Post-Thrombectomy Subarachnoid Hemorrhage: Incidence, Predictors, Clinical Relevance, and Effect Modulators
Abstract
1. Introduction
2. Methods
Statistical Methods
3. Results
3.1. Patient Characteristics
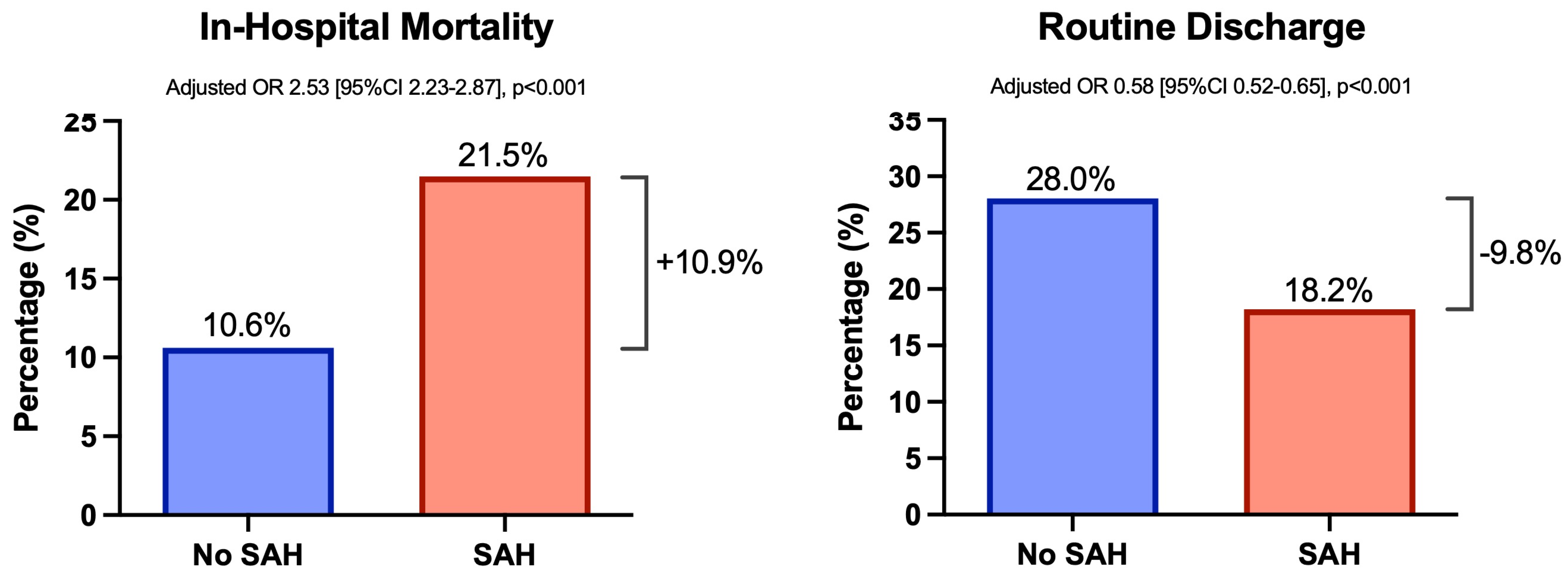
3.2. Impact of SAH on Patient Outcomes
3.3. Predictors of SAH Risk
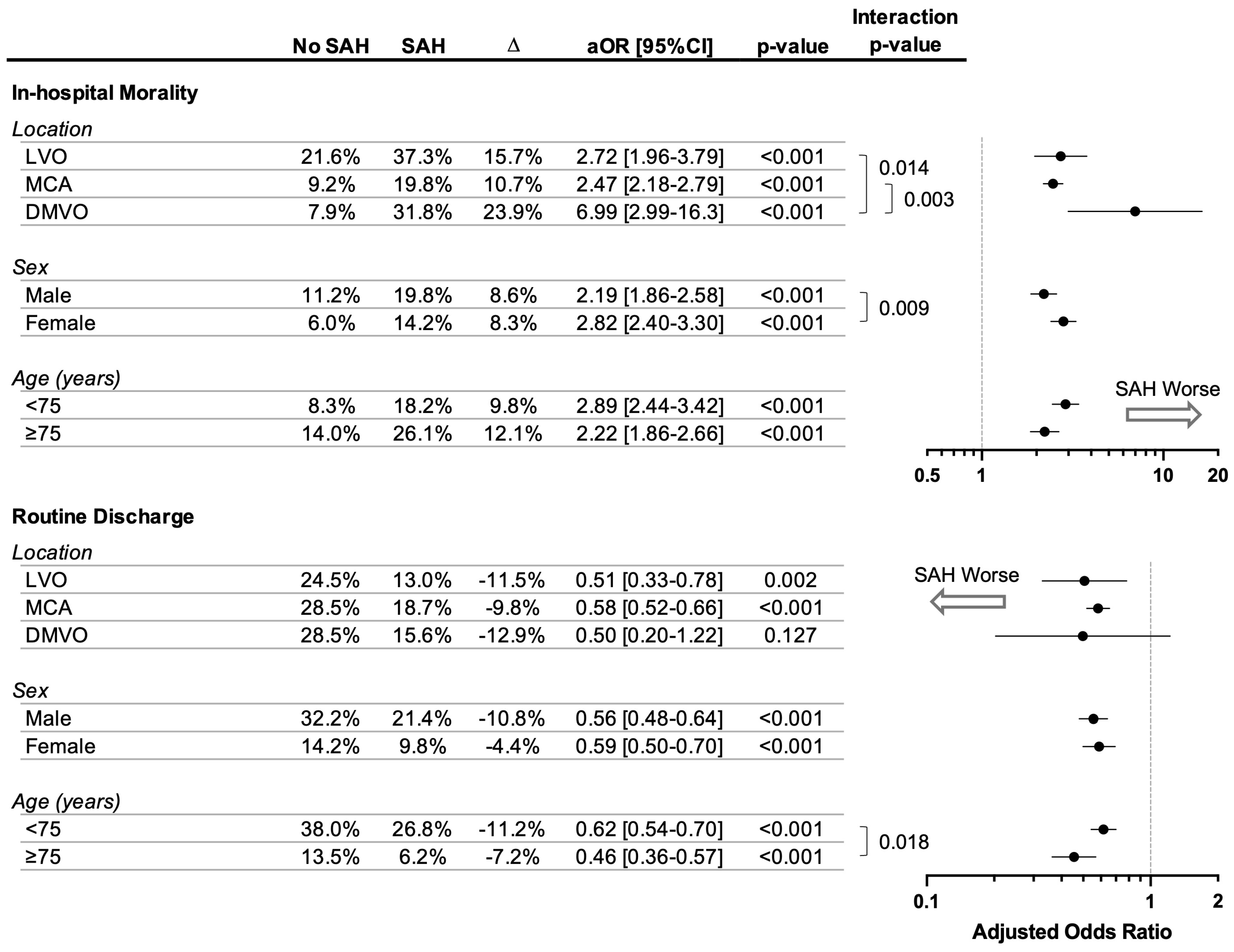
3.4. Modulators of SAH’s Association with Poorer Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischemic Stroke. J. Neurointerv. Surg. 2023, 15, e8. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, G.; Bonnet, L.; Biondi, A.; Moulin, T. Intracranial Bleeding after Reperfusion Therapy in Acute Ischemic Stroke. Front. Neurol. 2021, 11, 629920. [Google Scholar] [CrossRef]
- Costalat, V.; Jovin, T.G.; Albucher, J.F.; Cognard, C.; Henon, H.; Nouri, N.; Gory, B.; Richard, S.; Marnat, G.; Sibon, I.; et al. Trial of Thrombectomy for Stroke with a Large Infarct of Unrestricted Size. N. Engl. J. Med. 2024, 390, 1677–1689. [Google Scholar] [CrossRef]
- Yoshimura, S.; Sakai, N.; Yamagami, H.; Uchida, K.; Beppu, M.; Toyoda, K.; Matsumaru, Y.; Matsumoto, Y.; Kimura, K.; Takeuchi, M.; et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. N. Engl. J. Med. 2022, 386, 1303–1313. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.E.; Abraham, M.G.; Ortega-Gutierrez, S.; Kasner, S.E.; Hussain, M.S.; Chen, M.; Blackburn, S.; Sitton, C.W.; Churilov, L.; et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N. Engl. J. Med. 2023, 388, 1259–1271. [Google Scholar] [CrossRef]
- Huo, X.; Ma, G.; Tong, X.; Zhang, X.; Pan, Y.; Nguyen, T.N.; Yuan, G.; Han, H.; Chen, W.; Wei, M.; et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N. Engl. J. Med. 2023, 388, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Bendszus, M.; Fiehler, J.; Subtil, F.; Bonekamp, S.; Aamodt, A.H.; Fuentes, B.; Gizewski, E.R.; Hill, M.D.; Krajina, A.; Pierot, L.; et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: Multicentre, open-label, randomised trial. Lancet 2023, 402, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Colasurdo, M. Endovascular thrombectomy for large ischemic strokes: Meta-analysis of six multicenter randomized controlled trials. J. Neurointerv. Surg. 2024. [Google Scholar] [CrossRef]
