Diagnosis and Management of Scalp Metastases: A Review
Abstract
1. Introduction
2. Diagnosis
2.1. Clinical Features
2.2. Dermoscopy
- -
- Dermoscopy of vascular and/or pigmented features of the lesion;
- -
- Dermoscopy of the related scalp changes:
- (a)
- Preservation versus disruption of the hair follicles and associated changes (follicular patterns);
- (b)
- Interfollicular changes, including color changes, scaling, and vessels (interfollicular patterns);
- (c)
- Changes of the growing hair shaft.
2.3. Imaging
2.4. Cytology
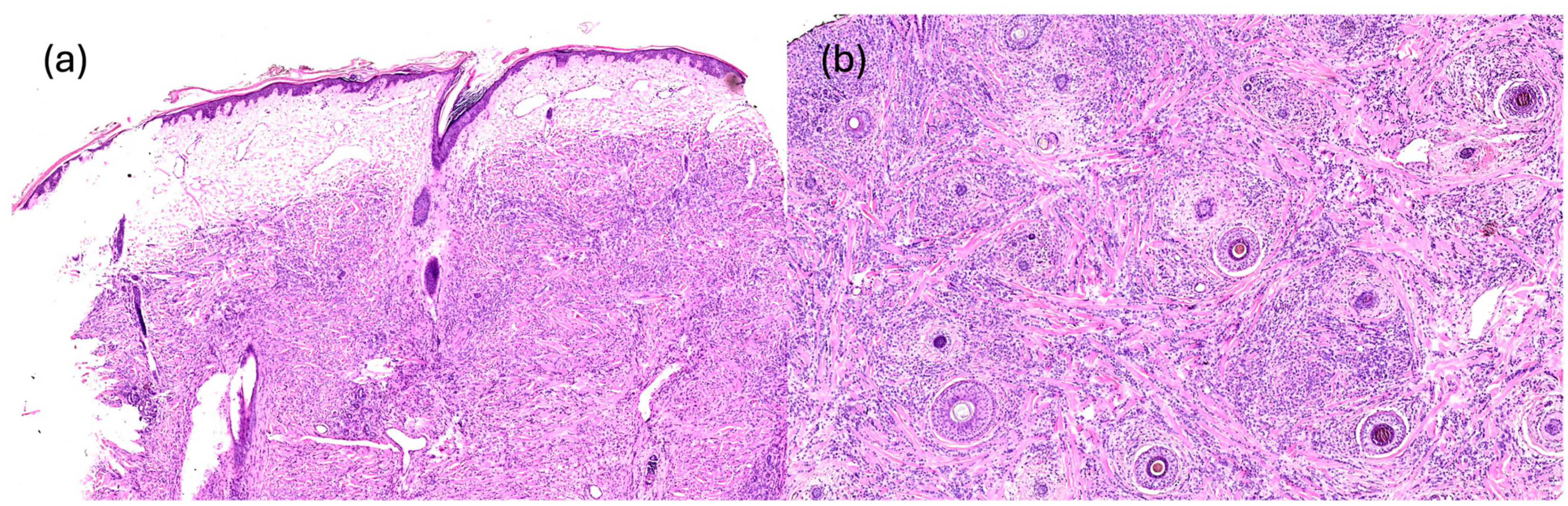
2.5. Histopathology
3. Management
4. Prognosis
5. Conclusions
| First Author, Year | Study Design | Primary Tumor | Clinical Presentation | Dermoscopy | Imaging | Histopathology | Treatment |
|---|---|---|---|---|---|---|---|
| Abdulraheem et al. (2023) [80] | Case report | Breast adenocarcinoma | Erythematous, firm, non-tender, and immobile nodules | NA | MR: soft tissue nodules | Metastatic invasive ductal carcinoma. IHC: ER+, PR+, HER2−, CK7+, p63−, KIT− | Incisional biopsy. Septic shock and death |
| Aguiar et al. (2016) [18] | Case report | Oesophageal squamous cell carcinoma | Firm, nodular, erythematous lesions with a keratinized center, mimicking keratoacanthoma | NA | NA | Nodular dermal-based proliferation of atypical squamous cells and carcinomatous vascular emboli at the peritumoral dermis | Surgical excision. Palliative treatment |
| Alharbi et al. (2023) [71] | Case report | Phylloid breast cancer | Enlarged nodules with blood and serous discharge | NA | CT/MR: multiple subcutaneous soft tissue masses | NA | Doxorubicin, ifosfamide, and radiotherapy |
| AlSubait et al. (2021) [53] | Case report | Sigmoid colon adenocarcinoma | Solitary, firm, asymptomatic pink nodule | Polymorphic vessels (dotted, linear, and serpentine) and a white structureless area on a pink-to-red background | NA | Tumor cells with clear cytoplasm occupying the dermis. IHC: CDX2+, CK7−, CK20+ | Incisional biopsy. Patient refusal to exeresis |
| Avecillas-Chasin et al. (2015) [10] | Retrospective review | Meningioma | Round, firm lesions | NA | CT/MR: extra-axial enhancing mass; “hourglass” configuration with subgaleal extension | Meningioma with loss of architecture infiltrating the soft tissues. IHC: Ki67 10% | Surgical excision. Radiotherapy. Temozolamide |
| Çetinarslan et al. (2020) [54] | Case report | Sarcomatoid renal cell carcinoma | Violaceous-erythematous, scaly, subcutaneous nodule, with telangiectasias | White scale, polymorphic vessels (linear and hairpin), and yellowish structureless area on a light red background | NA | Tumor cells fill the dermis, with clear cytoplasm, vesicular nuclei, nucleoli-specific cells, and a tendency to spindle. IHC: CD10+, CK PAN-AE1-AE3+, PAX8+, vimentin+ | Pazopanib + everolimus |
| Dai et al. (2023) [24] | Case report | Cervical cancer | White, discrete with a rubbery consistency nodule, fixed to the skin | NA | NA | IHC: p16+ | Cadonilimab and palliative chemotherapy |
| Dika et al. (2020) [25] | Review | Breast cancer | Erythematous, well-defined plaques | Irregular vascular pattern, hair loss, and “macrocomedo-like” black dots | NA | NA | Surgical excision |
| Doan et al. (2022) [76] | Retrospective review | Colorectal adenocarcinoma, upper gastrointestinal tract tumors, pancreatic tumors, lung carcinomas, melanoma, diffuse large B-cell, lymphoma, squamous cell carcinoma from the skin of the ear canal, alveolar soft part sarcoma | NA | NA | NA | Melanoma: amelanotic tumor cells with eccentric nuclei and prominent nucleoli. IHC: pan-melanoma cocktail+, SOX10+, pan-CK− | NA |
| Huang et al. (2023) [26] | Case report | Follicular thyroid carcinoma | Large growing mass | NA | PET/CT and MR: irregular solid-cystic mass displaying extensive bony destruction | Sheets of cohesive, small, round, uniform cells intermingled with abundant capillaries and vessels; follicular and microfollicular patterns; subtle pink-red colloid-like secretions in the lumen. IHC: Vimentin+, TTF1+, Napsin A−, PAX8+, thyroglobulin+, Ki-67 10–20% | Surgical excision |
