The Role of Oxidative Stress as a Mechanism in the Pathogenesis of Acute Heart Failure in Acute Kidney Injury
Abstract
:1. Introduction
1.1. Oxidative Stress and Mechanisms of Its Occurrence
1.2. Oxidative Stress in Patients with AKI
1.3. The Role of Mitochondria in Acute Cardiorenal Syndrome
1.4. Mechanisms of Mitochondrial Dysfunction in Cardiorenal Syndrome Type 3
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AICAR | 5-Aminoimidazole-4-carboxamide ribonucleotide |
| AKI | Acute Kidney Injury |
| Akt/mTOR | Serine/threonine protein kinase/mammalian target of Rapamycin |
| AMPK | 5’ adenosine monophosphate-activated protein kinase |
| AOPP | Advanced Oxidation Protein Product |
| ATP | Adenosine triphosphate |
| Cyt C | Cytochrome C |
| DNA | Deoxyribonucleic acid |
| DRP1 | Dyamin-related protein 1 |
| FIS1 | Mitochondrial fission 1 protein |
| Grb2 | Growth Factor receptor Bound protein 2 |
| GSK-3β | Glycogen synthase kinase-3 beta |
| GTP-ases | Guanosine triphosphate-hydrolase enzymes |
| H2O2 | Hydrogen peroxide |
| HIF | Hypoxia-inducible factor |
| KDIGO | Kidney Disease Improving Global Outcomes |
| LMW | Low molecular weight thiols |
| MA-5 | Mitochondrial acid 5 |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malonyl dialdehyde |
| MFN1 | Mitofusin 1 |
| MFN2 | Mitofusin 2 |
| MitoQ | Mitoquinone |
| mPT | Mitochondrial permeability transition |
| mPTP | Mitochondrial permeability transition pore |
| mtDNA | Mitochondrial double-stranded deoxyribonucleic acid |
| mtROS | Mitochondrial reactive oxygen species |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| O2− | Superoxide anion |
| OPA1 | Optic atrophy 1 |
| OXPHOS | Oxidative phosphorylation |
| RNS | Reactive nitrogen species |
| ROS | Reactive Oxygen Species |
| R-SH | Sulfhydryl group |
| SOD | Superoxidase dismutase |
| SS-31 | Elamipretide |
| TBARS | Thio barbituric Acid Reactive Substance |
| TDZD-8 | Thiadiazolidinone-8 |
| TGFβ | Transforming growth factor beta |
| TMA | Mitochondria-targeted antioxidant or agents |
| TPP+ | Tetraphenylphosphonium |
References
- K/DIGO AKI Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Intern. Suppl. 2012, 2, S3–S106. [Google Scholar]
- Sawhney, S.; Fraser, D.S. Epidemiology of AKI: Utilizing Large Database to determine the Burden of AKI. ACKD 2017, 24, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Topete, M.L.; Rosner, H.M.; Ronco, C. Acute Kidney Insjury Risk Assessment and the Nephrology Rapids Response Team. Blood Purif. 2017, 43, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Macedo, E.; Mehta, R.L. Preventing Acute Kidney Injury. Crit. Care Clin. 2015, 31, 773–784. [Google Scholar] [CrossRef]
- Soumalainen, A.; Nunnari, J. Mitochondria at the crossroads of health and disease. Cell 2024, 187, 2601–2627. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Ronco, C.; Bellomo, R. Acute kidney disease and the community. Lancet 2016, 387, 974–1976. [Google Scholar] [CrossRef]
- Hertzberg, D.; Ryden, L.; Pickering, J.W.; Sartipy, U.; Holzmann, M.J. Acute kidney injury-an overview of diagnostic methods and clinical management. CKJ 2017, 10, 323–331. [Google Scholar] [CrossRef]
- Feltes, C.M.; Van Eyk, J.; Rabb, H. Distant-organ changes after acute kidney injury. Nephron. Physiol. 2008, 109, 80–84. [Google Scholar] [CrossRef]