- Chen, H.; Lee, J.S.; Michel, P.; Yan, B.; Chaturvedi, S. Endovascular Stroke Thrombectomy for Patients with Large Ischemic Core. JAMA Neurol. 2024. [Google Scholar] [CrossRef]
- Saber, H.; Hinman, J.; Mun, K.; Kaneko, N.; Szeder, V.; Tateshima, S.; Nour, M.; Raychev, R.; Ooi, Y.C.; Jahan, R.; et al. Mechanical Thrombectomy for Acute Ischemic Stroke in Patients with Dementia. Stroke Vasc. Interv. Neurol. 2022, 2, e000164. [Google Scholar] [CrossRef]
- Salwi, S.; Cutting, S.; Salgado, A.D.; Espaillat, K.; Fusco, M.R.; Froehler, M.T.; Chitale, R.V.; Kirshner, H.; Schrag, M.; Jasne, A.; et al. Mechanical Thrombectomy in Patients with Ischemic Stroke with Prestroke Disability. Stroke 2020, 51, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Aloizou, A.-M.; Richter, D.; Charles James, J.; Lukas, C.; Gold, R.; Krogias, C. Mechanical Thrombectomy for Acute Ischemic Stroke in Patients with Malignancy: A Systematic Review. J. Clin. Med. 2022, 11, 4696. [Google Scholar] [CrossRef] [PubMed]
- Regenhardt, R.W.; Young, M.J.; Etherton, M.R.; Das, A.S.; Stapleton, C.J.; Patel, A.B.; Lev, M.H.; Hirsch, J.A.; Rost, N.S.; Leslie-Mazwi, T.M. Toward a more inclusive paradigm: Thrombectomy for stroke patients with pre-existing disabilities. J. Neurointerv. Surg. 2021, 13, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, F.; Schirmer, C.M.; Starke, R.M.; Chaudhary, N.; De Leacy, R.; Tjoumakaris, S.I.; Haranhalli, N.; Abecassis, I.J.; Amuluru, K.; Bulsara, K.R.; et al. Thrombectomy in special populations: Report of the Society of NeuroInterventional Surgery Standards and Guidelines Committee. J. Neurointerv. Surg. 2022, 14, 1033–1041. [Google Scholar] [CrossRef]
- Chen, H.; Jindal, G.; Miller, T.R.; Gandhi, D.; Chaturvedi, S. Stroke Thrombectomy in the Elderly: Efficacy, Safety, and Special Considerations. Stroke Vasc. Interv. Neurol. 2023, 3, e000634. [Google Scholar] [CrossRef]
- Desai, S.M.; Haussen, D.C.; Aghaebrahim, A.; Al-Bayati, A.R.; Santos, R.; Nogueira, R.G.; Jovin, T.G.; Jadhav, A.P. Thrombectomy 24 hours after stroke: Beyond DAWN. J. Neurointerv. Surg. 2018, 10, 1039–1042. [Google Scholar] [CrossRef]