| Lee et al. (2023) [73] | Case report | Pulmonary large cell neuroendocrine carcinoma | Round and firm mass, with a hemispherical, raised protrusion, light pink in color, without any associated pain or tenderness, and exhibited ulceration | NA | NA | Intradermal-cord-like tumor invasion. IHC: CK7+, EMA+, CEA+, S100−, melanA−, CK20, ER−, PR−, TTF1−, napsin A−, CK5/6− | Cisplatin, gemcitabine |
| Lim et al. (2021) [60] | Review | Breast cancer, lung cancer, gastrointestinal tract cancer, melanoma | Destroyed hair follicles, indurated skin, and telangiectasia | NA | PET/CT: FDG-avid skin thickenings or subcutaneous nodules | NA | NA |
| Oh et al. (2023) [72] | Case report | Prostate cancer | Multiple red, non-tender, dome-shaped papules, and nodules | NA | NA | Sheet-like infiltration of small crowded acini with round monomorphic nuclei in the reticular dermis. IHC: PSA+, P504S+ | Leuprolide acetate, abiraterone acetate |
| Paolino et al. (2019) [17] | Review article | Breast, thyroid, gastrointestinal, kidney, lung, uterus, central nervous system tumors | Localized asymptomatic red-violaceous nodules | NA | NA | NA | NA |
| Pipal et al. (2023) [20] | Case report | Ovarian cancers | Soft to firm, non-tender, fixed nodular swelling | NA | NA | Large, singly scattered, and diffusely infiltrating tumor cells | Chemotherapy |
| Quijano Moreno et al. (2022) [27] | Short article | Mesothelioma | Subcutaneous lesion, non-ulcerated, mobile, and painful | NA | NA | Tubules and strands through the proliferation of loosely cohesive atypical epithelioid cells with eosinophilic cytoplasm, prominent nucleoli and mild nuclear pleomorphism, with the presence of mitotic figures and apoptotic bodies. IHC: CK5/6+, WT1+, S100−, MelanA−, HMB45−, CK7−, CD31−, CD34− | Surgical excision |
| Ravaioli et al. (2019) [55] | Case report | Brest cancer | Multiple, well-demarcated, nonulcerated, erythematous, alopecic plaques | Erythema, erosions, peripheral black dots, “macrocomedo-like” structures, atypical vascular pattern, with dilated, serpentine and polymorphic vessels | NA | NA | NA |
| Riahi et al. (2012) [2] | Review | Bladder, breast, lung, rectal, renal cancers | Nodules, cystic lesions, or erythematous plaques. Asymptomatic or painful. Flesh-colored or red | NA | NA | NA | NA |
| Richmond et al. (2010) [37] | Review | Melanoma, breast, ovarian, lung, colon, oral, renal cell, and gastric cancers | Melanoma: firm dermal or subcutaneous nodules, skin-colored or faintly erythematous, visible pigment. Others: Skin-colored, erythematous, or purple dermal or subcutaneous nodules, or erythematous patches or plaques. Sometimes hemorrhagic, ulcerated, or with a zosteriform pattern. | NA | NA | NA | NA |
| Rudnicka et al. (2023) [15] | Review | Lung, prostate, breast cancers | NA | Polymorphic vessels, white structureless area, pink background | NA | NA | NA |
| Salari et al. (2019) [28] | Case report | Pancreatic adenosquamous carcinoma | Violaceous nodule | NA | NA | Solid nests of tumor cells with abundant eosinophilic cytoplasms and poorly formed ductal structures were identified. IHC: CK5/6+, p63+, EMA+, CK19+, CA 19−9+, CK7+, CEA+. | FOLFIRINOX, gemcitabine, paclitaxel |
| Sallman et al. (2020) [29] | Case report | Cholangiocarcinoma | Indurated pink nodular plaque with hemorrhagic crust and smaller satellite pink papule | NA | NA | Dermis was replaced by an infiltrative poorly differentiated carcinoma with an unremarkable epidermis. IHC: CK7+, CK20− | NA |
| Scalia et al. (2023) [30] | Case report | Anaplastic ependymoma | Subcutaneous lesion | NA | CT-PET 11C-methionine: increased uptake at the level of the lesion. MR: homogeneous contrast enhancement in T1W 3D- TFE sequences | Diffuse dermal and subdermal infiltration by poorly differentiated neoplastic cells, characterized by lobular growth patterns and focal areas of necrosis | Surgical excision + cisplatin, etoposide, cyclophosphamide |
| Sciscent et al. (2024) [23] | Review | Thyroid carcinoma | Slow-growing nodules, erythematous papules, and ulcerated lesions | NA | NA | NA | Surgical excision + radioactive iodine or radiation |
| Shastri et al. (2023) [31] | Case report | Pulmonary blastoma | Firm, immobile, and painless increasing swelling | NA | NA | Round to oval cells, with moderate nuclear pleomorphism, coarse chromatin, and tiny visible chromocenters/nucleoli, high nucleocytoplasmic ratio. IHC: β-catenin+, Ki-67 90%. | NA |
