Influencing Factors Regarding the Severity of Peri-Implantitis and Peri-Implant Mucositis
Abstract
1. Introduction
1.1. The Success Rate of the Implantation
1.2. Peri-Implant Mucositis
1.3. Peri-Implantitis
1.4. Risk Factors for Developing Peri-Implant Inflammations
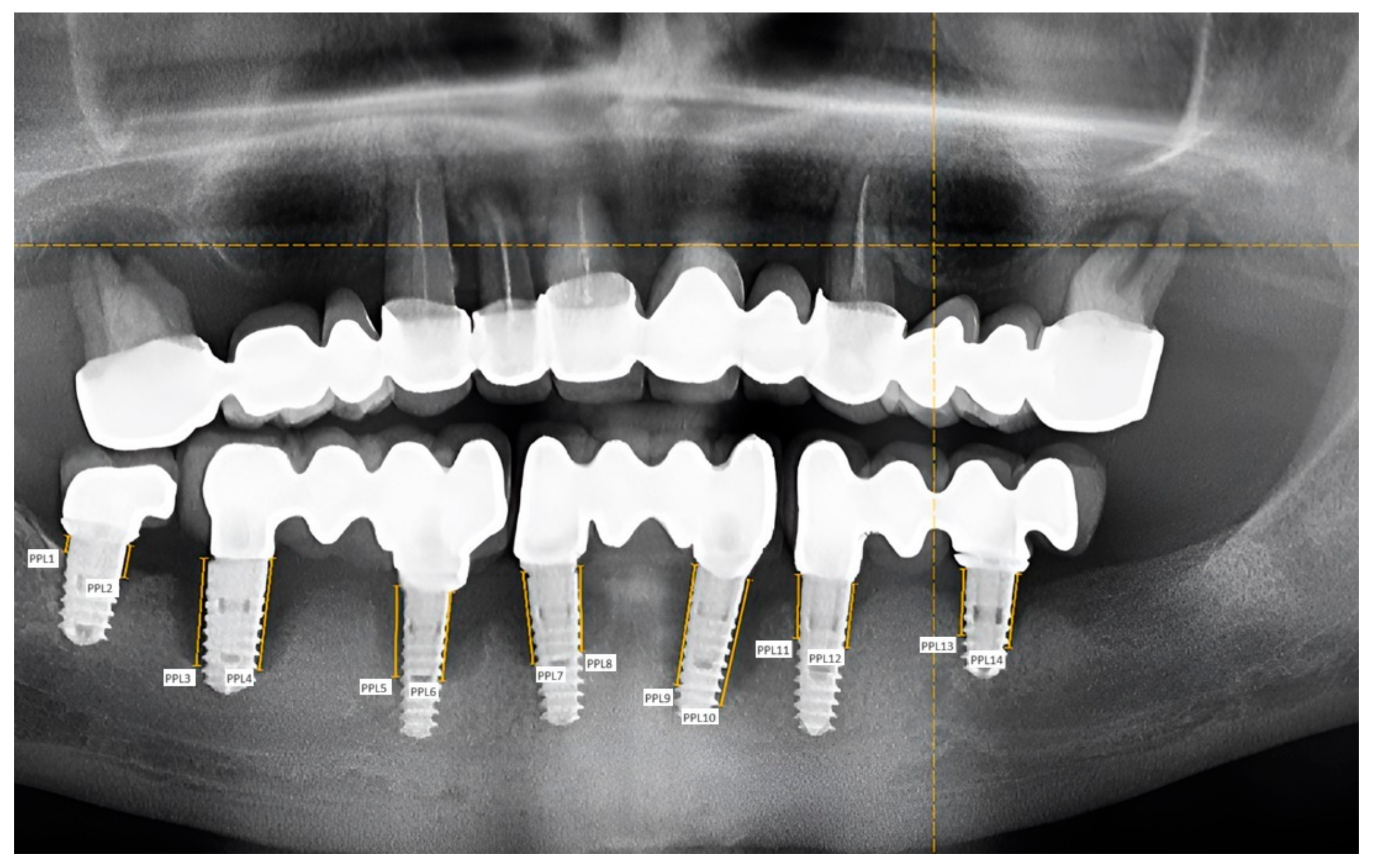
2. Materials and Methods
- -
- At least one dental implant placed.
