Left Ventricular Diastolic Dysfunction Predicts Global Longitudinal Strain Recovery after Surgical Aortic Valve Replacement
Abstract
1. Introduction
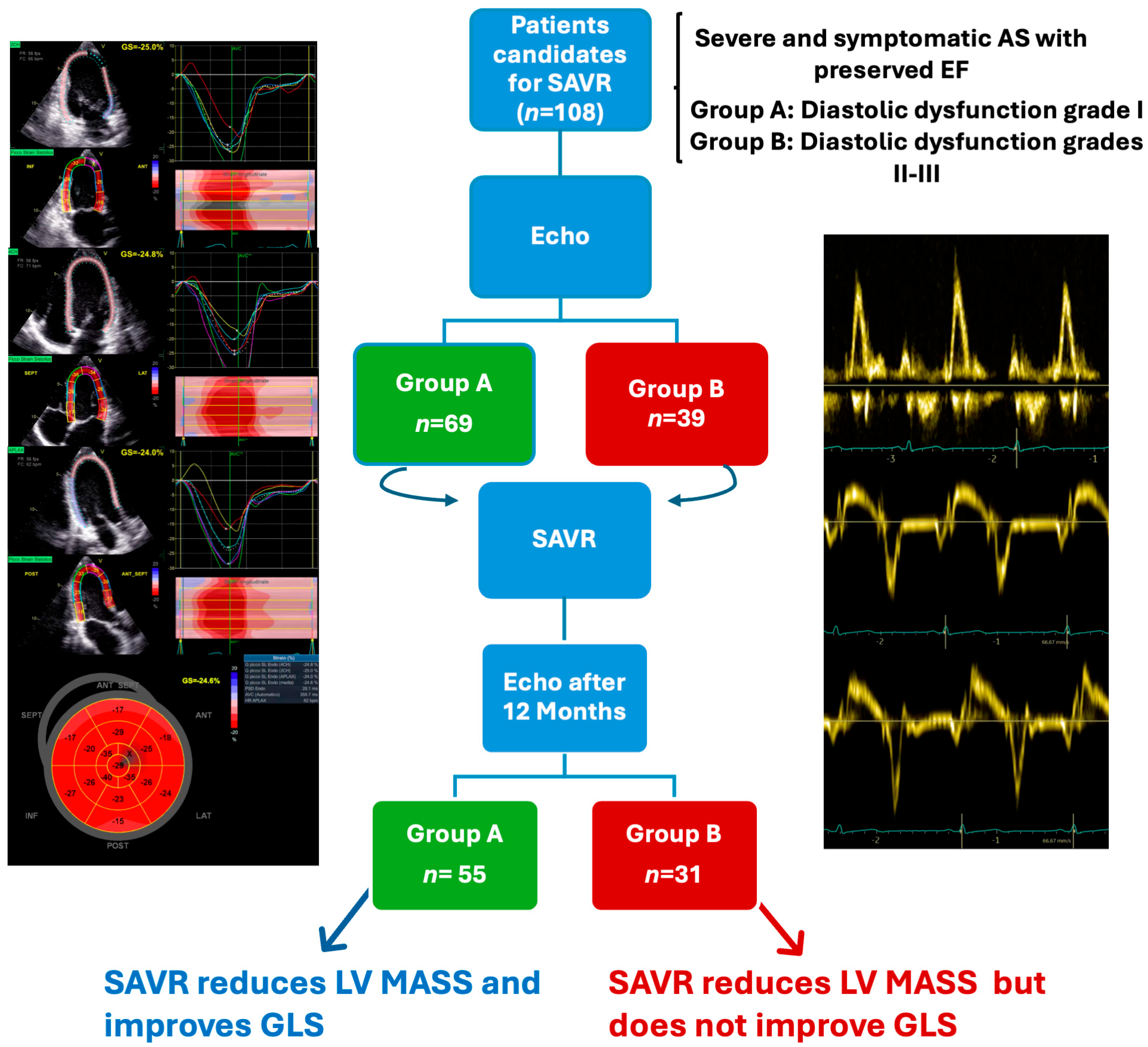
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Evaluation
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Naqvi, S.Y.; Giri, J.; Goldberg, S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am. J. Med. 2017, 130, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.S.; Duggan, J.P.; Trachiotis, G.D.; Antevil, J.L. Epidemiology of Valvular Heart Disease. Surg. Clin. N. Am. 2022, 102, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Chahal, N.; Senior, R. Assessing systolic function in aortic stenosis: The earlier the better? Heart 2020, 106, 1200–1201. [Google Scholar] [CrossRef]
- Delgado, V.; Tops, L.F.; Bommel, R.J.; Van Kley, F.; Van Der Marsan, N.A.; Klautz, R.J.; Versteegh, M.I.M.; Holman, E.R.; Schalij, M.J.; Bax, J.J. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur. Heart J. 2009, 30, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart diseaseDeveloped by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- O’Toole, J.D.; Geiser, E.A.; Sudhakar Reddy, P.; Curtiss, E.I.; Landfair, R.M. Effect of Preoperative Ejection Fraction on Survival and Hemodynamic Improvement Following Aortic Valve Replacement. Circulation 1978, 58, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.S.; Eleid, M.F.; Michelena, H.I.; Scott, C.G.; Suri, R.M.; Schaff, H.V.; Pellikka, P.A. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ. Cardiovasc. Imaging 2015, 8, e002917. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Chandrashekhar, Y. Echocardiography: The New Gold Standard for Imaging Ventricular Function? J. Am. Coll. Cardiol. 2017, 70, 955–957. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Prihadi, E.A.; Antoni, M.L.; Bertini, M.; Ewe, S.H.; Marsan, N.A.; Leung, D.Y.; Delgado, V.; Bax, J.J. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 859–867. [Google Scholar] [CrossRef]