| Stanganelli et al. (2012) [36] | Retrospective analysis | Melanoma | NA | Structureless blue-white pigmentation and atypical vessels | NA | NA | NA |
| Subasinghe et al. (2015) [66] | Case report | Hepatocellular carcinoma | Non-tender, hemispherical, subcutaneous lump over the occipital region | NA | CT: destruction of the adjacent skull vault and intracranial extension but no penetration of the meninges | IHC: alpha fetoproteins+, Hep par1+ | Surgical excision |
| Tung-Hahn et al. (2024) [21] | Case report | Small bowel NET | Flesh-colored subcutaneous papule | NA | NA | Well-circumscribed and focally infiltrative dermal nodule composed of closely packed nests and ductal appearing/pseudo rosetting structures. Tumor cells were cuboidal to columnar with ample amounts of amphophilic cytoplasm, round nuclei, and powdery chromatin. IHC: INSM1+, CDX2+ | Surgical excision |
| Vezzoni et al. (2021) [16] | Review | Breast cancer | Single or multiple reddish painless patches/plaques, or flesh-colored nodules | Giant un-focused arborizing vessels and fine telangiectasias on a pink-whitish background and a single well-defined orangish lesion with polymorphic vessels surrounded by a yellow-white crust | NA | NA | Radiotherapy, chemotherapy, and hormonal therapy |
| Wang et al. (2014) [22] | Case report | Colonic NET | Multiple reddish papules/nodules | NA | MR: multiple space-occupying lesions with a rich blood supply in the soft tissue | Irregular and small to medium-sized tumor cells with scanty cytoplasm, hyperchromatic nuclei and distinct nucleoli in some cells, arranged in diffuse and nesting patterns in the subcutis. IHC: Syn+, CDX2+, CD56+ | Etoposide, cisplatin |
| Wu et al. (2019) [64] | Case report | Anaplastic oligodendroglioma | Subcutaneous mass | NA | MR: homogeneously marked enhancing nodular lesion with restricted diffusion in the subcutaneous tissue | Sheets of tumor cells with round nuclei and perinuclear haloes. Necrotic areas showed increased cellularity, cellular pleomorphism, and necrotic foci. IHC: glial fibrillary acidic protein+, Olig-2+, Syn+, EGFR+, ATRX+ | Surgical excision |
| Wu et al. (2023) [35] | Case report | Adrenocortical carcinoma | Soft, sharply demarcated, round subcutaneous nodule | NA | CT: subcutaneous soft tissue mass | IHC to exclude non-adrenocortical tumors with similar histological features | Surgical excision |
| Yan et al. (2022) [47] | Case report | Hepatocellular carcinoma | Exophytic nodule | NA | NA | Highly atypical cells | NA |
| Yuen et al. (1998) [32] | Case report | Placental site trophoblastic tumor | Non-cicatricial, alopecic patches with slight erythema and elevated plaques | NA | NA | Diffuse dermal infiltrate of cords and sheets of large, pleomorphic, polyhedral cells with abundant eosinophilic cytoplasm. Destruction of hair follicles and extensive deposition of fibrinoid material. IHC: human placental lactogen+, CK+ | Etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine |
| Zhang et al. (2023) [33] | Case report | Lung carcinoma | Motionless, skin-colored, without hair, painless mass | NA | MR: soft tissue nodule | IHC: panCK+, CK7+, CD56+, P40+, NapsinA− | Paclitaxel and xindirizumab |
Author Contributions
Funding
Conflicts of Interest
References
- Habermehl, G.; Ko, J. Cutaneous Metastases: A Review and Diagnostic Approach to Tumors of Unknown Origin. Arch. Pathol. Lab. Med. 2019, 143, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.R.; Cohen, P.R. Clinical manifestations of cutaneous metastases: A review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am. J. Clin. Dermatol. 2012, 13, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lookingbill, D.P.; Spangler, N.; Sexton, F.M. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 1990, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lookingbill, D.P.; Spangler, N.; Helm, K.F. Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J. Am. Acad. Dermatol. 1993, 29 Pt 1, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.; Chen, G.S.; Wu, C.S.; Chai, C.Y.; Chen, W.T.; Lan, C.C. Rates of cutaneous metastases from different internal malignancies: Experience from a Taiwanese medical center. J. Am. Acad. Dermatol. 2009, 60, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Keehn, C.A.; Morgan, M.B. Cutaneous metastasis: A clinical, pathological, and immunohistochemical appraisal. J. Cutan. Pathol. 2004, 31, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Krathen, R.A.; Orengo, I.F.; Rosen, T. Cutaneous metastasis: A meta-analysis of data. South. Med. J. 2003, 96, 164–167. [Google Scholar] [CrossRef]
