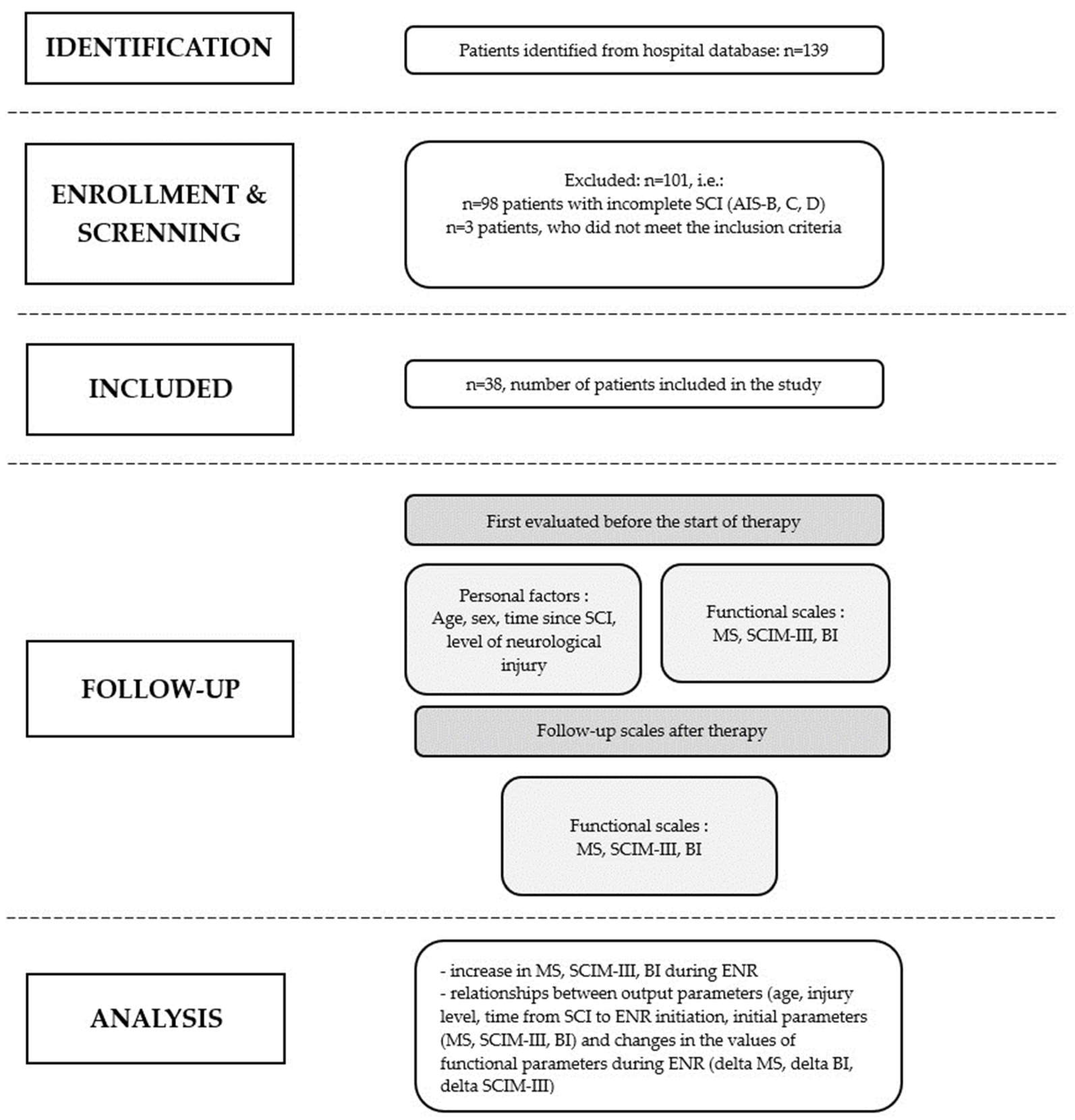
Can the Initial Parameters of Functional Scales Predict Recovery in Patients with Complete Spinal Cord Injury? A Retrospective Cohort Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Setting and Ethics Statement
2.2. Study Population
- -
- Patients with complete quadriplegia (AIS-A);
- -
- Patients after surgery due to a SCI (decompression and stabilization);
- -
- Patients admitted directly from the acute phase center (spinal surgery unit or intensive care unit) to the ward providing ENR.