- Lafitte, S.; Perlant, M.; Reant, P.; Serri, K.; Douard, H.; Demaria, A.; Roudaut, R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur. J. Echocardiogr. 2009, 10, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Cosyns, B.; Vannan, M.A. Global longitudinal strain in severe aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Twing, A.H.; Slostad, B.; Anderson, C.; Konda, S.; Groves, E.M.; Kansal, M.M. Improvements in global longitudinal strain after transcatheter aortic valve replacement according to race. Am. J. Cardiovasc. Dis. 2021, 11, 203. [Google Scholar]
- Grund, F.F.; Myhr, K.A.; Visby, L.; Hassager, C.; Mogelvang, R. Impact of surgical aortic valve replacement on global and regional longitudinal strain across four flow gradient patterns of severe aortic stenosis. Int. J. Cardiovasc. Imaging 2021, 37, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; Lefevre, M.; Miller, F.; Otto, C.M.; et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Chen, H.; Li, H. Prognostic Value of Global Longitudinal Strain in Asymptomatic Aortic Stenosis: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Vollema, E.M.; Sugimoto, T.; Shen, M.; Tastet, L.; Ng, A.C.T.; Abou, R.; Marsan, N.A.; Mertens, B.; Dulgheru, R.; Lancellotti, P.; et al. Association of Left Ventricular Global Longitudinal Strain with Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol. 2018, 3, 839–847. [Google Scholar] [CrossRef]
- Kafa, R.; Kusunose, K.; Goodman, A.L.; Svensson, L.G.; Sabik, J.F.; Griffin, B.P.; Desai, M.Y. Association of Abnormal Postoperative Left Ventricular Global Longitudinal Strain with Outcomes in Severe Aortic Stenosis Following Aortic Valve Replacement. JAMA Cardiol 2016, 1, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.S.; Videbæk, L.; Poulsen, M.K.; Rudbæk, T.R.; Pellikka, P.A.; Maller, J.E. Global strain in severe aortic valve stenosis relation to clinical outcome after aortic valve replacement. Circ. Cardiovasc. Imaging 2012, 5, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Lancellotti, P.; Sengupta, P.P.; Donal, E. LV Mechanics in Mitral