- Tiodorovic, D.; Stojkovic-Filipovic, J.; Marghoob, A.; Argenziano, G.; Puig, S.; Malvehy, J.; Tognetti, L.; Pietro, R.; Akay, B.N.; Zalaudek, I.; et al. Dermatoscopic patterns of cutaneous metastases: A multicentre cross-sectional study of the International Dermoscopy Society. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1432–1438. [Google Scholar] [CrossRef]
- Mueller, T.J.; Wu, H.; Greenberg, R.E.; Hudes, G.; Topham, N.; Lessin, S.R.; Uzzo, R.G. Cutaneous metastases from genitourinary malignancies. Urology 2004, 63, 1021–1026. [Google Scholar] [CrossRef]
- Avecillas-Chasin, J.M.; Saceda-Gutierrez, J.; Alonso-Lera, P.; Garcia-Pumarino, R.; Issa, S.; López, E.; Barcia, J.A. Scalp Metastases of Recurrent Meningiomas: Aggressive Behavior or Surgical Seeding? World Neurosurg. 2015, 84, 121–131. [Google Scholar] [CrossRef]
- La Placa, M.; Lambertini, M.; Veneziano, L.; Patrizi, A. Visual Dermatology: An Asymptomatic Periumbilical Nodule. J. Cutan. Med. Surg. 2019, 23, 459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Subedi, S.; Tong, Y.; Wei, Q.; Xu, H.; Wang, Y.; Gong, Y.; Shi, Y. Scalp metastases as first presentation of pulmonary adenocarcinomas: A case report. OncoTargets Ther. 2018, 11, 6147–6151. [Google Scholar] [CrossRef] [PubMed]
- Katz, T.M.; Silapunt, S.; Goldberg, L.H.; Jih, M.H.; Kimyai-Asadi, A. Analysis of 197 female scalp tumors treated with Mohs micrographic surgery. J. Am. Acad. Dermatol. 2005, 52, 291–294. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; Sim, W.Y.; Lew, B.L. Alopecia Neoplastica due to Gastric Adenocarcinoma Metastasis to the Scalp, Presenting as Alopecia: A Case Report and Literature Review. Ann. Dermatol. 2014, 26, 624–627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rudnicka, L.; Chrostowska, S.; Kamiński, M.; Waśkiel-Burnat, A.; Michalczyk, A.; Rakowska, A.; Olszewska, M. The role of trichoscopy beyond hair and scalp diseases. A review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Vezzoni, R.; Toffoli, L.; Conforti, C.; Dri, A.; Retrosi, C.; Meo, N.; Magaton Rizzi, G.; Signoretto, D.; Zalaudek, I. Breast Cancer-Related Neoplastic Alopecia: A Case Report and Review of the Literature. Ski. Appendage Disord. 2021, 7, 339–345. [Google Scholar] [CrossRef]
- Paolino, G.; Pampena, R.; Grassi, S.; Mercuri, S.R.; Cardone, M.; Corsetti, P.; Moliterni, E.; Muscianese, M.; Rossi, A.; Frascione, P.; et al. Alopecia neoplastica as a sign of visceral malignancies: A systematic review. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 1020–1028. [Google Scholar] [CrossRef]
- Aguiar, H.R.; Calderoni, D.R.; Stelini, R.F.; Andreollo, N.A.; Kharmandayan, P. Multiple cutaneous metastases of oesophageal squamous cell carcinoma that mimic keratoacanthoma. JPRAS Open 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Flanagan, K.E.; Burns, L.J.; Pathoulas, J.T.; Walker, C.J.; Pupo Wiss, I.; Cornejo, K.M.; Senna, M.M. Primary Alopecia Neoplastica: A Novel Case Report and Literature Review. Ski. Appendage Disord. 2021, 7, 499–509. [Google Scholar] [CrossRef]
- Pipal, V.R.; Singh, P.; Pipal, D.K.; Elhence, P. Skin Metastases in Ovarian Malignancy: A Case Report with Literature Review. J. Mid-Life Health 2023, 14, 49–52. [Google Scholar] [CrossRef]
- Tung-Hahn, E.; El-Haddad, G.; Strosberg, J. Cutaneous Neuroendocrine Metastases of Visceral Origin Responsive to Surgical Resection and Targeted Radionuclide Therapy. Case Rep. Dermatol. Med. 2024. [Google Scholar] [CrossRef]