- Rodriguez-Calienes, A.; Galecio-Castillo, M.; Vivanco-Suarez, J.; Mohamed, G.A.; Toth, G.; Sarraj, A.; Pujara, D.; Chowdhury, A.A.; Farooqui, M.; Ghannam, M.; et al. Endovascular thrombectomy beyond 24 hours from last known well: A systematic review with meta-analysis. J. Neurointerv. Surg. 2024, 16, 670–676. [Google Scholar] [CrossRef]
- Kobeissi, H.; Ghozy, S.; Adusumilli, G.; Kadirvel, R.; Brinjikji, W.; Rabinstein, A.A.; Kallmes, D.F. Endovascular Therapy for Stroke Presenting beyond 24 Hours. JAMA Netw. Open 2023, 6, e2311768. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Doheim, M.F.; Al-Bayati, A.R.; Lee, J.S.; Haussen, D.C.; Mohammaden, M.; Lang, M.; Starr, M.; Rocha, M.; da Câmara, C.P.; et al. Distal Medium Vessel Occlusion Strokes: Understanding the Present and Paving the Way for a Better Future. J. Stroke 2024, 26, 190–202. [Google Scholar] [CrossRef]
- Ospel, J.M.; Nguyen, T.N.; Jadhav, A.P.; Psychogios, M.-N.; Clarençon, F.; Yan, B.; Goyal, M. Endovascular Treatment of Medium Vessel Occlusion Stroke. Stroke 2024, 55, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Stracke, C.P.; Jungi, N.; Wallocha, M.; Broocks, G.; Sporns, P.B.; Maegerlein, C.; Dorn, F.; Zimmermann, H.; Naziri, W.; et al. Thrombectomy for Primary Distal Posterior Cerebral Artery Occlusion Stroke. JAMA Neurol. 2021, 78, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Qureshi, M.M.; Strambo, D.; Strbian, D.; Räty, S.; Herweh, C.; Abdalkader, M.; Olive-Gadea, M.; Ribo, M.; Psychogios, M.; et al. Endovascular Versus Medical Management of Posterior Cerebral Artery Occlusion Stroke: The PLATO Study. Stroke 2023, 54, 1708–1717. [Google Scholar] [CrossRef]
- Sabben, C.; Charbonneau, F.; Delvoye, F.; Strambo, D.; Heldner, M.R.; Ong, E.; Ter Schiphorst, A.; Henon, H.; Ben Hassen, W.; Agasse-Lafont, T.; et al. Endovascular Therapy or Medical Management Alone for Isolated Posterior Cerebral Artery Occlusion: A Multicenter Study. Stroke 2023, 54, 928–937. [Google Scholar] [CrossRef]
- Maulucci, F.; Disanto, G.; Bianco, G.; Pileggi, M.; Fischer, U.; Padlina, G.; Strambo, D.; Michel, P.; Kahles, T.; Nedeltchev, K.; et al. Endovascular therapy outcome in isolated posterior cerebral artery occlusion strokes: A multicenter analysis of the Swiss Stroke Registry. Eur. Stroke J. 2023, 8, 575–580. [Google Scholar] [CrossRef]