- -
- Absence of systemic diseases in the background, especially those that directly affect the success and survival rate of dental implants, such as osteoporosis, diabetes, neurological disorders, HIV, hypothyroidism, and cardiovascular diseases.
- -
- Absence of oral cavity changes, such as autoimmune mucocutaneous diseases, necrotizing periodontal diseases, scurvy, and extensive necrosis in agranulocytosis.
- -
- Absence of medication: Only patients who were not taking any medication were included in this study. The most important drugs that could affect the survival rate of dental implants are bisphosphonates, non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, proton pump inhibitors (PPIs), and serotonin reuptake inhibitors (SSRIs).
- -
- Non-smoking patients.
- -
- Good or acceptable patient oral hygiene protocol, performed at least twice daily. Oral hygiene habits were considered by designing a questionnaire.
- -
- No inserted dental implant;
- -
- Presence of systemic diseases;
- -
- Presence of oral cavity changes;
- -
- Long-term daily medication of the patient;
- -
- Smoking patients;
- -
- Poor oral hygiene;
- -
- Absence of evaluable digital panoramic X-rays.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Block, M.S. Dental implants: The last 100 years. J. Oral Maxillofac. Surgery 2018, 76, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol. 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Nervins, M. Implant dentistry: A continuing evolution. Int. J. Periodontics Restor. Dent. 2014, 34, 7. [Google Scholar] [CrossRef] [PubMed]
- Vajdovich, I.; Orosz, M. Realization of tissue care concept by the use of Denti bone level implants. Three years of clinical experience in applying Denti BL implants. Fogorvosi Szle. 2012, 105, 155–156. [Google Scholar]
- Karthik, K.; Sivaraj, S. Evaluation of implant success: A review of past and present concepts. J. Pharm. Bioallied Sci. 2013, 5, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Makary, C.; Menhall, A. Primary stability optimization by using fixtures with different thread depth according to bone density: A clinical prospective study on early loaded implants. Materials 2019, 12, 2398. [Google Scholar] [CrossRef]
- Cicciu, M. Bioengineering methods of analysis and medical devices: Current trends and state of the art. Materials 2020, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.J.; Dhinakarsamy, V. Osseoinegration. J. Pharm. Bioallied Sci. 2015, 7, 226–229. [Google Scholar] [CrossRef]
- Iacono, V.J.; Bassir, S.H.; Wang, H.H.; Myneni, S.R. Peri-implantitis: Effects of periodontitis and its risk factors—A narrative review. FOMM 2023, 5, 27. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G. Success criteria in implants dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J. Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L. Peri-implant mucositis and peri-implantitis: Key features and differences. Br. Dent. J. 2024, 236, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.; Salvi, G.E. Peri-implant mucositis. J. Periodontol. 2018, 89, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Aglietta, M.; Eick, S.; Sculean, A.; Lang, N.P.; Ramseier, C.A. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin. Oral Implant. Res. 2012, 23, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Chen, S.T.; Wilson, T.G. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int. J. Oral Maxillofac. Implant. 2004, 19, 26–28. [Google Scholar]
- Derks, J.; Schaller, D.; Hakansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Peri-implantitis—Onset and pattern of progression. J. Clin. Periodontol. 2016, 43, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Lapinska, B.; Nissan, J.; Lukomska-Szymanska, M. Factors influencing marginal bone loss around dental implants: A narrative review. Coatings 2021, 11, 865. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Catena, A.; Pérez-Sayáns, M.; Fernández-Barbero, J.E.; O’Valle, F.; Padial-Molina, M. Early marginal bone loss around dental implants to define success in implant dentistry: A retrospective study. Clin. Implant. Dent. Relat. Res. 2022, 24, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.E.; Putz, M.; Patzelt, S.; Alkalak, A.; Uelkuemen, S.; Boeker, M. Marginal bone loss around oral implants supporting fixed versus removable prostheses: A systematic review. Int. J. Implant. Dent. 2020, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, N.; Reed, D.N.; Walters, J.D.; Kumar, P. Periodontal and peri-implant diseases: Identical or fraternal infections? Mol. Oral Microbiol. 2016, 31, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H. Peri-implantitis. J. Clin. Periodontol. 2018, 45, 246–266. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Wang, Z.; Li, L.; Zhang, J.; Zhang, Q.; Chen, T.; Lin, J.; Chen, F. Subgingival microbiome in patients with healthy and ailing dental implants. Sci. Rep. 2015, 16, 10948. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.D.; Silva, G.L.; Cortelli, J.R.; Costa, J.E.; Costa, F.O. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J. Clin. Periodontol. 2006, 33, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, H.Y.; Sun, T.C.; Karimbux, N. Impact of patient compliance on tooth loss during supportive periodontal therapy: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis update: Risk indicators, diagnosis, and treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef]
- Gera, I. Periodontology, 1st ed.; Semmelweis Kiadó: Budapest, Hungary, 2009; pp. 30, 421. [Google Scholar]
- Causes and Treatment of the Failure of Dental Implant Restorations. Available online: https://www.semmelweis.hu/szajsebeszet (accessed on 29 June 2018).