- Wang, S.M.; Ye, M.; Ni, S.M. Multiple scalp metastases from colonic neuroendocrine carcinoma: Case report and literature review. BMC Cancer 2014, 14, 305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sciscent, B.Y.; Eberly, H.W.; Goyal, N.; Goldenberg, D. Thyroid Cancer with Cutaneous Metastases. Ear Nose Throat J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, Y.; Ke, X.; Liu, Y.; Zang, C. Cutaneous metastasis from cervical cancer to the scalp and trunk: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 435. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Patrizi, A.; Veronesi, G.; Manuelpillai, N.; Lambertini, M. Malignant cutaneous tumours of the scalp: Always remember to examine the head. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ji, P.; Peng, S.-K. Unmasking the Silent Invader: A Rare Case of Follicular Thyroid Carcinoma With Skull Metastasis and an Uncommon KRAS Q61R Mutation. Cureus 2023, 15, e47641. Available online: https://www.cureus.com/articles/188786-unmasking-the-silent-invader-a-rare-case-of-follicular-thyroid-carcinoma-with-skull-metastasis-and-an-uncommon-kras-q61r-mutation (accessed on 22 June 2024). [CrossRef] [PubMed]
- Quijano Moreno, S.L.; García de Lacoba, M. Metastasis of malignant pleural mesothelioma to the scalp following chemotherapy: A case report and review of the literature. Rev. Esp. Patol. 2022, 55 (Suppl. S1), S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Salari, B.; Sheinbein, D.M.; Rosman, I.S.; Dehner, L.P. Metastatic pancreatic adenosquamous carcinoma to the scalp: A case report and review of the literature. J. Cutan. Pathol. 2020, 47, 263–268. [Google Scholar] [CrossRef]
- Sallman, M.A.; Li, J.Y.; Swaby, M.; Chon, S.Y. Cutaneous scalp metastasis of cholangiocarcinoma in hepatitis C. JAAD Case Rep. 2020, 6, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Scalia, G.; Ferini, G.; Chaurasia, B.; Graziano, F.; Priola, S.; Amico, P.; Umana, G.E. Recurrent intracranial anaplastic ependymoma with late-onset giant scalp metastasis. Clin. Case Rep. 2023, 11, e8324. [Google Scholar] [CrossRef]
- Shastri, M.; Kundu, R. Scalp metastasis of pulmonary blastoma—A rare entity: Cytomorphology with differential diagnoses. Cytopathology 2023, 34, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Yuen, Y.F.; Lewis, E.J.; Larson, J.T.; Wilke, M.S.; Rest, E.B.; Zachary, C.B. Scalp metastases mimicking alopecia areata. First case report of placental site trophoblastic tumor presenting as cutaneous metastasis. Dermatol. Surg. 1998, 24, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Yang, S.; Lu, L.; Yu, X. Nasal Alar and Scalp Metastases From Lung Carcinoma: A Case Report and Literature Review. Ear Nose Throat J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Carson, H.J.; Pellettiere, E.V.; Lack, E. Alopecia neoplastica simulating alopecia areata and antedating the detection of primary breast carcinoma. J. Cutan. Pathol. 1994, 21, 67–70. [Google Scholar] [CrossRef]
- Wu, K.; Wu, K.; Zhang, M.; Li, X. Scalp nodule in a patient with adrenocortical cancer. Asian J. Surg. 2023, 46, 4582–4583. [Google Scholar] [CrossRef] [PubMed]
- Stanganelli, I.; Argenziano, G.; Sera, F.; Blum, A.; Ozdemir, F.; Karaarslan, I.K.; Piccolo, D.; Peris, K.; Kirchesch, H.; Bono, R.; et al. Dermoscopy of scalp tumours: A multi-centre study conducted by the international dermoscopy society. J. Eur. Acad. Dermatol. Venereol. JEADV 2012, 26, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Richmond, H.M.; Duvic, M.; Macfarlane, D.F. Primary and metastatic malignant tumors of the scalp: An update. Am. J. Clin. Dermatol. 2010, 11, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Maglie, R.; Vannucchi, M.; Quintarelli, L.; Caproni, M.; Massi, D.; Antiga, E. At the Root: Cutaneous Langerhans Cell Histiocytosis. Am. J. Med. 2018, 131, 922–926. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Grazzini, M.; Alfaioli, B.; Savarese, I.; Corciova, S.A.; Guerriero, G.; Lotti, T. Cutaneous manifestations of breast carcinoma. Dermatol. Ther. 2010, 23, 581–589. [Google Scholar] [CrossRef]
- Skafida, E.; Triantafyllopoulou, I.; Flessas, I.; Liontos, M.; Koutsoukos, K.; Zagouri, F.; Dimopoulos, A.M. Secondary Alopecia Neoplastica Mimicking Alopecia Areata following Breast Cancer. Case Rep. Oncol. 2020, 13, 627–632. [Google Scholar] [CrossRef]
