Impacts and Correlations on Corneal Biomechanics, Corneal Optical Density and Intraocular Pressure after Cataract Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Surgical Procedures of Cataract Surgery
2.3. Clinical Assessment
2.4. Sample Size Estimation
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Corneal Biomechanics
3.2.1. Basic Parameters of the Corvis ST
3.2.2. Integrated Parameters of Corvis ST
3.2.3. Biomechanics Changes during Extended Observation
3.3. COD and CT
3.3.1. COD
3.3.2. CT
3.4. Correlation between COD and Biomechanics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kling, S.; Hafezi, F. Corneal biomechanics–A review. Ophthalmic Physiol. Opt. 2017, 37, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Marshall, J. A review of corneal biomechanics: Mechanisms for measurement and the implications for refractive surgery. Indian J. Ophthalmol. 2020, 68, 2679. [Google Scholar]
- Rahmati, S.M.; Razaghi, R.; Karimi, A. Biomechanics of the keratoconic cornea: Theory, segmentation, pressure distribution, and coupled FE-optimization algorithm. J. Mech. Behav. Biomed. Mater. 2021, 113, 104155. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Lopes, B.T.; Zheng, X.; Ji, Y.; Wang, J.; Elsheikh, A. Corneal biomechanics losses caused by refractive surgery. Curr. Eye Res. 2023, 48, 137–143. [Google Scholar] [CrossRef]
- Chan, E.; Mahroo, O.A.; Spalton, D.J. Complications of cataract surgery. Clin. Exp. Optom. 2010, 93, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Lyle, W.A.; Jin, G.J. Prospective evaluation of early visual and refractive effects with small clear corneal incision for cataract surgery. J. Cataract. Refract. Surg. 1996, 22, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmood, A.M.; Al-Swailem, S.A.; Behrens, A. Clear corneal incision in cataract surgery. Middle East Afr. J. Ophthalmol. 2014, 21, 25. [Google Scholar] [PubMed]
- Kato, Y.; Nakakura, S.; Asaoka, R.; Matsuya, K.; Fujio, Y.; Kiuchi, Y.; on behalf of the Ocular Biomechanics Study Group. Cataract surgery causes biomechanical alterations to the eye detectable by Corvis ST tonometry. PLoS ONE 2017, 12, e0171941. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, K.; Nakakura, S.; Nakao, Y.; Fujino, Y.; Matsuura, M.; Murata, H.; Kiuchi, Y.; Asaoka, R. Changes in corneal biomechanics and intraocular pressure following cataract surgery. Am. J. Ophthalmol. 2018, 195, 26–35. [Google Scholar] [CrossRef]
- Wallace, H.B.; Misra, S.L.; Li, S.S.; McKelvie, J. Biomechanical changes in the cornea following cataract surgery: A prospective assessment with the Corneal Visualisation Scheimpflug Technology. Clin. Exp. Ophthalmol. 2019, 47, 461–468. [Google Scholar] [CrossRef]
- Kunzmann, B.C.; Wenzel, D.A.; Bartz-Schmidt, K.U.; Spitzer, M.S.; Schultheiss, M. Effects of ultrasound energy on the porcine corneal endothelium–Establishment of a phacoemulsification damage model. Acta Ophthalmol. 2020, 98, e155–e160. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.S.; Wälti, R.; Böhnke, M. Corneal thickness and endothelial density before and after cataract surgery. Br. J. Ophthalmol. 2001, 85, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Oki, K.; Takahashi, K.; Shiwa, T.; Takahashi, H. Functional evaluation of corneal endothelium by combined measurement of corneal volume alteration and cell density after phacoemulsification. J. Cataract. Refract. Surg. 2007, 33, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Yu, H.-J.; Yang, I.-H.; Ho, R.-W.; Hsiao, Y.-T.; Fang, P.-C.; Kuo, M.-T. Simultaneously monitoring whole corneal injury with corneal optical density and thickness in patients undergoing cataract surgery. Diagnostics 2021, 11, 1639. [Google Scholar] [CrossRef] [PubMed]
- Ávila, F.J.; Marcellán, M.C.; Remón, L. On the relationship between corneal biomechanics, macrostructure, and optical properties. J. Imaging 2021, 7, 280. [Google Scholar] [CrossRef] [PubMed]
- Iancu, R.C.; Bujor, I.A.; Iliuță, C.; Tudor, Ș.; Ungureanu, E.; Pașca, I.G.; Istrate, S. Correlations between corneal biomechanics and specular microscopy in patient with cataract. Rom. J. Ophthalmol. 2020, 64, 132. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Han, T.; Jhanji, V.; Shang, J.; Zhao, J.; Li, M.; Zhou, X. Correlation between corneal topographic, densitometry, and biomechanical parameters in keratoconus eyes. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.T.; Roberts, C.J.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Reisdorf, S.; Berger, S.; Koprowski, R.; Ambrósio, R. Repeatability and reproducibility of intraocular pressure and dynamic corneal response parameters assessed by the Corvis ST. J. Ophthalmol. 2017, 2017, 851572. [Google Scholar] [CrossRef] [PubMed]