- Chen, H.; Khunte, M.; Colasurdo, M.; Malhotra, A.; Gandhi, D. Thrombectomy vs. Medical Management for Posterior Cerebral Artery Stroke: Systematic Review, Meta-Analysis, and Real-World Data. Neurology 2024, 102, e209315. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Cimflova, P.; Ospel, J.M.; Chapot, R. Endovascular treatment of anterior cerebral artery occlusions. J. Neurointerv. Surg. 2021, 13, 1007–1011. [Google Scholar] [CrossRef]
- Chen, H.; Khunte, M.; Malhotra, A.; Gandhi, D.; Colasurdo, M. Endovascular thrombectomy versus medical management for moderate-to-severe anterior cerebral artery occlusion stroke. J. Neurol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Stracke, P.; Broocks, G.; Elsharkawy, M.; Sporns, P.; Piechowiak, E.I.; Kaesmacher, J.; Maegerlein, C.; Hernandez Petzsche, M.R.; Zimmermann, H.; et al. Thrombectomy versus Medical Management for Isolated Anterior Cerebral Artery Stroke: An International Multicenter Registry Study. Radiology 2023, 307, e220229. [Google Scholar] [CrossRef]
- Salim, H.A.; Yedavalli, V.; Musmar, B.; Adeeb, N.; Naamani, K.E.L.; Henninger, N.; Sundararajan, S.H.; Kühn, A.L.; Khalife, J.; Ghozy, S.; et al. Endovascular therapy versus best medical management in distal medium middle cerebral artery acute ischaemic stroke: A multinational multicentre propensity score-matched study. J. Neurol. Neurosurg. Psychiatry 2024. [Google Scholar] [CrossRef]
- Lee, H.; Qureshi, A.M.; Mueller-Kronast, N.H.; Zaidat, O.O.; Froehler, M.T.; Liebeskind, D.S.; Pereira, V.M. Subarachnoid Hemorrhage in Mechanical Thrombectomy for Acute Ischemic Stroke: Analysis of the STRATIS Registry, Systematic Review, and Meta-Analysis. Front. Neurol. 2021, 12, 663058. [Google Scholar] [CrossRef] [PubMed]
- Tarkiainen, J.; Hovi, V.; Pyysalo, L.; Ronkainen, A.; Frösen, J. The clinical course and outcomes of non-aneurysmal subarachnoid hemorrhages in a single-center retrospective study. Acta Neurochir. 2023, 165, 2843–2853. [Google Scholar] [CrossRef]
- Zöllner, J.P.; Konczalla, J.; Stein, M.; Roth, C.; Krakow, K.; Kaps, M.; Steinmetz, H.; Rosenow, F.; Misselwitz, B.; Strzelczyk, A. Acute symptomatic seizures in intracerebral and subarachnoid hemorrhage: A population study of 19,331 patients. Epilepsy Res. 2020, 161, 106286. [Google Scholar] [CrossRef] [PubMed]