- Archer, C.B.; Smith, N.P. Alopecia neoplastica responsive to tamoxifen. J. R. Soc. Med. 1990, 83, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Baum, E.M.; Omura, E.F.; Payne, R.R.; Little, W.P. Alopecia neoplastica–a rare form of cutaneous metastasis. J. Am. Acad. Dermatol. 1981, 4, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Lin, W.C.; Jung, S.M.; Yang, C.H.; Hong, H.S. Breast cancer metastasized to the scalp mimicking alopecia areata: Alopecia neoplastica. Breast J. 2007, 13, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Levy, E. SCHREIBER H: Alopecia neoplastica due to breast carcinoma. Arch. Dermatol. 1961, 84, 490–492. [Google Scholar] [CrossRef]
- Paolino, G.; Panetta, C.; Didona, D.; Donati, M.; Donati, P. Folliculotropic Cutaneous Metastases and Lymphangitis Carcinomatosa: When Cutaneous Metastases of Breast Carcinoma Are Mistaken for Cutaneous Infections. Acta Dermatovenerol. Croat. ADC 2016, 24, 154–157. [Google Scholar] [PubMed]
- Salemis, N.S.; Veloudis, G.; Spiliopoulos, K.; Nakos, G.; Vrizidis, N.; Gourgiotis, S. Scalp metastasis as the first sign of small-cell lung cancer: Management and literature review. Int. Surg. 2014, 99, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Deng, M.; Fan, H. Scalp and Finger Metastasis From Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2022, 20, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M.; Tagor, V.; Myint, A.S.; Memon, A. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J. Am. Acad. Dermatol. 2003, 48, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Brasanac, D.; Boricic, I.; Todorovic, V. Epidermotropic metastases from breast carcinoma showing different clinical and histopathological features on the trunk and on the scalp in a single patient. J. Cutan. Pathol. 2003, 30, 641–646. [Google Scholar] [CrossRef]
- Scheinfeld, N. Review of scalp alopecia due to a clinically unapparent or minimally apparent neoplasm (SACUMAN). Acta Derm. Venereol. 2006, 86, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Crotty, K.; McCarthy, W.; Quinn, M.; McCarthy, S. Alopecia neoplastica caused by desmoplastic melanoma. Australas. J. Dermatol. 2003, 44, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Primary alopecia neoplastica versus secondary alopecia neoplastica: A new classification for neoplasm-associated scalp hair loss. J. Cutan. Pathol. 2009, 36, 917–918. [Google Scholar] [CrossRef] [PubMed]
- AlSubait, N.A.; BinJadeed, H.F.; AlSaleh, M.R.; AlFaifi, F.S.; AlSaif, F.M.; Arafah, M.A. Dermoscopy of scalp cutaneous metastasis of sigmoid adenocarcinoma. JAAD Case Rep. 2021, 14, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Çetinarslan, T.; Ermertcan, A.T.; Temiz, P.; Evrenos, M.K.; Müezzinoğlu, T. Dermoscopy of scalp cutaneous metastasis of sarcomatoid renal cell carcinoma. Dermatol. Ther. 2020, 33, 14189. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, G.M.; Starace, M.; Alessandrini, A.M.; Guicciardi, F.; Moustafa, F.; Brandi, N.; Piraccini, B.M. Trichoscopy of Scalp Metastases. Int. J. Trichology 2019, 11, 86–87. [Google Scholar] [CrossRef]
- Costa, J.; Ortiz-Ibañez, K.; Salerni, G.; Borges, V.; Carrera, C.; Puig, S.; Malvehy, J. Dermoscopic patterns of melanoma metastases: Interobserver consistency and accuracy for metastasis recognition. Br. J. Dermatol. 2013, 169, 91–99. [Google Scholar] [CrossRef]
- Fagotti, S.; Pizzichetta, M.A.; Corneli, P.; Toffolutti, F.; Serraino, D.; di Meo, N.; Zalaudek, I. Dermoscopic features of face and scalp basal and squamous cell carcinomas according to clinical histopathologic characteristics and anatomical location. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e237–e239. [Google Scholar] [CrossRef]
- Lallas, A.; Apalla, Z.; Ioannides, D.; Argenziano, G.; Castagnetti, F.; Moscarella, E.; Longo, C.; Palmieri, T.; Ramundo, D.; Zalaudek, I. Dermoscopy in the diagnosis and management of basal cell carcinoma. Future Oncol. 2015, 11, 2975–2984. [Google Scholar] [CrossRef]
- Moscarella, E.; Piana, S.; Specchio, F.; Kyrgidis, A.; Nazzaro, G.; Eliceche, M.L.; Savoia, F.; Bugatti, L.; Filosa, G.; Zalaudek, I.; et al. Dermoscopy features of atypical fibroxanthoma: A multicenter study of the International Dermoscopy Society. Australas. J. Dermatol. 2018, 59, 309–314. [Google Scholar] [CrossRef]