- Tecson, K.M.; Hashemi, H.; Afzal, A.; Gong, T.A.; Kale, P.; Mc Cullough, P.A. Community-acquired acute kidney injury s a risk factor of de novo heart failure hospitalization. Cardiorenal. Med. 2019, 9, 252–260. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2021. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, R.U. Cardiorenal Syndrome:An Updated Classification Based on Clinical Hallmarks. J. Clin. Med. 2022, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Breglia, A.; Castellani Ch Ankawi, G.; Bolin, C.; De Cal, M.; Cianci, V.; Angelini, A.; Vescovo, G.; Ronco, C. Lipopolysacharide in systemic circulation induces activation of inflammatory responce and oxidative stress in cardiorenal syndrome type 1. J. Nephrol. 2019, 32, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Breglia, A.; Brocca, A.; De Cal, M.; Bolin, C.; Vescovo, G.; Ronco, C. Oxidative Stress, and Tissue damage markers in patients with Acute heart failure with and without cardiorenal Syndrome Type 1. Cardiorenal. Med. 2018, 8, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Clementi, A.; De Cal, M.; Brocca, A.; Day, S.; Pastori, S.; Bolin, C.; Vescovo, G.; Ronco, C. Oxidative Stress:Dual Pathway Induction in Cardiorenal Syndrome Type 1 Pathogenesis. Oxid. Med. Cell Longev. 2015, 2015, 391790. [Google Scholar] [CrossRef]
- Bansal, N.; Matheny, M.E.; Greevy, R.A.; Eden, S.K.; Perkis, A.M.; Parr, S.K.; Fly, J.; Abdel-Kader, K.; Himmelfarb, J.; Hung, A.M.; et al. Acute kidney injury and risk of incident heart failure among US veterans. Am. J. Kidney Dis. 2018, 71, 236–245. [Google Scholar] [CrossRef]
- Gammelager, H.; Christiansen, C.F.; Johansen, M.B.; Tonnesen, E.; Jespersen, B.; Sorensen, H.T. Three-year risk of cardiovascular disease amnog intensive care patients with acute kidney injury: Apopulation-based cohort study. Crit. Care 2014, 18, 492. [Google Scholar] [CrossRef]
- Dieter, B.P.; Daratha, K.B.; McPherson, S.M.; Short, R.; Alicic, R.Z.; Tuttle, K.R. Association of acute kidney injury with cardiovascular events and death in systolic blood pressure intervention trial. Am. J. Nephrol. 2019, 49, 359–367. [Google Scholar] [CrossRef]
- Sohel, B.M.; Rumana, N.; Ohsawa, M.; Turin, T.C.; Kelly, M.A.; Al Mamun, M. Renal Function trajectory over time and adverse clinical outcomes. Clin. Exp. Nephrol. 2016, 20, 379–393. [Google Scholar] [CrossRef]
- Di Lullo, L.; Reeves, P.B.; Bellasi, A.; Ronco, C. Cardiorenal syndrome in acute kidney injury. Semin. Nephrol. 2019, 39, 31–40. [Google Scholar] [CrossRef]
- Mehta, S.; Chauhan, K.; Patel, A.; Patel, S.; Pinotti, R.; Nadkarni, G.N.; Parikh, C.R.; Coca, S.G. The prognostic importance of duration of AKI:a systematic review and meta-analysis. BMC Nephrol. 2018, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G. Ptolemy and copernicus revisited:the complex interplay between the kidneys and heart failure. CJASN 2018, 13, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Hsu, C.; Parikh, R.V.; Leong, T.K.; Tan, T.C.; Walia, S.; Liu, K.D.; Hsu, R.K.; Go, A.S. Non-recovery from dialysis-requiring acute kidney injury and short-term mortality and cardiovascular risk: A cohort study. BMC Nephrol. 2018, 19, 134. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Zelnick, L.R.; Chinchilli, M.V.; Moledina, G.D.; Coca, G.S.; Parikh, R.C.; Garg, A.X.; Hsu, C.-Y.; Go, A.S.; Liu, K.D.; et al. Association Btween Early Recovery of Kidney Function After Acute Kindey Injury and Long-term Clinical Outcomes. JAMA Netw. Open 2020, 3, e202682. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L. Renal recovery After Acute Kidney Injury and Long-term Outcomes:Is Time of the Essence? JAMA Netw. Open 2020, 3, e202676. [Google Scholar] [CrossRef]
- Vaara, S.T.; Bhatraju, P.K.; Stanski, N.L.; McMahon, B.A.; Liu, K.; Joannidis, M.; Bagshaw, S.M. Subphenotypes in acute kidney injury:a narrative review. Crit. Care 2022, 26, 251. [Google Scholar] [CrossRef]