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Duraisamy, R. Crestal Bone Loss in Implants Postloading and Its Association with Age, Gender, and Implant Site: A Retrospective Study. J. Long-Term Eff. Med. Implant. 2020, 30, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Vajdovich, I. Dental Implantology, 1st ed.; Semmelweis: Budapest, Hungary, 2017; pp. 40–52, 245–259. [Google Scholar]
- Ogata, Y.; Nakayama, Y.; Tatsumi, J.; Kubota, T.; Sato, S.; Nishida, T.; Takeuchi, Y.; Onitsuka, T.; Sakagami, R.; Nozaki, T.; et al. Prevalence and risk factors for peri-implant diseases in Japanese adult dental patients. J. Oral Sci. 2017, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dental Implants—Esthetic Complications. Available online: https://www.dental.hu/fogaszati-implantatumok-esztetikai-komplikaciok (accessed on 6 February 2024).
- Ferreira, C.F.; Buttendorf, A.R.; de Souza, J.G.; Dalago, H.; Guenther, S.F.; A Bianchini, M. Prevalence of peri-implant diseases: Analyses of associated factors. Eur. J. Prosthodont. Restor. Dent. 2015, 23, 199–206. [Google Scholar] [PubMed]
- Mahardawi, B.; Jiaranuchart, S.; Damrongsirirat, N.; Arunjaroensuk, S.; Mattheos, N.; Somboonsavatdee, A.; Pimkhaokham, A. The lack of keratinized mucosa as a risk factor for peri-implantitis: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 3778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afrashtehfar, K.I.; Oh, K.C.; Jurado, C.A.; Lee, H. Lack of keratinized mucosa increases peri-implantitis risk. Evid.-Based Dent. 2023, 24, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Staubli, N.; Walter, C.; Schmidt, J.C.; Weiger, R.; Zitzmann, N.U. Excess cement and the risk of peri-implant disease—A systematic review. Clin. Oral Implant. Res. 2017, 28, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.; Zhang, L.; Gaillard, P.; Raedel, M.; Walter, M.; Konstantinidis, I. Investigation of the Association Between Cement-Retention and Prevalent Peri-Implant Diseases: A Cross-Sectional Study. J. Periodontol. 2015, 87, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, S.F.; Land, M.F.; Fujimoto, J. Contemporary Fixed Prosthodontics, 5th ed.; Elsevier Inc.: St. Louis, MO, USA, 2016; pp. 334, 358–361. [Google Scholar]
- Chio, A.; Hatai, Y. Restoration of two implants using custom abutments and transverse screw-retained zirconia crowns. Am. J. Esthet. Dent. 2012, 2, 264–280. [Google Scholar]
- Wittneben, J.G.; Joda, T.; Weber, H.P.; Bragger, U. Screw retained vs. cement retained implant-supported fixed dental prosthesis. Periodontol. 2000 2017, 73, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Jánosi, K.M.; Cerghizan, D.; Berneanu, F.D.; Kovács, A.; Szász, A.; Mureșan, I.; Hănțoiu, L.G.; Albu, A.I. Full-mouth rehabilitation of a patient with gummy smile—Multidisciplinary approach: Case Report. Medicina 2023, 59, 197. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Tarnow, D. The etiology of hard- and soft-tissue deficiencies at dental implants: A narrative review. J. Periodontol. 2018, 89, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Petkovic-Curcin, A.; Zeljic, K.; Cikota-Aleksic, B.; Dakovic, D.; Tatic, Z.; Magic, Z. Association of cytokine gene polymorphism with peri-implantitis risk. Int. J. Oral Maxillofac. Implant. 