- -
- Patients who suffered peripheral nerve damage in the upper limbs along with SCI;
- -
- Patients who suffered other severe injuries of the upper limbs or their amputation along with SCI;
- -
- Patients who had suffered a central nervous system injury in the past resulting in paresis (partial loss of motor function) or plegia (complete loss of motor function);
- -
- Patients who had already had paresis or plegia of limbs in the course of chronic diseases prior to their SCI.
2.3. Patient Selection and Data Collection
2.4. Early Neurological Rehabilitation
- -
- Urological management: regulation of urination, intermittent catheterization/cystostomy, learning appropriate behaviors, pharmacotherapy and prevention/treatment of urinary tract infections;
- -
- Treatment of concomitant spasticity: physiotherapy, pharmacotherapy and learning appropriate behaviors;
- -
- Prevention/treatment of cardiorespiratory complications: respiratory infections, orthostatic hypotonia, autonomic dysreflexia and venous thromboembolism (bronchial toilet, physiotherapy, pharmacotherapy and learning appropriate behaviors);
- -
- Prevention/treatment of intestinal complications, management of bowel movements: dietary treatment, physiotherapy, pharmacological management and learning appropriate behaviors;
- -
- Anti-bedsore prophylaxis: changing body position, using pressure-relieving devices, i.e., mattress, wheelchair cushion and learning appropriate behaviors;
- -
- Pain management: pharmacotherapy and physiotherapy;
- -
- Others, e.g., treatment of concomitant diseases (according to patients’ needs).
2.5. Research Tool and Outcome Measures
- Self-service: feeding and bathing (upper and lower body half), dressing (upper and lower body), hygiene, grooming and caring for the appearance;
- Respiration and sphincter management: assisted or unassisted breathing, urination and the assessment of residual urine volume, regularity/irregularity of bowel movements, assisted bowel movements and use of the toilet;
- Mobility: changing position in bed and preventing pressure sores, independent/assisted transfer from wheelchair to bed and vice versa, and independent/assisted transfer from wheelchair to bathtub;
- Locomotion: inability to move, assisted transfer, e.g., via wheelchair, rehabilitation devices, depending on the distance (>10 m, 10–100 m, <100 m), the possibility of walking up the stairs, and transfer from wheelchair to car and from the floor to wheelchair.
- -
- Initial parameters: age, time elapsed from SCI to ENR initiation (corresponding to the length of the acute stay), the level of neurological injury and initial (before the start of ENR) parametric values of the MS, BI and SCIM scales
- -
- Final parameters (after ending ENR): parametric values of the MS, BI and SCIM scales;
- -
- Change of functional parameters: increase in MS, BI and SCIM functional parameters in the course of ENR;
- -
- Correlations between the initial values of scales (MS, BI and SCIM), age, time since SCI and the level of neurological injury, and changes in functional parameters in the course of ENR.