and Aortic Valve Diseases: Value of Functional Assessment Beyond Ejection Fraction. JACC Cardiovasc. Imaging 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Thellier, N.; Altes, A.; Appert, L.; Binda, C.; Leman, B.; Marsou, W.; Debry, N.; Joly, C.; Ennezat, P.V.; Tribouilloy, C.; et al. Prognostic Importance of Left Ventricular Global Longitudinal Strain in Patients with Severe Aortic Stenosis and Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1454–1464. [Google Scholar] [CrossRef]
- Stassen, J.; Pio, S.M.; Ewe, S.H.; Singh, G.K.; Hirasawa, K.; Butcher, S.C.; Cohen, D.J.; Généreux, P.; Leon, M.B.; Marsan, N.A.; et al. Left Ventricular Global Longitudinal Strain in Patients with Moderate Aortic Stenosis. J. Am. Soc. Echocardiogr. 2022, 35, 791–800.e4. [Google Scholar] [CrossRef] [PubMed]
- Kampaktsis, P.N.; Kokkinidis, D.G.; Wong, S.C.; Vavuranakis, M.; Skubas, N.J.; Devereux, R.B. The role and clinical implications of diastolic dysfunction in aortic stenosis. Heart 2017, 103, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.A.; Park, P.W.; Sung, K.; Lee, S.C.; Park, S.W.; Lee, Y.T.; Oh, J.K. Noninvasive estimate of left ventricular filling pressure correlated with early and midterm postoperative cardiovascular events after isolated aortic valve replacement in patients with severe aortic stenosis. J. Thorac. Cardiovasc. Surg. 2010, 140, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.S.; Videbæk, L.; Poulsen, M.K.; Pellikka, P.A.; Veien, K.; Andersen, L.I.; Haghfelt, T.; Møller, J.E. Noninvasive assessment of filling pressure and left atrial pressure overload in severe aortic valve stenosis: Relation to ventricular remodeling and clinical outcome after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2011, 142, e77–e83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Cheng, L.; Fan, L.; Wang, C.; Shu, X. The Early Variation of Left Ventricular Strain after Aortic Valve Replacement by Three-Dimensional Echocardiography. PLoS ONE 2015, 10, e0140469. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Rouge, J.; Burtin, P.; Roussiaux, A.; Ducrocq, N.; Halchini, C. Left ventricular strain variations in cardiac surgery; The role of the type of surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, S130. [Google Scholar] [CrossRef]