- Perinel, S.; Vincent, F.; Lautrette, A.; Dellamonica, J.; Mariat, C.; Zeni, F.; Cohen, Y.; Tardy, B.; Souweine, B.; Darmon, M. Transient and Persistent Acute Kidney Injury and the Risk of Hospital Mortality in Critically Ill Patients: Results of a Multicenter Cohort Study. Crit. Care Med. 2015, 43, e269–e275. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Mukherjee, P.; Robinson-Cohen, C.; O’Keefe, G.E.; Frank, J.A.; Christie, D.J.; Meyer, N.J.; Liu, K.D.; Matthay, M.A.; Calfee, C.S.; et al. Acute kidney injury subphenotypes based on creainine trajectory identifies patients at increased risk of death. Crit. Care 2016, 20, 372. [Google Scholar] [CrossRef]
- Flannery, A.H.; Bosler, K.; Ortiz-Sorano, V.M.; Gianella, F.; Prado, V.; Lambert, J.; Toto, R.D.; Moe, O.W.; Neyra, J.A. Kidney Biomarkers and major Adverse Kidney Events in Critically Ill Patients. Kidney360 2021, 2, 26–32. [Google Scholar] [CrossRef]
- Johnson, A.C.M.; Zager, R.A. Catalytic iron mediated renal stress responses during experimental cardiorenal syndrome 1 (CRS-1). Transl. Res. 2021, 237, 53–62. [Google Scholar] [CrossRef]
- Johnson, A.C.M.; Delrow, J.J.; Zager, R.A. Tin protoporphyrin activates the oxidant-dependent NRF2-cytoprotective pathway and mitigates acute kidney injury. Transl. Res. 2017, 186, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Clementi, A.; Brocca, A.; de Cal, M.; Ronco, C. Molecular and genetic mechanisms involved in the pathogenesis of cardiorenal cross talk. Pathobiology 2016, 83, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; McMonagle, E.; Freedman, S.; Klenzak, J.; Mc Menamin, E.; Le, P.; Pupim, L.B.; Ikizler, T.A.; The Picard Group. Oxidative stress is increased in critically ill patients with acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Kasuno, K.; Shirakawa, K.; Yoshida, H.; Mori, K.; Kimra, H.; Takahashi, N.; Nobukawa, Y.; Shigemi, K.; Tanabe, S.; Yamada, N.; et al. Renal redox dysregulation in AKI: Application for oxidative stress marker of AKI. Am. J. Physiol. Renal. Physiol. 2014, 307, F1342–F1351. [Google Scholar] [CrossRef]
- Just, A. Nitric oxide and renal autoregulation. Kidney Blood Press Res. 1997, 20, 201–204. [Google Scholar] [CrossRef]
- Tomsa, A.M.; Alexa, A.L.; Junie, M.L.; Rachisan, A.L.; Ciumarnean, L. Oxidative stress as a potential target in acute kidney injury. PeerJ 2019, 7, e8046. [Google Scholar] [CrossRef]
- Schön, S.K.; Steven, S.; Kossmann, S.; Scholz, A.; Daub, S.; Oelze, M.; Xia, N.; Hausding, M.; Mikhed, Y.; Zinßius, E.; et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014, 20, 247–266. [Google Scholar] [CrossRef]
- Palomeque, J.; Rueda, O.V.; Sapia, L.; Valverde, C.A.; Salas, M.; Petroff, V.M.; Mattiazzi, A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ. Res. 2009, 105, 1204–1212. [Google Scholar] [CrossRef]
- Silva-Caio, W.; da Silva Dias, D.; Junho, C.V.C.; Panico, K.; Neres-Santos, R.S.; Pelegrino, T.M.; Pieretti, J.C.; Seabra, A.B.; De Angelis, K.; Carneiro-Ramos, M.S. Characterization of the Oxidative stress in renal Ischemia/Reperfusion-Induced cardiorenal Syndrome Tupe 3. Biomed. Res. Int. 2020. [Google Scholar] [CrossRef]
- Dennis, J.M.; Witting, P.K. Protective role for antioxidants in acute kidney disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef]
- Araujo, M.; Welch, W.J. Oxidative stress and nitric oxide in kidney function. Curr. Opin. Nephrol. Hypert. 2006, 15, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Nilakantan, V.; Hilton, G.; Maenpaa, C.; van Why, S.K.; Pieper, G.M.; Johnson, C.P.; Shames, B.D. Favorable balance of anti-oxidant/pro-oxidant systems and ablated oxidative stress in Brown Norway rats in renal ischemia-reperfusion injury. Mol. Cell Biochem. 2007, 304, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 337–388. [Google Scholar] [CrossRef] [PubMed]