2.6. Statistical Methods
3. Results
Participants
4. Discussion
4.1. Functional Parameters
4.1.1. Motor Score from ASIA Impairment Scale
4.1.2. The Barthel Index
4.1.3. Spinal Cord Independence Measure
4.2. Other Parameters
4.3. Conversion from Complete to Incomplete SCI
5. Strengths and Limitations of This Study
5.1. Strengths of This Study
5.2. Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability from the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Tarnacka, B.; Korczyński, B.; Frasuńska, J. Long-term complications following spinal cord injury and aging. Adv. Psychiatry Neurol./Postępy Psychiatr. I Neurol. 2020, 29, 234–245. [Google Scholar] [CrossRef]
- Kirshblum, S.; Snider, B.; Eren, F.; Guest, J. Characterizing Natural Recovery after Traumatic Spinal Cord Injury. J. Neurotrauma 2021, 38, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.J.; Ditunno, J.F.; Donovan, W.H.; Maynard, F. Neurologic recovery after traumatic spinal cord injury: Data from the model spinal cord injury systems. Arch. Phys. Med. Rehabil. 1999, 80, 1391–1396. [Google Scholar] [CrossRef]
- Chay, W.; Kirshblum, S. Predicting Outcomes After Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.; Botticello, A.; Lammertse, D.P.; Marino, R.J.; Chiodo, A.E.; Jha, A. The impact of sacral sensory in motor complete spinal cord injury. Arch. Phys. Med. Rehabil. 2011, 92, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; Noonan, V.K.; Smith, D.E.; Wing, P.C.; Dvorak, M.F.; Kwon, B. Motor Recovery, Functional Status, and Health-Related Quality of Life in Patients with Complete Spinal Cord Injuries. Spine 2005, 30, 2200–2207, Erratum in Spine. 2006, 31, 1289. [Google Scholar] [CrossRef]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.; Wolfe, D.L.; Spinal Cord Injury Rehabilitation Evidence Scire Research Team. The health and life priorities of individuals with spinal cord injury: A systematic review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef]
- Al-Habib, A.F.; Attabib, N.; Ball, J.; Bajammal, S.; Casha, S.; Hurlbert, R.J. Clinical predictors of recovery after blunt spinal cord trauma: Systematic review. J. Neurotrauma 2011, 28, 1431–1443. [Google Scholar] [CrossRef]
- Mputu Mputu, P.; Beauséjour, M.; Richard-Denis, A.; Mac-Thiong, J.M. Early Predictors of Neurological Outcomes after Traumatic Spinal Cord Injury: A Systematic Review and Proposal of a Conceptual Framework. Am. J. Phys. Med. Rehabil. 2021, 100, 700–711. [Google Scholar] [CrossRef]
- Yang, Z.; Apiliogullari, S.; Fu, Y.; Istanbouli, A.; Kaur, S.; Jabbal, I.S.; Moghieb, A.; Irfan, Z.; Patterson, R.L.; Kurup, M.; et al. Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury. Diagnostics 2023, 13, 1814. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Huber, J. Comparing Parameters of Motor Potentials Recordings Evoked Transcranially with Neuroimaging Results in Patients with Incomplete Spinal Cord Injury: Assessment and Diagnostic Capabilities. Biomedicines 2023, 11, 2602. [Google Scholar] [CrossRef] [PubMed]