- Magne, J.; Cosyns, B.; Popescu, B.A.; Carstensen, H.G.; Dahl, J.; Desai, M.Y.; Kearney, L.; Lancellotti, P.; Marwick, T.H.; Sato, K.; et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis: An Individual Participant Data Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 84–92. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 108) | Group A (n = 69) | Group B (n = 39) | p-Value |
|---|---|---|---|---|
| Sex (male), n (%) | 57 (52.8) | 42 (60.9) | 15 (38.5) | 0.029 |
| Age, (years), mean ± SD | 71.3 ± 7.2 | 70.5 ± 7.6 | 72.6 ± 6.3 | 0.147 |
| BMI (kg/m2), mean ± SD | 26.87 ± 4 | 26.18 ± 3.7 | 28.08 ± 4.1 | 0.017 |
| Hypertension, n (%) | 76 (70.4) | 46 (66.7) | 30 (76.9) | 0.283 |
| Diabetes mellitus, n (%) | 24 (22.2) | 14 (20.3) | 10 (25.6) | 0.631 |
| Dyslipidemia, n (%) | 67 (62) | 43 (62.3) | 24 (61.5) | 1.000 |
| Chronic kidney disease, n (%) | 9 (8.3) | 3 (4.3) | 6 (15.4) | 0.069 |
| Smoke, n (%) | 51 (47.2) | 28 (40.6) | 23 (59) | 0.074 |
| Indication to CABG, n (%) | 22 (20.4) | 15 (21.7) | 7 (17.9) | 0.804 |
| Euroscore II (%), median [IQR] | 1.43 [1–2.2] | 1.44 [1–2.2] | 1.43 [1–2.2] | 1.000 |
| Hospital stay (days), median [IQR] | 11 [8–14.8] | 10 [8–13.5] | 12 [10–17] | 0.187 |
| Preoperative | Early Postoperative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total (n = 108) | Group A (n = 69) | Group B (n = 39) | p A1 vs. B1 | Total (n = 108) | Group A (n = 6) | Group B (n = 39) | p A2 vs. B2 | p Total 1 vs. 2 | p A1 vs. A2 | p B1 vs. B2 |
| LVEF (%) | 63 [58–65] | 61 [57.5–65] | 64 [60–66] | 0.562 | 59 [56–61] | 60 [56–60] | 60 [57–63] | 0.721 | <0.001 | <0.001 | 0.002 |
| LV/mass/BSA (g/m2) | 130.4 ± 30.1 | 127.8 ± 30.2 | 134 ± 29.6 | 0.238 | 116.6 ± 35.6 | 111.9 ± 38.3 | 124.7 ± 29.2 | 0.074 | <0.001 | <0.001 | 0.027 |