- van Landeghem, N.; Ziegenfuß, C.; Demircioglu, A.; Dammann, P.; Jabbarli, R.; Haubold, J.; Forsting, M.; Wanke, I.; Köhrmann, M.; Frank, B.; et al. Impact of post-thrombectomy isolated subarachnoid hemorrhage on neurological outcomes in patients with anterior ischemic stroke—A retrospective single-center observational study. Neuroradiology 2024. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Baik, S.H.; Jung, C.; Kim, J.Y.; Han, S.-G.; Kim, B.J.; Kang, J.; Bae, H.-J.; Kim, J.H. Predictors and Impact of Sulcal SAH after Mechanical Thrombectomy in Patients with Isolated M2 Occlusion. Am. J. Neuroradiol. 2022, 43, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Benalia, V.H.; Aghaebrahim, A.; Cortez, G.M.; Sauvageau, E.; Hanel, R.A. Evaluation of pure subarachnoid hemorrhage after mechanical thrombectomy in a series of 781 consecutive patients. Interv. Neuroradiol. 2023. [Google Scholar] [CrossRef]
- Yoon, W.; Jung, M.Y.; Jung, S.H.; Park, M.S.; Kim, J.T.; Kang, H.K. Subarachnoid Hemorrhage in a Multimodal Approach Heavily Weighted toward Mechanical Thrombectomy with Solitaire Stent in Acute Stroke. Stroke 2013, 44, 414–419. [Google Scholar] [CrossRef]
- Deb-Chatterji, M.; Pinnschmidt, H.; Flottmann, F.; Leischner, H.; Alegiani, A.; Brekenfeld, C.; Fiehler, J.; Gerloff, C.; Thomalla, G. Stroke patients treated by thrombectomy in real life differ from cohorts of the clinical trials: A prospective observational study. BMC Neurol. 2020, 20, 81. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Dicpinigaitis, A.J.; Dick-Godfrey, R.; Gellerson, O.; Shapiro, S.D.; Kamal, H.; Ghozy, S.; Kaur, G.; Desai, S.M.; Ortega-Gutierrez, S.; Yaghi, S.; et al. Real-World Outcomes of Endovascular Thrombectomy for Basilar Artery Occlusion: Results of the BArONISStudy. Ann. Neurol. 2023, 94, 55–60. [Google Scholar] [CrossRef]
- Dmytriw, A.A.; Musmar, B.; Salim, H.; Ghozy, S.; Siegler, J.E.; Kobeissi, H.; Shaikh, H.; Khalife, J.; Abdalkader, M.; Klein, P.; et al. Incidence and clinical outcomes of perforations during mechanical thrombectomy for medium vessel occlusion in acute ischemic stroke: A retrospective, multicenter, and multinational study. Eur. Stroke J. 2024, 9, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Dmytriw, A.A.; Ku, J.C.; Yang, V.X.D.; Hui, N.; Uchida, K.; Morimoto, T.; Spears, J.; Marotta, T.R.; Diestro, J.D.B. Do Outcomes between Women and Men Differ after Endovascular Thrombectomy? A Meta-analysis. AJNR Am. J. Neuroradiol. 