- Lim, E.J.; Leong, N.; McAdory, L.E.; Ho, C.L. Multimodality imaging and treatment strategy for malignant scalp neoplasms in adults. Clin. Imaging 2021, 77, 48–57. [Google Scholar] [CrossRef]
- Hayman, L.A.; Shukla, V.; Ly, C.; Taber, K.H. Clinical and imaging anatomy of the scalp. J. Comput. Assist. Tomogr. 2003, 27, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Jouvet, J.C.; Thomas, L.; Thomson, V.; Yanes, M.; Journe, C.; Morelec, I.; Bracoud, L.; Durupt, F.; Giammarile, F.; Berthezene, Y. Whole-body MRI with diffusion-weighted sequences compared with 18 FDG PET-CT, CT and superficial lymph node ultrasonography in the staging of advanced cutaneous melanoma: A prospective study. J. Eur. Acad. Dermatol. Venereol. JEADV 2014, 28, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Onufer, J.; MacFarlane, D.F. Imaging of head and neck skin cancer. In Skin Cancer Management: A Practical Approach; MacFarlane, D.F., Ed.; Springer: New York, NY, USA, 2010; pp. 239–258. [Google Scholar]
- Wu, L.; Ou, Y.; Liu, B.; Liu, W. Scalp Metastasis of Anaplastic Oligodendroglioma. World Neurosurg. 2019, 128, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Ginat, D.T.; Kelly, H.R.; Schaefer, P.W.; Davidson, C.J.; Curry, W. Recurrent scalp metastasis from glioblastoma following resection. Clin. Neurol. Neurosurg. 2013, 115, 461–463. [Google Scholar] [CrossRef]
- Subasinghe, D.; Keppetiyagama, C.T.; Sudasinghe, H.; Wadanamby, S.; Perera, N.; Sivaganesh, S. Solitary scalp metastasis—A rare presentation of hepatocellular carcinoma. Ann. Surg. Innov. Res. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.G.; Oudsema, R.; Masseaux, J.A.; Rosenberg, H.K. US of Pediatric Superficial Masses of the Head and Neck. Radiographics: A review publication of the Radiological Society of North America. Radiographics 2018, 38, 1239–1263. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Wortsman, J.; Matsuoka, L.; Saavedra, T.; Mardones, F.; Saavedra, D.; Guerrero, R.; Corredoira, Y. Sonography in pathologies of scalp and hair. Br. J. Radiol. 2012, 85, 647–655. [Google Scholar] [CrossRef]
- Kumar, R.; Mavi, A.; Bural, G.; Alavi, A. Fluorodeoxyglucose-PET in the management of malignant melanoma. Radiol. Clin. N. Am. 2005, 43, 23–33. [Google Scholar] [CrossRef]
- Sager, S.; Yilmaz, S.; Doner, R.K.; Niyazoglu, M.; Halac, M.; Kanmaz, B. A rare case of solitary subcutaneous scalp metastasis from follicular thyroid carcinoma revealed with positron emission tomography/computed tomography: A case report and review. J. Cancer Res. Ther. 2014, 10, 431–433. [Google Scholar] [CrossRef]
- Alharbi, M.; Ashkar, L.; Abusanad, A. A Rare Presentation of Cutaneous Scalp Metastasis From a Malignant Phyllodes Tumor of the Breast: A Case Report and Literature Review. Cureus 2023, 15, 50009. [Google Scholar] [CrossRef]
- Oh, T.H.; Kim, H.S.; Park, S.C. Scalp nodules as the first presentation of prostate cancer: A CARE-compliant article. Medicine 2023, 102, 36570. [Google Scholar] [CrossRef]
- Lee, H.W.; Hwang, Y.J.; Jung, S.G.; Hong, I.P. Nodular scalp mass as the first presentation of pulmonary large cell neuroendocrine carcinoma: A case report. Arch. Craniofacial Surg. 2023, 24, 240–243. [Google Scholar] [CrossRef]
- Handa, U.; Kundu, R.; Dimri, K. Cutaneous Metastasis: A Study of 138 Cases Diagnosed by Fine-Needle Aspiration Cytology. Acta Cytol. 2017, 61, 47–54. [Google Scholar] [CrossRef]
- Mondal, A.; Ray, C.K. Clinically malignant scalp and underlying bone tumours diagnosed by aspiration cytology: A study of 121 cases. J. Indian. Med. Assoc. 1992, 90, 175–178. [Google Scholar]
- Doan, M.; Ramani, N.S.; Arbab, F.; Green, L.K. Fine-needle aspiration of scalp masses: A review of 30 cases. Diagn. Cytopathol. 2023, 51, 140–145. [Google Scholar] [CrossRef]
- Sharma, P.; Pathak, P.; Goyal, A.; Sharma, S. Cytomorphological spectrum of scalp lesions in the population of a developing country: A retrospective study. Diagn. Cytopathol. 2019, 47, 571–578. [Google Scholar] [CrossRef]
- Alsaad, K.O.; Obaidat, N.A.; Ghazarian, D. Skin adnexal neoplasms–part 1: An approach to tumours of the pilosebaceous unit. J. Clin. Pathol. 2007, 60, 129–144. [Google Scholar] [CrossRef]