- Anderstam, B.; Ann-Christin, B.H.; Valli, A.; Stenvinkel, P.; Lindholm, B.; Suliman, M.E. Modification of the oxidative stress biomarker AOPP assey: Application in uremic samples. Clin. Chim. Acta 2008, 393, 114–118. [Google Scholar] [CrossRef]
- Selmeci, L.; Seres, L.; Antal, M.; Lukacs, J.; Regoly-Merei, A.; Acsady, G. Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients:a simple, fast and inexpensive automated technique. Clin. Chem. Lab. Med. 2005, 43, 294–297. [Google Scholar] [CrossRef]
- Mahzari, S.; Hossseinian, S.; Hadjzadeh, M.A.; Mohebbati, R.; Noshahr, Z.S.; Rad, A.K. Kidney dysfunction and oxidative stress in doxorubicin-induced nephritic rat:protective role of sesame oil. Saudi. J. Kidney Dis. Transpl. 2021, 32, 1243–1252. [Google Scholar] [CrossRef]
- Irigaraz, P.; Caccamo, D.; Belpomme, D. Oxidative stress in electrohzpersensitivity self-reporting patients:results of a prospective in vivo investigation with comprehensive molecular analysis. Int. J. Mol. Med. 2018, 42, 1885–1898. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malonaldehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Pfaff, A.R.; Beltz, J.; King, E.; Ercal, N. Medicinal Thiols: Current Status and new perspectives. Mini Rev. Med. Chem. 2020, 20, 513–529. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Gillis, B.S.; Arbiva, Z.; Gavin, I.M. Analysis of lead toxicity in human cells. BMC Genom. 2012, 13, 344. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C.; Qiu, C. Importance of nitric oxide in the control of renal hemodynamics. Kidney Int. 1996, 49, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-J.; Wang, Z.-Y.; Xu, L.; Chen, X.-H.; Li, X.-X.; Liao, W.-T.; Ma, H.-K.; Jiang, M.-D.; Xu, T.-T.; Xu, J.; et al. HIF-1α-BNIP3-mediated mitophagy in tubular cells protect against renal ischemia/reperfsion injury. Redox Biol. 2020, 36, 101671. [Google Scholar] [CrossRef]
- Nezu, M.; Souma, T.; Yu, L.; Suzuki, T.; Saigusa, D.; Ito, S.; Suzuki, N.; Yamamoto, M. Transcription factor Nrt2 hyperactivation in erly-phase renl ischemia-reperfusion injury prevents tubular damage progression. Kidney Int. 2017, 91, 387–401. [Google Scholar] [CrossRef]
- Lasségue, B.; Griendling, K.K. NADPH Oxidaes: Functions and Pathologies in the Vasculature. Arterioscler. Thromb. Vasc. Bol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Héebert, R.L. NADPH Oxidases, Reactive Oxygen Species, and the Kindey: Friend and Foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef]
- Révész, C.; Kaucsàr, T.; Godó, M.; Bocskai, K.; Krenàcs, T.; Mócsai, A.; Szénási, G.; Hamar, P. Neutrophills and NADPH Oxidase Are Major Contributors to Mild but Not Severe Ischemic Acute Kindey Injury in Mice. Int. J. Sci. 2024, 25, 2948. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Lushchak, V.I. Classification of oxidative stress based on its intensity. EXCLI J. 2014, 13, 922–937. [Google Scholar] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Ann. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tomşa, M.A.; Răchisan, A.L.; Pandrea, S.L.; Benea, A.; Uifălean, A.; Parvu, A.E.; Junie, L.M. Accelerated lipid peroxidation in a rat model of gentamicin nephrotoxicity. Exp. Ther. Med. 2021, 22, 1218. [Google Scholar] [CrossRef]