| IVS (mm) | 13 [12–14] | 13 [12–14] | 13 [12–14] | 0.338 | 12 [11–13] | 12 [11–13] | 13 [11–13] | 0.292 | <0.001 | 0.032 | 0.010 |
| PWT (mm) | 11 [11–12] | 11 [10.25–12] | 12 [11–12] | 0.010 | 11 [10–12] | 11 [10–12] | 11 [10–12] | 0.478 | 0.320 | 0.683 | 0.022 |
| LVD (mm) | 50 [45–53] | 50 [45.5–54] | 48 [45–52] | 0.253 | 47 [45–53] | 47 [45–52] | 48 [45–54] | 0.592 | 0.04 | <0.001 | 0.837 |
| LAVI (mL/m2) | 39.1 ± 12.9 | 35.9 ± 12.1 | 44.8 ± 12.4 | <0.001 | 34.4 ± 13 | 30.9 ± 11.5 | 40.9 ± 13.2 | <0.001 | <0.001 | 0.001 | 0.036 |
| TAPSE (mm) | 22.5 ± 3.6 | 22.3 ± 3.5 | 23 ± 3.8 | 0.310 | 16 ± 3.1 | 16.6 ± 3.1 | 16.5 ± 3.3 | 0.905 | <0.001 | <0.001 | <0.001 |
| PAPS (mmHg) | 29 [25–34] | 28.5 [24–32.8] | 33 [28–36] | 0.001 | 26 [22–33] | 26 [22–31] | 28 [25–35] | 0.071 | 0.009 | 0.263 | 0.010 |
| RVD2 (mm) | 28.4 ± 6.7 | 28.8 ± 6.6 | 27.9 ± 6.9 | 0.530 | 28.5 ± 5.9 | 28.8 ± 5.8 | 27.9 ± 6.2 | 0.435 | 0.975 | 0.988 | 0.966 |
| Grad-Peak (mmHg) | 86 ± 27.9 | 80.7 ± 26.2 | 95.3 ± 28.8 | 0.010 | 13.3 ± 4.3 | 13.0 ± 3.9 | 13.7 ± 4.8 | 0.630 | <0.001 | <0.001 | <0.001 |
| Grad-Mean (mmHg) | 56.7 ± 19.1 | 54.11 ± 18.1 | 61.2 ± 20.1 | 0.065 | 9.2 ± 3.1 | 9.4 ± 3.15 | 9 ± 3.8 | 0.435 | <0.001 | <0.001 | <0.001 |
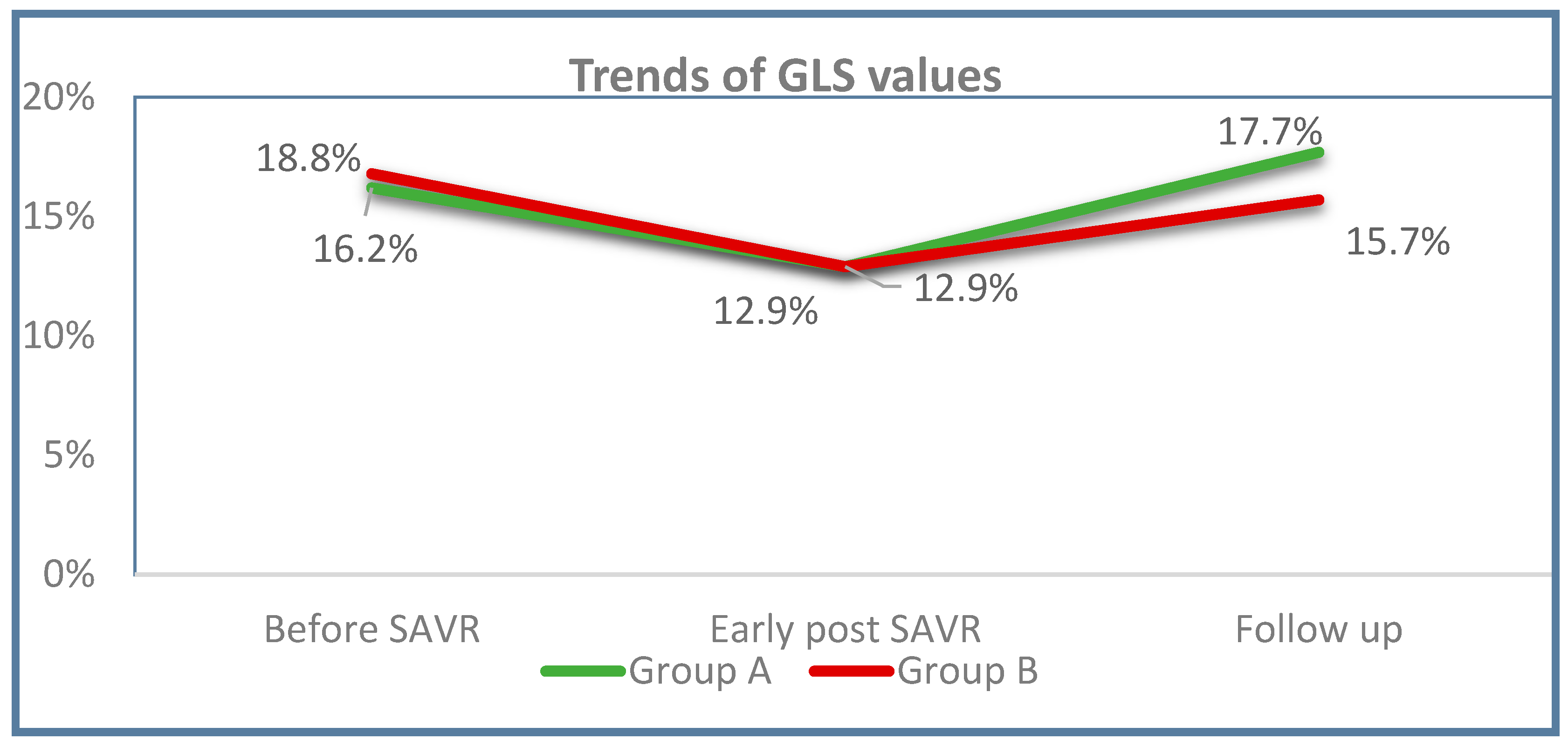
| Mean GLS (%) | 16 ± 4.3 | 15.6 ± 4.3 | 16.7 ± 4.2 | 0.185 | 12.8 ± 3.4 | 12.7 ± 3.3 | 12.8 ± 3.5 | 0.854 | <0.001 | <0.001 | <0.001 |
| Variables | Total (n = 86) | Group A (n = 55) | Group B (n = 31) | p A3 vs. B3 | p Total 1 vs. 3 | p A1 vs. A3 | p B1 vs. B3 |
|---|---|---|---|---|---|---|---|
| EF (%) | 60 [59–65] | 60 [60–65] | 60 [58–63] | 0.034 | 0.050 | 0.478 | 0.025 |
| LV/mass/BSA (g) | 95.1 ± 25.7 | 90.2 ± 26.1 | 103.4 ± 20.1 | 0.026 | <0.001 | <0.001 | <0.001 |
| IVS (mm) | 11 [11,12] | 11 [10–12] | 12 [11–13] | 0.050 | <0.001 | <0.001 | 0.003 |
| PWT (mm) | 10 [10–11] | 10 [9–11] | 11 [10–11] | 0.010 | <0.001 | <0.001 | 0.827 |
| LVD (mm) | 45 [43–48] | 45 [43–48] | 45 [42–50] | 0.825 | <0.001 | <0.001 | 0.016 |
| LAVI (mL/m2) | 35.2 ± 12.0 | 33.4 ± 12.0 | 38.3 ± 11.5 | 0.077 | 0.021 | 0.289 | 0.018 |