- Fernandez-Flores, A. Cutaneous metastases: A study of 78 biopsies from 69 patients. Am. J. Dermatopathol. 2010, 32, 222–239. [Google Scholar] [CrossRef]
- Abdulraheem, A.M.; Naji, D.; Al Heyasat, A.N.; Alhasan, M.; Almasri, N.M.; Odeh, R. Breast cancer with scalp metastases: A case report. J. Med. Case Rep. 2023, 17, 203. [Google Scholar] [CrossRef]
- Rekhtman, N.; Pietanza, C.M.; Sabari, J.; Montecalvo, J.; Wang, H.; Habeeb, O.; Kadota, K.; Adusumilli, P.; Rudin, C.M.; Ladanyi, M.; et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: Napsin A expression and genomic alterations. Mod. Pathol. 2018, 31, 111–121. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, H.; Cagle, P.T. Utility of immunohistochemistry in the diagnosis of pleuropulmonary and mediastinal cancers: A review and update. Arch. Pathol. Lab. Med. 2014, 138, 1611–1628. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Value of the Ber-EP4 Antibody in Differentiating Epithelial Pleural Mesothelioma From Adenocarcinoma: The M.D. Anderson Experience and a Critical Review of the Literature. Am. J. Clin. Pathol. 1998, 109, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Compton, L.A.; Murphy, G.F.; Lian, C.G. Diagnostic Immunohistochemistry in Cutaneous Neoplasia: An Update. Dermatopathology 2015, 2, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, M.; Nguyen, L.P.; Richards, J.E.; Muzikansky, A.; Hoang, M.P. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: An immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod. Pathol. 2010, 23, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Conner, K.B.; Cohen, P.R. Cutaneous metastasis of breast carcinoma presenting as alopecia neoplastica. South. Med. J. 2009, 102, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.P.; Forder, A.; Brockley, L.J.; Pewarchuk, M.E.; Telkar, N.; de Araújo, R.P.; Trejo, J.; Benard, K.; Seneda, A.L.; Minutentag, I.W.; et al. Liquid Biopsy in Lung Cancer: Biomarkers for the Management of Recurrence and Metastasis. Int. J. Mol. Sci. 2023, 24, 8894. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Marchetti, D.; Lang, J.E. Liquid biopsy: From concept to clinical application. Sci. Rep. 2023, 13, 21685. [Google Scholar] [CrossRef] [PubMed]
- Benati, E.; Ribero, S.; Longo, C.; Piana, S.; Puig, S.; Carrera, C.; Cicero, F.; Kittler, H.; Deinlein, T.; Zalaudek, I.; et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: An International Dermoscopy Society study. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 732–736. [Google Scholar] [CrossRef]
- Xie, C.; Pan, Y.; McLean, C.; Mar, V.; Wolfe, R.; Kelly, J.W. Scalp melanoma: Distinctive high risk clinical and histological features. Australas. J. Dermatol. 2017, 58, 181–188. [Google Scholar] [CrossRef]
- Namin, A.W.; Cornell, G.E.; Thombs, L.A.; Zitsch, R.P. Patterns of recurrence and retreatment outcomes among clinical stage I and II head and neck melanoma patients. Head. Neck 2019, 41, 1304–1311. [Google Scholar] [CrossRef]
- Unsal, A.A.; Patel, V.R.; Chung, S.Y.; Zhou, A.H.; Baredes, S.; Eloy, J.A. Head and neck sweat gland adenocarcinoma: A population-based perspective of a rare entity. Laryngoscope 2017, 127, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.J. Management of malignant tumors of the scalp. Ann. N. Y. Acad. Sci. 1964, 114, 976–984. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedirian, S.; Rapparini, L.; Sechi, A.; Piraccini, B.M.; Starace, M. Diagnosis and Management of Scalp Metastases: A Review. Diagnostics 2024, 14, 1638. https://doi.org/10.3390/diagnostics14151638
Cedirian S, Rapparini L, Sechi A, Piraccini BM, Starace M. Diagnosis and Management of Scalp Metastases: A Review. Diagnostics. 2024; 14(15):1638. https://doi.org/10.3390/diagnostics14151638
Chicago/Turabian StyleCedirian, Stephano, Luca Rapparini, Andrea Sechi, Bianca Maria Piraccini, and Michela Starace. 2024. "Diagnosis and Management of Scalp Metastases: A Review" Diagnostics 14, no. 15: 1638. https://doi.org/10.3390/diagnostics14151638
APA StyleCedirian, S., Rapparini, L., Sechi, A., Piraccini, B. M., & Starace, M. (2024). Diagnosis and Management of Scalp Metastases: A Review. Diagnostics, 14(15), 1638. https://doi.org/10.3390/diagnostics14151638