- Rau, Y.; Thietje, R.; Schulz, A.P.; Auerswald, M.; Böthig, R.; Hirschfeld, S. The Correlation between Cervical Fusion Length and Functional Outcomes in Patients with Traumatic Spinal Cord Damage—A Registry-Based Cohort Study. J. Clin. Med. 2022, 11, 5867. [Google Scholar] [CrossRef] [PubMed]
- Na, B.-R.; Seo, H.-Y. Adult Spinal Cord Injury without Major Bone Injury: Effects of Surgical Decompression and Predictors of Neurological Outcomes in American Spinal Injury Association Impairment Scale A, B, or C. J. Clin. Med. 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury; 2002 Revision; American Spinal Injury Association: Chicago, IL, USA, 2002. [Google Scholar]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL index: A reliability study. Int. Disabil. Stud. 1988, 2, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Itzkovich, M.; Gelernter, I.; Biering-Sorensen, F.; Weeks, C.; Laramee, M.T.; Craven, B.C.; Tonack, M.; Hitzig, S.L.; Glaser, E.; Zeilig, G.; et al. The Spinal Cord Independence Measure (SCIM) version III: Reliability and validity in a multi-center international study. Disabil. Rehabil. 2007, 29, 1926–1933. [Google Scholar] [CrossRef]
- Burns, A.S.; Marino, R.J.; Kalsi-Ryan, S.; Middleton, J.W.; Tetreault, L.A.; Dettori, J.R.; Mihalovich, K.E.; Fehlings, M.G. Type and Timing of Rehabilitation Following Acute and Subacute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7 (Suppl. S3), 175–194. [Google Scholar] [CrossRef]
- Wincek, A.; Huber, J.; Leszczyńska, K.; Fortuna, W.; Okurowski, S.; Tabakow, P. Results of a long-term uniform system of neurorehabilitation in patients with incomplete thoracic spinal cord injury. Ann. Agric. Environ. Med. 2022, 29, 94–102. [Google Scholar] [CrossRef]
- Walker, J.R.; Detloff, M.R. Plasticity in Cervical Motor Circuits following Spinal Cord Injury and Rehabilitation. Biology 2021, 10, 976. [Google Scholar] [CrossRef]
- Anderson, K.; Aito, S.; Atkins, M.; Biering-Sørensen, F.; Charlifue, S.; Curt, A.; Ditunno, J.; Glass, C.; Marino, R.; Marshall, R.; et al. Functional Recovery Outcome Measures Work Group. Functional recovery measures for spinal cord injury: An evidence-based review for clinical practice and research. J. Spinal Cord Med. 2008, 31, 133–144. [Google Scholar] [CrossRef]
- Dvorak, M.F.; Fisher, C.G.; Hoekema, J.; Boyd, M.; Noonan, V.; Wing, P.C.; Kwon, B.K. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: A long-term follow-up. Spine 2005, 30, 2303–2311, Erratum in Spine 2006, 31, 1289. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Xu, C. Spinal Cord Injury AIS Predictions Using Machine Learning. eNeuro. 2023, 10, ENEURO.0149-22.2022. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Shin, J.J.; Lee, J.H.; Cho, W.H. Prognostic factors for surgical outcome in spinal cord injury associated with ossification of the posterior longitudinal ligament (OPLL). J. Orthop. Surg. Res. 2015, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.J.; Graves, D.E. Metric properties of the ASIA motor score: Subscales improve correlation with functional activities. Arch. Phys. Med. Rehabil. 2004, 85, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, G.; Li, G.; Kalsi-Ryan, S.; Abel, R.; Maier, D.; Kalke, Y.B.; Weidner, N.; Rupp, R.; Schubert, M.; Curt, A.; et al. Segmental motor recovery after cervical spinal cord injury relates to density and integrity of corticospinal tract projections. Nat. Commun. 2023, 14, 723. [Google Scholar] [CrossRef]