| TAPSE (mm) | 20.1 ± 3.2 | 19.7 ± 2.8 | 20.6 ± 3.7 | 0.208 | <0.001 | <0.001 | 0.003 |
| PAPS (mmHg) | 25 [20–29] | 24 [20–26] | 27 [22–33] | 0.036 | <0.001 | 0.004 | 0.031 |
| RVD2 (mm) | 30.0 ± 5.3 | 30.9 ± 5.0 | 28.7 ± 5.7 | 0.080 | 0.011 | 0.012 | 0.412 |
| Grad-Peak (mmHg) | 20.1 ± 7.4 | 19.3 ± 6.1 | 21.5 ± 8.9 | 0.198 | <0.001 | <0.001 | <0.001 |
| Grad-Mean (mmHg) | 11.3 ± 4.5 | 10.8 ± 3.8 | 11.0 ± 5.5 | 0.216 | <0.001 | <0.001 | <0.001 |
| Mean GLS (%) | 17.0 ± 3.4 | 17.7 ± 3.4 | 15.7 ± 3.2 | 0.011 | 0.087 | 0.018 | 0.221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanni, F.; Caciolli, S.; Berteotti, M.; Grasso Granchietti, A.; Tozzetti, V.; Cenni, N.; Servoli, C.; Bandini, M.; Marchi, E.; Del Pace, S.; et al. Left Ventricular Diastolic Dysfunction Predicts Global Longitudinal Strain Recovery after Surgical Aortic Valve Replacement. Diagnostics 2024, 14, 2176. https://doi.org/10.3390/diagnostics14192176
Bonanni F, Caciolli S, Berteotti M, Grasso Granchietti A, Tozzetti V, Cenni N, Servoli C, Bandini M, Marchi E, Del Pace S, et al. Left Ventricular Diastolic Dysfunction Predicts Global Longitudinal Strain Recovery after Surgical Aortic Valve Replacement. Diagnostics. 2024; 14(19):2176. https://doi.org/10.3390/diagnostics14192176
Chicago/Turabian StyleBonanni, Francesca, Sabina Caciolli, Martina Berteotti, Andrea Grasso Granchietti, Valentina Tozzetti, Noemi Cenni, Chiara Servoli, Marta Bandini, Enrico Marchi, Stefano Del Pace, and et al. 2024. "Left Ventricular Diastolic Dysfunction Predicts Global Longitudinal Strain Recovery after Surgical Aortic Valve Replacement" Diagnostics 14, no. 19: 2176. https://doi.org/10.3390/diagnostics14192176
APA StyleBonanni, F., Caciolli, S., Berteotti, M., Grasso Granchietti, A., Tozzetti, V., Cenni, N., Servoli, C., Bandini, M., Marchi, E., Del Pace, S., Stefano, P., & Marchionni, N. (2024). Left Ventricular Diastolic Dysfunction Predicts Global Longitudinal Strain Recovery after Surgical Aortic Valve Replacement. Diagnostics, 14(19), 2176. https://doi.org/10.3390/diagnostics14192176