- Zhou, H.; Kato, A.; Miyaji, T.; Ysuda, H.; Fujigaki, Y.; Yammamoto, T.; Yonemura, K.; Takebayashi, S.; Mineta, H.; Hishida, A. Urinary marker for oxidative stress in kidneys in cisplatin-induced acute renal failure in rats. Nephrol. Dial. Transplant. 2006, 21, 616–623. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive oxygen species a key hallmark of cardiovascular disease. Adv. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, L.T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiologica. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, M.C.; Westerweel, P.E.; Van Zonneveld, A.J.; Rabelink, T.J. Free radical production by dysfunctional eNOS. Heart 2004, 90, 494–495. [Google Scholar] [CrossRef]
- Becker, T.; Wagner, R. Mitochondrial outer membrane channels: Emerging Diversity in Transport Processes. Bioessays 2018, 40, e1800013. [Google Scholar] [CrossRef]
- Krüger, V.; Becker, T.; Becker, L.; Montilla-Martinez, M.; Ellenrieder, L.; Vögtle, F.-N.; Meyer, H.E.; Ryan, M.T.; Wiedemann, N.; Warscheid, B.; et al. Identification of new channels by systematic analysis of the mitochondrial outer membrane. J. Cell Biol. 2017, 216, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-T.; See, D.H.; Huang, Y.-J.; Jao, T.-M.; Liu, S.-Y.; Chou, C.-Y.; Lai, C.-F.; Lin, W.-C.; Wang, C.-Y.; Huang, J.-W.; et al. LTBP4 Protects Against Renal Fibrosis via Mitochondrial and vascular impacts. Circ. Res. 2023, 133, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Domnguez, J.H.; Xie, D.; Kelly, K.J. Cardiac effect of renal ischemia. Am. J. Physiol. Renal. Physiol. 2023, 324, F64–F74. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, A.; Qi, J.; Wang, H.; Liu, X.; Zhao, M.; Duan, S.; Huang, Z.; Zhang, C.; Wu, L.; et al. P53/DRP1-dependent mitochondrial fission mediates aldosteron-induced injury and mitochondrial dysfunction. Am. J. Physiol. Renal. Physiol. 2018, 314, F798–F808. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.W.; Fang, Y.H.; Han, M.; Zhang, H.J.; Hong, Z.; Banathy, A.; Morrow, E.; Ryan, J.J.; Archer, S.L. Dyamin-related protein 1 (Drp1)-mediated diastolic dysfunction in mzocardial ischemia-reperfusion injury:therapeutic benefits of DRP1 inhibition to reduce mitochondrial fission. FASEB J. 2014, 28, 316–326. [Google Scholar] [CrossRef]
- Haileselassie, B.; Mukherjee, R.; Joshi, A.U.; Napier, B.A.; Massis, L.M.; Ostberg, N.P.; Queliconi, B.B.; Monack, D.; Bernstein, D.; Mochly-Rosen, D. Drp1/fisInteraction mediates Mitochondrial Dysfunction in Septic Cardiomyopathy. J. Mol. Cell Cardiol. 2019, 130, 160–169. [Google Scholar] [CrossRef]
- Perry, H.M.; Huang, L.; Wilson, R.J.; Bajwa, A.; Sesaki, H.; Yan, Z.; Rosin, D.L.; Kashatus, D.F.; Okusa, M.D. Dyamin-Realted Protein 1 Deficiency promotes recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 194–206. [Google Scholar] [CrossRef]
- Sumida, M.; Doi, K.; Ogasawara, E.; Yamashita, T.; Hamasaki, Y.; Kariya, T.; Takimoto, E.; Yahagi, N.; Nangaku, M.; Noiri, E. Regulation of Mitochondrial Dynamics by Dynamin-Related Protein-1 in Acute Cardiorenal Syndrome. J. Am. Soc. Nephrol. 2015, 26, 2378–2387. [Google Scholar] [CrossRef]
- IkeIkeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 mediates Mitochondrial Autophagy and Protects the heart Against Energy Stress. Circ. Res. 2014, 116, 264–278. [Google Scholar] [CrossRef]
- Baines, C.P.; Gutierrez-Aguilar, M. The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium. 2018, 73, 121–130. [Google Scholar] [CrossRef]