- Küçükdeveci, A.A.; Yavuzer, G.; Tennant, A.; Süldür, N.; Sonel, B.; Arasil, T. Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand. J. Rehabil. Med. 2000, 32, 87–92. [Google Scholar] [CrossRef]
- Roth, E.; Davidoff, G.; Haughton, J.; Ardner, M. Functional assessment in spinal cord injury: A comparison between the Modified Bathel Index and the “adapted” Functional Independence Measure. Clin. Rehabil. 1990, 4, 277–285. [Google Scholar] [CrossRef]
- Unai, K.; Uemura, O.; Takemura, R.; Kawakami, M.; Liu, M. Association Between SCIM III Total Scores and Individual Item Scores to Predict Independence with ADLs in Persons with Spinal Cord Injury. Arch. Rehabil. Res. Clin. Transl. 2019, 1, 100029. [Google Scholar] [CrossRef]
- Richard-Denis, A.; Chatta, R.; Thompson, C.; Mac-Thiong, J.M. Patterns and predictors of functional recovery from the subacute to the chronic phase following a traumatic spinal cord injury: A prospective study. Spinal Cord 2020, 58, 43–52. [Google Scholar] [CrossRef]
- van Hedel, H.J.; Curt, A. Fighting for each segment: Estimating the clinical value of cervical and thoracic segments in SCI. J. Neurotrauma 2006, 23, 1621–1631. [Google Scholar] [CrossRef]
- Wichmann, T.O.; Jensen, M.H.; Kasch, H.; Rasmussen, M.M. Early clinical predictors of functional recovery following traumatic spinal cord injury: A population-based study of 143 patients. Acta Neurochir. 2021, 163, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, B.; Alexander, M.; Mirvis, S.E.; Shanmuganathan, K.; Chesler, D.; Maulucci, C.; Iguchi, M.; Aresco, C.; Blacklock, T. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J. Neurosurg. Spine 2011, 14, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gedde, M.H.; Lilleberg, H.S.; Aßmus, J.; Gilhus, N.E.; Rekand, T. Traumatic vs non-traumatic spinal cord injury: A comparison of primary rehabilitation outcomes and complications during hospitalization. J. Spinal Cord Med. 2019, 42, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Pavese, C.; Kessler, T.M. Prediction of Lower Urinary Tract, Sexual, and Bowel Function, and Autonomic Dysreflexia after Spinal Cord Injury. Biomedicines 2023, 11, 1644. [Google Scholar] [CrossRef]
- Pavese, C.; Bachmann, L.M.; Schubert, M.; Curt, A.; Mehnert, U.; Schneider, M.P.; Scivoletto, G.; FinazziAgrò, E.; Maier, D.; Abel, R.; et al. Bowel Outcome Prediction after Traumatic Spinal Cord Injury: Longitudinal Cohort Study. Neurorehabilit. Neural Repair 2019, 33, 902–910. [Google Scholar] [CrossRef]
- Abedi, A.; Montero, S.; Ojeda, L.M.; Gaburak, P.; Kohli, P.; Abedi, A.; Chapman, D.; Ginsberg, D.; Kreydin, E. Resilience as an Independent Predictor of Bowel Related Quality of Life after Spinal Cord Injury. J. Neurotrauma, 2023; epub ahead of print. [Google Scholar] [CrossRef]