2021, 42, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Khunte, M.; Colasurdo, M.; Malhotra, A.; Gandhi, D. Intravenous thrombolysis prior to endovascular thrombectomy in elderly stroke patients: An analysis of the National Inpatient Sample database. J. Neurol. Sci. 2023, 454, 120842. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Khunte, M.; Colasurdo, M.; Jindal, G.; Malhotra, A.; Gandhi, D.; Chaturvedi, S. Associations of Osteoarthritis With Thrombectomy Utilization and Outcomes for Large Vessel Acute Ischemic Stroke. Stroke 2023, 54, 518–526. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort | No SAH | SAH | ||
|---|---|---|---|---|
| Characteristic—% (n) or Mean (SD) | N = 99,219 (100%) | N = 93,045 (93.8%) | N = 6174 (6.2%) | SMD |
| Female sex | 51.1% (50,657) | 50.7% (47,191) | 56.1% (3466) | 0.109 |
| Age (years) | 69.1 (14.8) | 69.1 (14.8) | 69.6 (14.4) | 0.032 |
| NIH Stroke Scale | 15.0 (7.8) | 15.0 (7.8) | 15.1 (7.9) | 0.018 |
| Posterior circulation | 7.6% (7551) | 7.8% (7295) | 4.2% (257) | 0.185 |
| Site of occlusion | ||||
| Large vessel (ICA, BA, VA) | 11.7% (11,619) | 12.0% (11,119) | 8.1% (500) | 0.141 |
| Middle cerebral artery (M1, M2, M3 etc.) | 85.8% (85,167) | 85.6% (79,616) | 89.9% (5552) | 0.145 |
| Distal/medium vessel (ACA, PCA) | 2.5% (2433) | 2.5% (2311) | 2.0% (122) | 0.037 |
| Non-embolic etiology | 62.3% (61,822) | 62.3% (57,993) | 62.0% (3829) | 0.006 |
| Bleeding risk factors | ||||
| Intravenous tPA use | 33.9% (33,666) | 33.9% (31,566) | 34.0% (2100) | 0.002 |
| Anticoagulant use | 17.7% (17,580) | 17.8% (16,562) | 16.5% (1018) | 0.035 |
| Antiplatelet use | 27.1% (26,930) | 27.2% (25,338) | 25.8% (1592) | 0.033 |
| Coagulopathy | 10.4% (10,358) | 10.1% (9426) | 15.1% (932) | 0.139 |
| Stroke risk factors | ||||
| Atrial fibrillation or flutter | 41.3% (41,008) | 41.3% (38,468) | 41.1% (2540) | 0.004 |
| Hypertension | 82.5% (81,820) | 82.6% (76,817) | 81.0% (5002) | 0.039 |
| Smoking history | 18.4% (18,258) | 18.5% (17,251) | 16.3% (1006) | 0.061 |