2017, 32, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.A.; Tozoğlu, S.; Yıldırımyan, N.; Özarslan, M. Is history of periodontitis a risk factor for peri-implant disease? A pilot study. Int. J. Oral Maxillofac. Implant. 2018, 33, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.-X.; Zhong, J.-S.; Ouyang, X.-Y.; Iao, S.; Liu, J.; Xie, Y. Investigation of peri-implant diseases prevalence and related risk indicators in patients with treated severe periodontitis over 4 years after restoration. J. Dent. Sci. 2024, 19, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, V.; Ríos-Carrasco, B.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Bullón, B.; Herrero-Climent, M.; Bullón, P. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 4147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coli, P.; Christiaens, V.; Sennerby, L.; de Bruyn, H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol. 2000 2017, 73, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45, 230–236. [Google Scholar] [CrossRef] [PubMed]


| Age Group | PPD around Natural Teeth (Mean ± SD) | PD around Implants (Mean ± SD) | p Value | Peri-Implant Mucosa Width (Mean ± SD) |
|---|---|---|---|---|
| Under 35 years | 4.14 ± 1.16 | 4.75 ± 1.32 | 0.188 | 3.43 ± 1.82 |
| 35–55 years | 4.24 ± 0.83 | 5.08 ± 0.95 | 0.002 | 2.54 ± 1.32 |
| Over 55 years | 4.09 ± 0.91 | 5.12 ± 1.30 | 0.014 | 2.07 ± 2.10 |
| Insertion of the Implant (s) | PPD around Natural Teeth (Mean ± SD) | PD around Implants (Mean ± SD) | p Value | Peri-Implant Mucosa Width (Mean ± SD) |
|---|---|---|---|---|
| 1–3 years ago | 4.14 ± 0.88 | 4.86 ± 1.08 | 0.04 | 3.29 ± 1.52 |
| 4–7 years ago | 4.09 ± 0.91 | 5.12 ± 1.30 | 0.014 | 2.07 ± 2.10 |
| more than 7 years ago | 4.27 ± 0.95 | 5.13 ± 1.03 | 0.016 | 2.29 ± 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedek, C.; Kerekes-Máthé, B.; Bereșescu, L.; Buka, I.Z.; Bardocz-Veres, Z.; Geréb, I.; Mártha, K.I.; Jánosi, K.M. Influencing Factors Regarding the Severity of Peri-Implantitis and Peri-Implant Mucositis. Diagnostics 2024, 14, 1573. https://doi.org/10.3390/diagnostics14141573
Benedek C, Kerekes-Máthé B, Bereșescu L, Buka IZ, Bardocz-Veres Z, Geréb I, Mártha KI, Jánosi KM. Influencing Factors Regarding the Severity of Peri-Implantitis and Peri-Implant Mucositis. Diagnostics. 2024; 14(14):1573. https://doi.org/10.3390/diagnostics14141573
Chicago/Turabian StyleBenedek, Csilla, Bernadette Kerekes-Máthé, Liana Bereșescu, Imola Zsuzsa Buka, Zsuzsanna Bardocz-Veres, Ildikó Geréb, Krisztina Ildikó Mártha, and Kinga Mária Jánosi. 2024. "Influencing Factors Regarding the Severity of Peri-Implantitis and Peri-Implant Mucositis" Diagnostics 14, no. 14: 1573. https://doi.org/10.3390/diagnostics14141573
APA StyleBenedek, C., Kerekes-Máthé, B., Bereșescu, L., Buka, I. Z., Bardocz-Veres, Z., Geréb, I., Mártha, K. I., & Jánosi, K. M. (2024). Influencing Factors Regarding the Severity of Peri-Implantitis and Peri-Implant Mucositis. Diagnostics, 14(14), 1573. https://doi.org/10.3390/diagnostics14141573