- Morciano, G.; Giorgi, C.; Bonora, M.; Punzetti, S.; Pavasini, R.; Wieckowski, M.R.; Campo, G.; Pinton, P. Molecular identitiy of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Molec. Cell Card. 2015, 78, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H.; Inagi, R. Mitohondrial dysfunction in kidney disease and uremic sarcopenia. Front. Physiol. 2020, 11, 565023. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.; Kazachenko, A.; Vyssokikh, M.; Vasileva, A.; Tcvirkun, D.; Isaev, N.; Kirpatovsky, V.; Zorov, D. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007, 72, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, B.; Li, Y.; Xu, X.; Lv, J.; Jia, Q.; Chai, R.; Xue, W.; Li, Y.; Wang, Y.; et al. Mithochondrial Dysfunction:An Emerging Link in the pathophysiology of Cardiorenal Syndrome. Front. Cardiovasc. Med. 2022, 9, 837270. [Google Scholar]
- Ravarotto, V.; Bertoldi, G.; Innico, G.; Gobbi, L.; Calo, L.A. The Pivotal Role of oxidative Stress in the pathophysiology of Cardiovascular-Renal Remodeling in Kidney Disease. Antioxidants 2021, 10, 1041. [Google Scholar] [CrossRef]
- Morciano, G.; Boncompagni, C.; Ramaccini, D.; Pedriali, G.; Bouhamida, E.; Tremoli, E.; Giorgi, C.; Pinton, P. Comprehensive Analysis of Mitochondrial Dynamics Alterations in Heart Diseases. Int. J. Mol. Sci. 2023, 24, 3414. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2023, 19, 2084862. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar] [CrossRef]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-Chain Fatty Acids in the Metabolism of Heart Failure—Rethinking the Fat Stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef]
- Fontecha-barriuso, M.; Martin-sanchez, D.; Martinez-moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-niño, M.D.; Ruiz-ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Lopez-Diaz, A.M.; Guerrero-Mauvecin, J.; Miguel, V.; Ramos, A.M.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease. Antioxidants 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cai, J.; Yin, X.M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, M.; Xiong, L.; Fan, J.; Zhou, Y.; Li, H.; Peng, X.; Zhong, Z.; Wang, Y.; Huang, F.; et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020, 11, 29. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Shao, X.; Zhu, X.; Wu, J.; Wu, B.; Zhang, M.; Zhou, W.; Zhou, Y.; Jin, H.; et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic. Biol. Med. 2020, 152, 632–649. [Google Scholar] [CrossRef]
- Tagaya, M.; Kume, S.; Yasuda-Yamahara, M.; Kuwagata, S.; Yamahara, K.; Takeda, N.; Tanaka, Y.; Chin-Kanasaki, M.; Nakae, Y.; Yokoi, H.; et al. Inhibition of mitochondrial fission protects podocytes from albumin-induced cell damage in diabetic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166368. [Google Scholar] [CrossRef]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharmacol. 2021, 178, 2060–2076. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; An, P.; Luo, J.; Luo, Y. Mitochondrial Homeostasis in VSMCs as a Central Hub in Vascular Remodeling. Int. J. Mol. Sci. 2023, 24, 3483. [Google Scholar] [CrossRef]
- Cooper, H.A.; Cicalese, S.; Preston, K.J.; Kawai, T.; Okuno, K.; Choi, E.T.; Kasahara, S.; Uchida, H.A.; Otaka, N.; Scalia, R.; et al. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovasc. Res. 2021, 117, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Bajwa, A. Role of Mitochondrial Therapy for Ischemic-Reperfusion Injury and Acute Kidney Injury. Nephron 2022, 146, 253–258. [Google Scholar] [CrossRef]
- Walker, R.B.; Moreas, T.C. Nuclear-Mitochondrial Interactions. Biomolecules 2022, 12, 427. [Google Scholar] [CrossRef]