| Hyperlipidemia | 58.7% (58,284) | 58.9% (54,786) | 56.7% (3498) | 0.045 |
| Diabetes | 11.9% (11,815) | 12.0% (11,151) | 10.7% (663) | 0.040 |
| Diabetes (complicated) | 17.5% (17,404) | 17.5% (16,288) | 18.1% (1117) | 0.015 |
| Chronic heart failure | 26.8% (26,570) | 26.7% (24,853) | 27.8% (1716) | 0.024 |
| Angioplasty or stenting | 10.3% (10,180) | 10.1% (9404) | 12.6% (776) | 0.074 |
| Concurrent intraparenchymal hemorrhage | 17.5% (17,366) | 17.2% (15,958) | 22.8% (1408) | 0.135 |
| Predictor | Adjusted OR [95%CI] | p-Value |
|---|---|---|
| Higher risk | ||
| DMVO * | 1.50 [1.07–2.09] | 0.017 |
| Coagulopathy | 1.47 [1.33–1.62] | <0.001 |
| Angioplasty | 1.44 [1.22–1.70] | <0.001 |
| Concurrent intraparenchymal hemorrhage | 1.39 [1.26–1.55] | <0.001 |
| MCA * | 1.28 [1.08–1.52] | 0.004 |
| Female sex | 1.27 [1.17–1.37] | <0.001 |
| No change in risk | ||
| tPA | 1.03 [0.94–1.12] | 0.55 |
| Non-embolic etiology | 1.02 [0.94–1.10] | 0.65 |
| Age | 1.01 [0.98–1.05] | 0.46 |
| NIHSS | 0.99 [0.93–1.05] | 0.68 |
| Antiplatelet use | 0.98 [0.91–1.05] | 0.54 |
| Lower risk | ||
| Anticoagulant use | 0.89 [0.80–1.00] | 0.041 |
| Posterior cirrculation | 0.61 [0.48–0.77] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Colasurdo, M.; Khunte, M.; Malhotra, A.; Gandhi, D. Post-Thrombectomy Subarachnoid Hemorrhage: Incidence, Predictors, Clinical Relevance, and Effect Modulators. Diagnostics 2024, 14, 1856. https://doi.org/10.3390/diagnostics14171856
Chen H, Colasurdo M, Khunte M, Malhotra A, Gandhi D. Post-Thrombectomy Subarachnoid Hemorrhage: Incidence, Predictors, Clinical Relevance, and Effect Modulators. Diagnostics. 2024; 14(17):1856. https://doi.org/10.3390/diagnostics14171856
Chicago/Turabian StyleChen, Huanwen, Marco Colasurdo, Mihir Khunte, Ajay Malhotra, and Dheeraj Gandhi. 2024. "Post-Thrombectomy Subarachnoid Hemorrhage: Incidence, Predictors, Clinical Relevance, and Effect Modulators" Diagnostics 14, no. 17: 1856. https://doi.org/10.3390/diagnostics14171856
APA StyleChen, H., Colasurdo, M., Khunte, M., Malhotra, A., & Gandhi, D. (2024). Post-Thrombectomy Subarachnoid Hemorrhage: Incidence, Predictors, Clinical Relevance, and Effect Modulators. Diagnostics, 14(17), 1856. https://doi.org/10.3390/diagnostics14171856