| Mean ± SD or N (%) | Median (IQR) | Range | |
|---|---|---|---|
| Age, years | 39.5 ± 17.2 | 33.5 (25.0 to 48.0) | from 17.0 to 78.0 |
| Women | 4 (10.5%) | ||
| Men | 34 (89.5%) | ||
| Time from SCI to ENR initiation, weeks * | 7.2 ± 5.5 | 5.5 (4.0 to 8.0) | from 1.0 to 27.0 |
| Level of injury ** | |||
| C4 | 12 (31.6) | ||
| C5 | 7 (18.4) | ||
| C6 | 14 (36.8) | ||
| C7 | 4 (10.5) | ||
| C8 | 1 (2.6) | ||
| Duration of ENR, weeks | 14.8 ± 3.5 | 16.0 (15.0 to 14.0) | from 3.0 to 26.0 |
| Functional Parameters | Initial | Final | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Range | Mean ± SD | Median (IQR) | Range | |
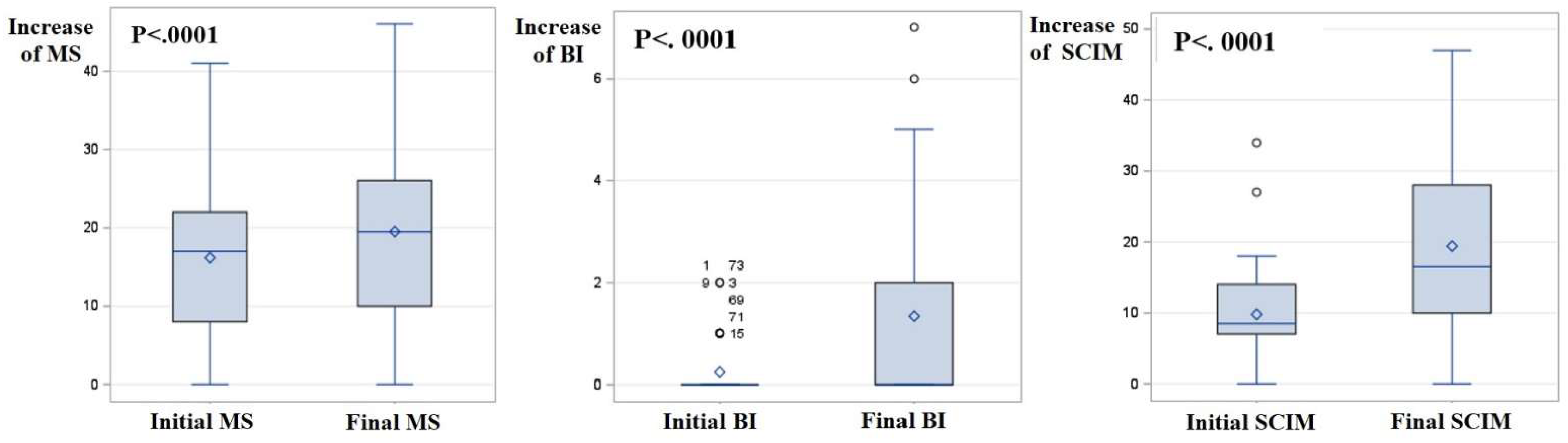
| MS of cervical SCI (range) | 16.2 ± 9.9 | 17.0 (8.0 to 22.0) | 0.0 to 41.0 | 19.5 ± 11.2 | 19.5 (10.0 to 26.0) | 0.0 to 46.0 |
| BI | 0.2 ± 0.5 | 0.0 (0.0 to 0.0) | 0.0 to 2.0 | 1.3 ± 2.0 | 0.0 (0.0 to 2.0) | 0.0 to 7.0 |
| SCIM | 9.8 ± 7.2 | 8.5 (7.0 to 14.0) | 0.0 to 34.0 | 19.4 ± 12.3 | 16.5 (10.0 to 28.0) | 0.0 to 47.0 |
| Mean ± SD | Median (IQR) | Range | p-Value | |
|---|---|---|---|---|
| MS of cervical SCI | 3.4 ± 3.6 | 2.0 (0.0 to 5.0) | 0.0 to 15.0 | <0.0001 |
| BI | 1.1 ± 1.7 | 0.0 (0.0 to 2.0) | 0.0 to 6.0 | <0.0001 |
| SCIM | 9.6 ± 10.2 | 7.5 (0.0 to 19.0) | 0.0 to 38.0 | <0.0001 |
| Initial Parameters | Increase in MS | Increase in BI | Increase in SCIM | |
|---|---|---|---|---|
| Initial MS | r | +0.20 | +0.65 | +0.36 |
| p | NS | <0.0001 | 0.028 | |
| Initial BI | r | +0.34 | +0.39 | +0.02 |
| p | 0.039 | 0.017 | NS | |
| Initial SCIM | r | +0.10 | +0.38 | −0.02 |
| p | NS | 0.018 | NS | |
| Age, years | r | +0.05 | −0.19 | −0.07 |
| p | NS | NS | NS | |
| Time since SCI *, weeks | r | −0.17 | −0.50 | −0.24 |
| p | NS | 0.002 | NS | |
| Level of SCI ** | r | +0.16 | +0.46 | +0.26 |
| p | NS | 0.004 | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasiak, K.; Frasuńska, J.; Tarnacka, B. Can the Initial Parameters of Functional Scales Predict Recovery in Patients with Complete Spinal Cord Injury? A Retrospective Cohort Study. Diagnostics 2024, 14, 129. https://doi.org/10.3390/diagnostics14020129
Wasiak K, Frasuńska J, Tarnacka B. Can the Initial Parameters of Functional Scales Predict Recovery in Patients with Complete Spinal Cord Injury? A Retrospective Cohort Study. Diagnostics. 2024; 14(2):129. https://doi.org/10.3390/diagnostics14020129
Chicago/Turabian StyleWasiak, Krzysztof, Justyna Frasuńska, and Beata Tarnacka. 2024. "Can the Initial Parameters of Functional Scales Predict Recovery in Patients with Complete Spinal Cord Injury? A Retrospective Cohort Study" Diagnostics 14, no. 2: 129. https://doi.org/10.3390/diagnostics14020129
APA StyleWasiak, K., Frasuńska, J., & Tarnacka, B. (2024). Can the Initial Parameters of Functional Scales Predict Recovery in Patients with Complete Spinal Cord Injury? A Retrospective Cohort Study. Diagnostics, 14(2), 129. https://doi.org/10.3390/diagnostics14020129