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders. Life 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Arzola, K.; Diaz-Quintana, A. Mitochondrial Factors in the Cell Nucleus. Int. J. Mol. Sci. 2023, 24, 13656. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liu, Y.; Sun, M.; Qin, S.; Xin, W.; Guan, X.; Zhang, B.; He, T.; Huang, Y. The molecular mechanisms and intervention strategies of mitophagy in cardiorenal syndrome. Front. Physiol. 2022, 13, 1008517. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Egea, V.; Bidzhekov, K.; Natarelli, L.; Mourão, A.; Blanchet, X.; Wichapong, K.; Aslani, M.; Brunßen, C.; Horckmans, M.; et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci. Transl. Med. 2020, 12, eaaz2294. [Google Scholar] [CrossRef] [PubMed]
- Bugga, P.; Alam, M.J.; Kumar, R.; Pal, S.; Chattopadyay, N.; Banerjee, S.K. Sirt3 ameliorates mitochondrial dysfunction and oxidative stress through regulating mitochondrial biogenesis and dynamics in cardiomyoblast. Cell Signal. 2022, 94, 110309. [Google Scholar] [CrossRef]
- Yu, R.; Lendahl, U.; Nistér, M.; Zhao, J. Regulation of mammalian mitochondrial dynamics: Opportunities and challenges. Front. Endocrinol. 2020, 11, 374. [Google Scholar] [CrossRef]
- Merry, T.L.; Chan, A.; Woodhead, J.S.T.; Reynolds, J.C.; Kumagai, H.; Kim, S.-J.; Lee, C. Mitochondrial-derived peptides in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E659–E666. [Google Scholar] [CrossRef]
- Gyuraszova, M.; Gurecka, R.; Babickova, J. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Zhang, X.; Agborbesong, E.; Li, X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int. J. Mol. Sci. 2021, 22, 20. [Google Scholar] [CrossRef]
- Duann, P.; Lin, P.H. Mitochondria Damage and Kidney Disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar] [CrossRef]
- Tan, Y.; Xia, F.; Li, L.; Peng, X.; Liu, W.; Zhang, Y.; Fang, H.; Zeng, Z.; Chen, Z. Novel Insights into the Molecular Features and Regulatory Mechanisms of Mitochondrial Dynamic Disorder in the Pathogenesis of Cardiovascular Disease. Oxid. Med. Cell. Longev. 2021, 2021, 6669075. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Graciani, K.M.; Chapa-Dubocq, X.R.; MacMillan-Crow, L.A.; Javadov, S. Association between L-OPA1 Cleavage and Cardiac Dysfunction. Cell. Physiol. Biochem. 2020, 54, 1101–1114. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Zhou, W.; Wu, Y.; Zhang, S.; Ding, G.; Zhang , Y.; Zhang, A.; Huang, S.; Jia, G.; et al. Maintaing homeostasis of mitochondria and endoplasmic reticulum with NSC228155 alleviates cisplatin-induced acute kidney injury. Free Rad. Biomed. 2022, 181, 270–287. [Google Scholar] [CrossRef]
- Wang, J.; Toan, S.; Li, R.; Zhou, H. Melatonin fine-tunes intracellular calcium signals and eliminates myocardial damage through the IP3R/MCU pathways in cardiorenal syndrome type 3. Biochem. Pharmacol. 2020, 174, 113832. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Wang, X.; Cui, S.; Liu, R.; Liu, J.; Fu, B.; Gong, M.; Wang, C.; Shi, Y.; et al. Grb2 induces cardiorenal syndrome type 3: Roles of IL-6, cardiomyocyte bioenergetics, and Akt/mTOR pathway. Front. Cell Dev. Biol. 2021, 9, 630412. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms-Biomarkers-Interventions, and Future perspectives. Oxidative Med. Cell. Longev. 2017, 6193694. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Jiang, D.; Fu, C.; Xiao, J.; Zhang, Z.; Zou, J.; Ye, Z.; Zhang, X. SGK1 Attenuates Oxidative Stress-Induced Renal Tubular Epithelial Cell Injury by Regulating Mitochondrial Function. Oxid. Med. Cell Longev. 2019, 2019, 2013594. [Google Scholar] [CrossRef]
- Popov, L. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lv, X.; Zhang, Y.; Han, Y.; Li, J.; Zeng, J.; Tang, D.; Meng, J.; Yuan, X.; Peng, Z.; et al. Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage. Oxid. Med. Cell Longev. 2022, 2022, 2453617. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Ferreira, I.B.; Hollstein, E.P.; Curtis, D.S.; Trefts, E.; Novak Weiser, S.; Yu, J.; Gilson, R.; Hellberg, K.; Fang, L.; et al. Induction of lysosomal and mitochondrial biogenesis by AMPK phosphrylation of FNIP1. Science 2023, 380. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Chung, K.-P.; Nakahira, K.; Patino, E.; Rice, M.C.; Torres, L.K.; Muthukumar, T.; Choi, A.M.; Akchurin, O.M.; Choi, M.E. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. J. Clin. Investig. 2019, 4, e132826. [Google Scholar] [CrossRef]
- Jia, Q.; Han, L.; Zhang, X.; Yang, W.; Gao, Y.; Shen, Y.; Li, B.; Wang, S.; Qin, M.; Lowe, S.; et al. Tongluo Yishen Decoction Ameliorates Renal Fibrosis via Regulating Mitochondrial Dysfunction Induced by Oxidative Stress in Unilateral Ureteral Obstruction Rats. Front. Pharmacol. 2021, 12, 762756. [Google Scholar] [CrossRef]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res. Clin. Pract. 2020, 39, 244–258. [Google Scholar] [CrossRef]
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fundam. Clin. Pharmacol. 2019, 33, 504–523. [Google Scholar] [CrossRef]
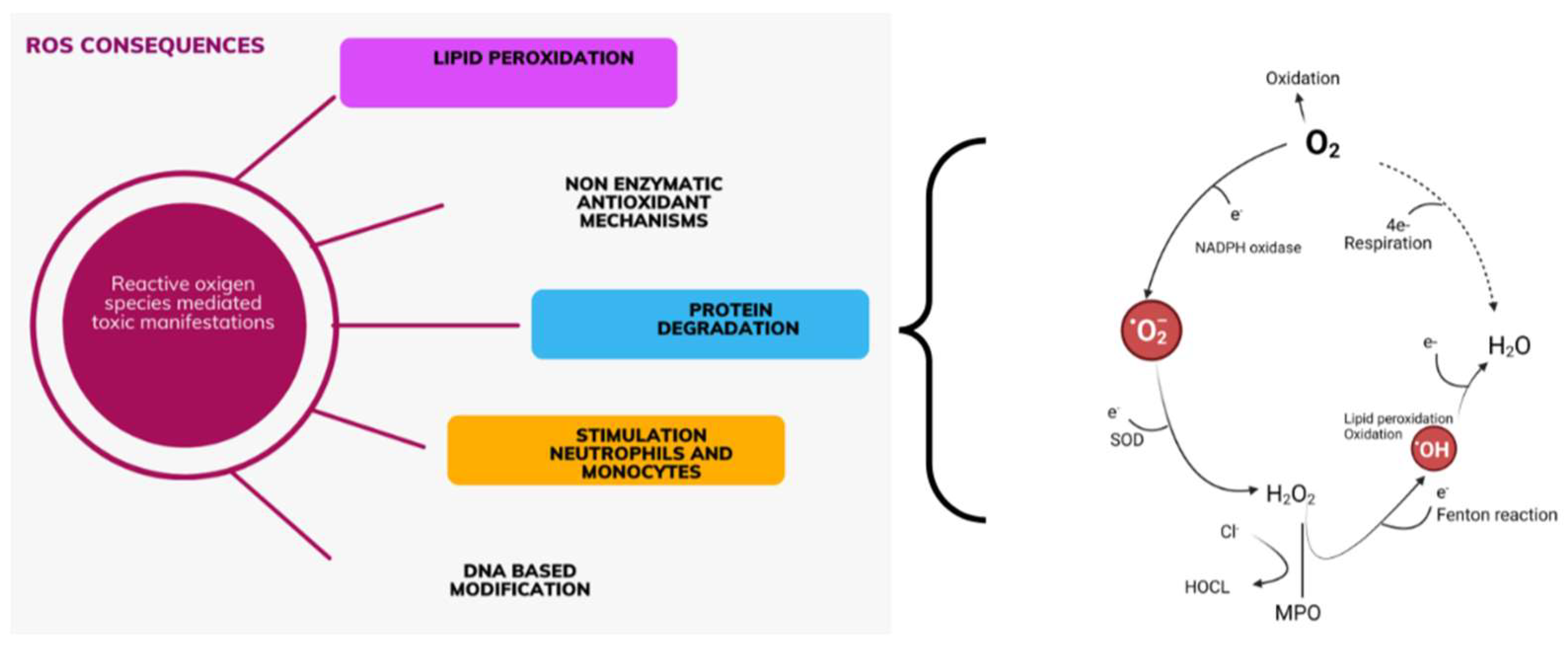
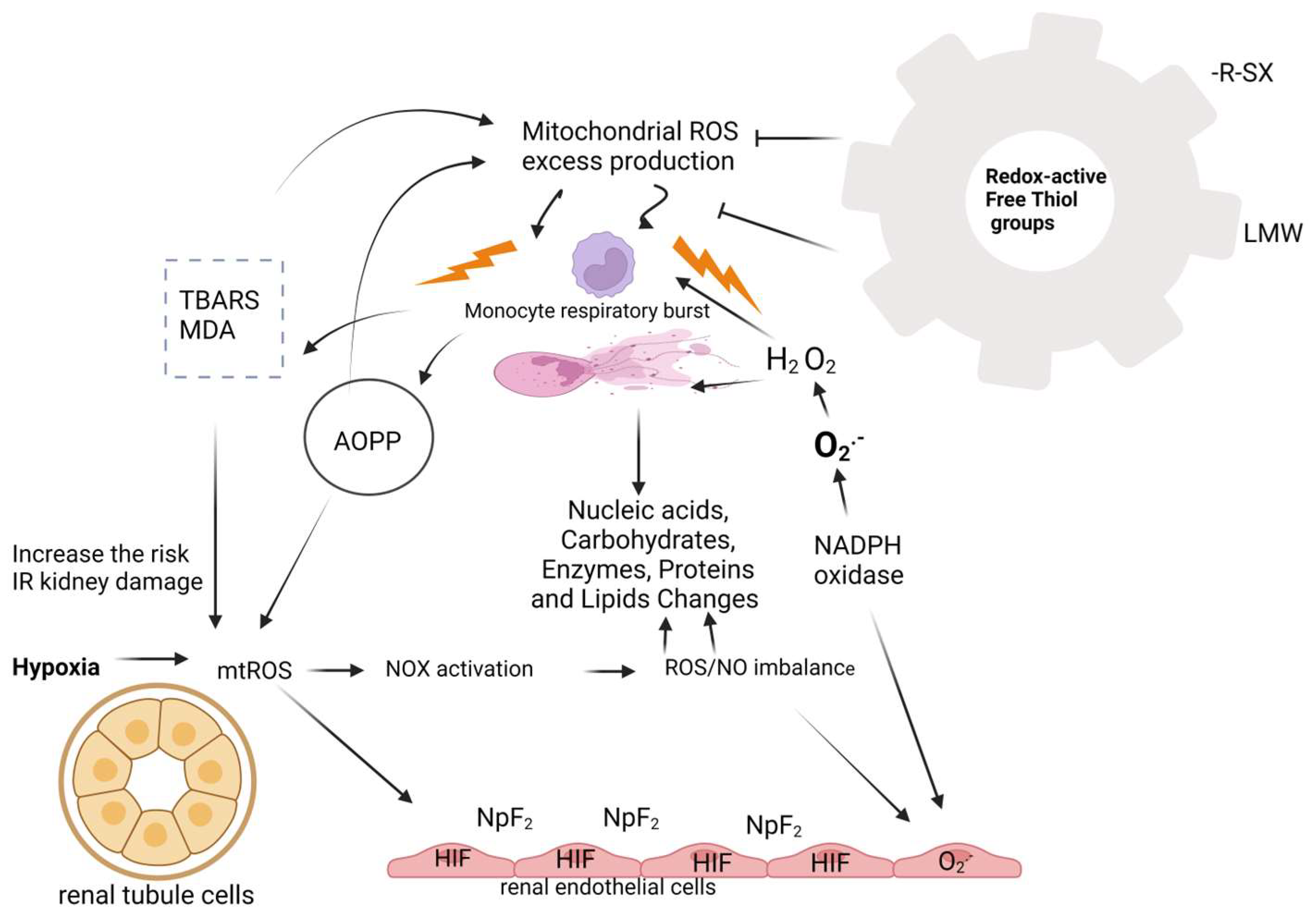
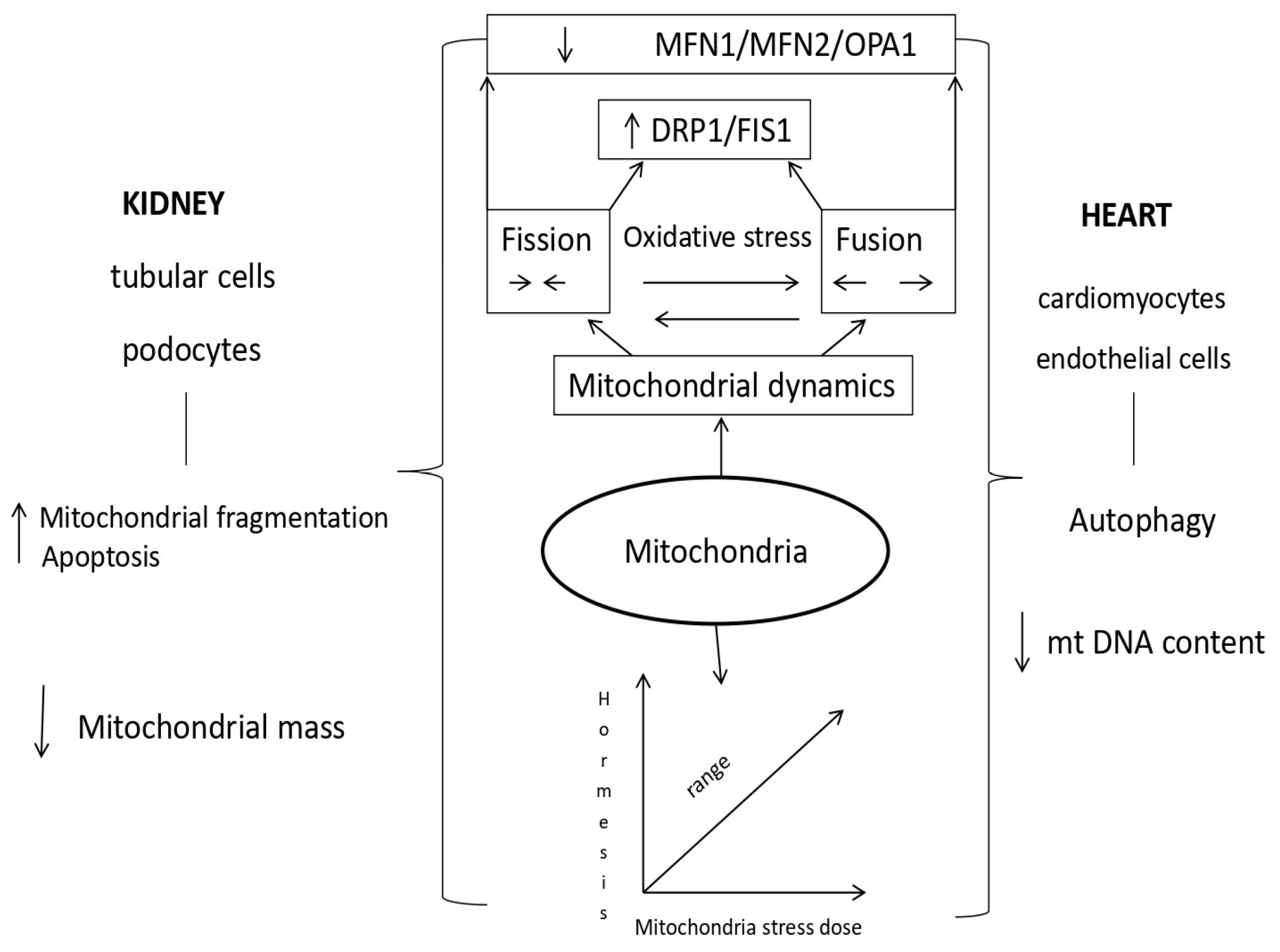
| Experimental Setting | Target | Main Finding | Reference | |
|---|---|---|---|---|
| Kidney | Ltbp4S-/- mouse model | AKI-IR/LTBP4 | AKI to CKD transition via DRP1 pathways | [70] |
| Kidney/Heart | Renal ischemia/ Doxorubicin rats | Systemic inflammation/heart damage after renal ischemia | ↑ DRP1 | [71] |
| Kidney | Aldosterone-induced podocytes injury/mouse/cultured podocytes | P53/DRP1 mitochondrial dysfunction | ↑ DRP1 | [72] |
| Heart | Neonatal murine cardiomyocytes/adult rat hearts | Activation of DRP1 by myocardial IR and LV impairment | DRP1 mediated diastolic dysfunction and therapeutic benefits DRP1 inhibition | [73] |
| Heart | In vivo mice models of septic cardiomyopathy/cardiac cell line model | Inhibition DRP1/FIS1 | Treatment P110 reduced cardiac mitochondrial fragmentation and improved ATP production | [74] |
| Kidney | In vivo genetic murine models in wild-type mice | IR/therapeutic potential DRP1 deletion in proximal tubule epithelium before IR | Protects against kidney injury | [75] |
| Acute cardiorenal syndrome | In vivo mice IR models/in vitro cell | IR by bilateral renal artery clamping | Cardiac dysfunction induced by renal IR improved by DRP1 inhibition | [76] |
| Heart | Tamoxifen-inducible/DRP1-hetCKO mice | Role endogenous DRP1 in the protection LV function against IR | Left ventricular dysfunction and increased susceptibility to IR | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasić, D.; Dimitrijević, Z. The Role of Oxidative Stress as a Mechanism in the Pathogenesis of Acute Heart Failure in Acute Kidney Injury. Diagnostics 2024, 14, 2094. https://doi.org/10.3390/diagnostics14182094
Tasić D, Dimitrijević Z. The Role of Oxidative Stress as a Mechanism in the Pathogenesis of Acute Heart Failure in Acute Kidney Injury. Diagnostics. 2024; 14(18):2094. https://doi.org/10.3390/diagnostics14182094
Chicago/Turabian StyleTasić, Danijela, and Zorica Dimitrijević. 2024. "The Role of Oxidative Stress as a Mechanism in the Pathogenesis of Acute Heart Failure in Acute Kidney Injury" Diagnostics 14, no. 18: 2094. https://doi.org/10.3390/diagnostics14182094
APA StyleTasić, D., & Dimitrijević, Z. (2024). The Role of Oxidative Stress as a Mechanism in the Pathogenesis of Acute Heart Failure in Acute Kidney Injury. Diagnostics, 14(18), 2094. https://doi.org/10.3390/diagnostics14182094