- Dhubhghaill, S.N.; Rozema, J.J.; Jongenelen, S.; Hidalgo, I.R.; Zakaria, N.; Tassignon, M.-J. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 162–168. [Google Scholar] [CrossRef]
- Ambrósio Jr, R.; Alonso, R.S.; Luz, A.; Velarde, L.G.C. Corneal-thickness spatial profile and corneal-volume distribution: Tomographic indices to detect keratoconus. J. Cataract. Refract. Surg. 2006, 32, 1851–1859. [Google Scholar] [CrossRef]
- Qin, B.; Tang, M.; Li, Y.; Zhang, X.; Chu, R.; Huang, D. Anterior segment dimensions in Asian and Caucasian eyes measured by optical coherence tomography. Ophthalmic Surg. Lasers Imaging Retin. 2012, 43, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Poley, B.J.; Lindstrom, R.L.; Samuelson, T.W.; Schulze, R., Jr. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: Evaluation of a causal relationship between the natural lens and open-angle glaucoma. J. Cataract. Refract. Surg. 2009, 35, 1946–1955. [Google Scholar] [CrossRef]
- Aoki, S.; Asaoka, R.; Fujino, Y.; Nakakura, S.; Murata, H.; Kiuchi, Y. Comparing corneal biomechanic changes among solo cataract surgery, microhook ab interno trabeculotomy and iStent implantation. Sci. Rep. 2023, 13, 19148. [Google Scholar] [CrossRef] [PubMed]
- Azuara-Blanco, A.; Burr, J.; Ramsay, C.; Cooper, D.; Foster, P.J.; Friedman, D.S.; Scotland, G.; Javanbakht, M.; Cochrane, C.; Norrie, J. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. Lancet 2016, 388, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wu, N.; Fu, Z.; Kong, X. Evaluation of corneal deformation parameters provided by the Corvis ST tonometer after trabeculectomy. J. Glaucoma 2017, 26, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Felletti, M.; Grasso, L.; Brusini, P. Corneal deformation parameters provided by the Corvis-ST pachy-tonometer in healthy subjects and glaucoma patients. J. Glaucoma 2015, 24, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Melancia, D.; Abegão Pinto, L.; Marques-Neves, C. Cataract surgery and intraocular pressure. Ophthalmic Res. 2015, 53, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lubbad, A.; Oluwatoba-Popoola, I.; Haar, M.; Framme, C.; Bajor, A. The influence of corneal density and thickness on tonometry measurement with goldmann applanation, non-contact and iCare tonometry methods. Int. Ophthalmol. 2022, 42, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Ma, X.; Qu, Z.; Eliasy, A.; Wu, B.; Xu, H.; Wang, P.; Zheng, X.; Wang, J.; Ye, Y. Performance of Updated Stress-Strain Index in Differentiating between Normal, Forme-Fruste, Subclinical and Clinical Keratoconic Eyes. Am. J. Ophthalmol. 2023, 258, 196–207. [Google Scholar] [CrossRef]
- Eliasy, A.; Chen, K.-J.; Vinciguerra, R.; Lopes, B.T.; Abass, A.; Vinciguerra, P.; Ambrósio, R., Jr.; Roberts, C.J.; Elsheikh, A. Determination of corneal biomechanical behavior in-vivo for healthy eyes using CorVis ST tonometry: Stress-strain index. Front. Bioeng. Biotechnol. 2019, 7, 105. [Google Scholar] [CrossRef]
- Herber, R.; Vinciguerra, R.; Lopes, B.; Raiskup, F.; Pillunat, L.E.; Vinciguerra, P.; Ambrósio, R., Jr. Repeatability and reproducibility of corneal deformation response parameters of dynamic ultra-high-speed Scheimpflug imaging in keratoconus. J. Cataract. Refract. Surg. 2020, 46, 86–94. [Google Scholar] [PubMed]
- Vinciguerra, R.; Ambrósio Jr, R.; Elsheikh, A.; Roberts, C.J.; Lopes, B.; Morenghi, E.; Azzolini, C.; Vinciguerra, P. Detection of keratoconus with a new biomechanical index. J. Refract. Surg. 2016, 32, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xu, L.; Fan, Q.; Gu, Y.; Yang, K. Accuracy of new Corvis ST parameters for detecting subclinical and clinical keratoconus eyes in a Chinese population. Sci. Rep. 2021, 11, 4962. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ma, B.-S.; Zeng, J.-H.; Ma, D.-J. Corneal optical density: Structural basis, measurements, influencing factors, and roles in refractive surgery. Front. Bioeng. Biotechnol. 2023, 11, 1144455. [Google Scholar] [CrossRef] [PubMed]
- Briceno-Lopez, C.; Burguera-Giménez, N.; García-Domene, M.C.; Díez-Ajenjo, M.A.; Peris-Martínez, C.; Luque, M.J. Corneal Edema after Cataract Surgery. J. Clin. Med. 2023, 12, 6751. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial–stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The corneal basement membranes and stromal fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef]
- Wang, X.; McAlinden, C.; Zhang, H.; Yan, J.; Wang, D.; Wei, W.; Mi, S. Assessment of corneal biomechanics, tonometry and pachymetry with the Corvis ST in myopia. Sci. Rep. 2021, 11, 3041. [Google Scholar] [CrossRef]

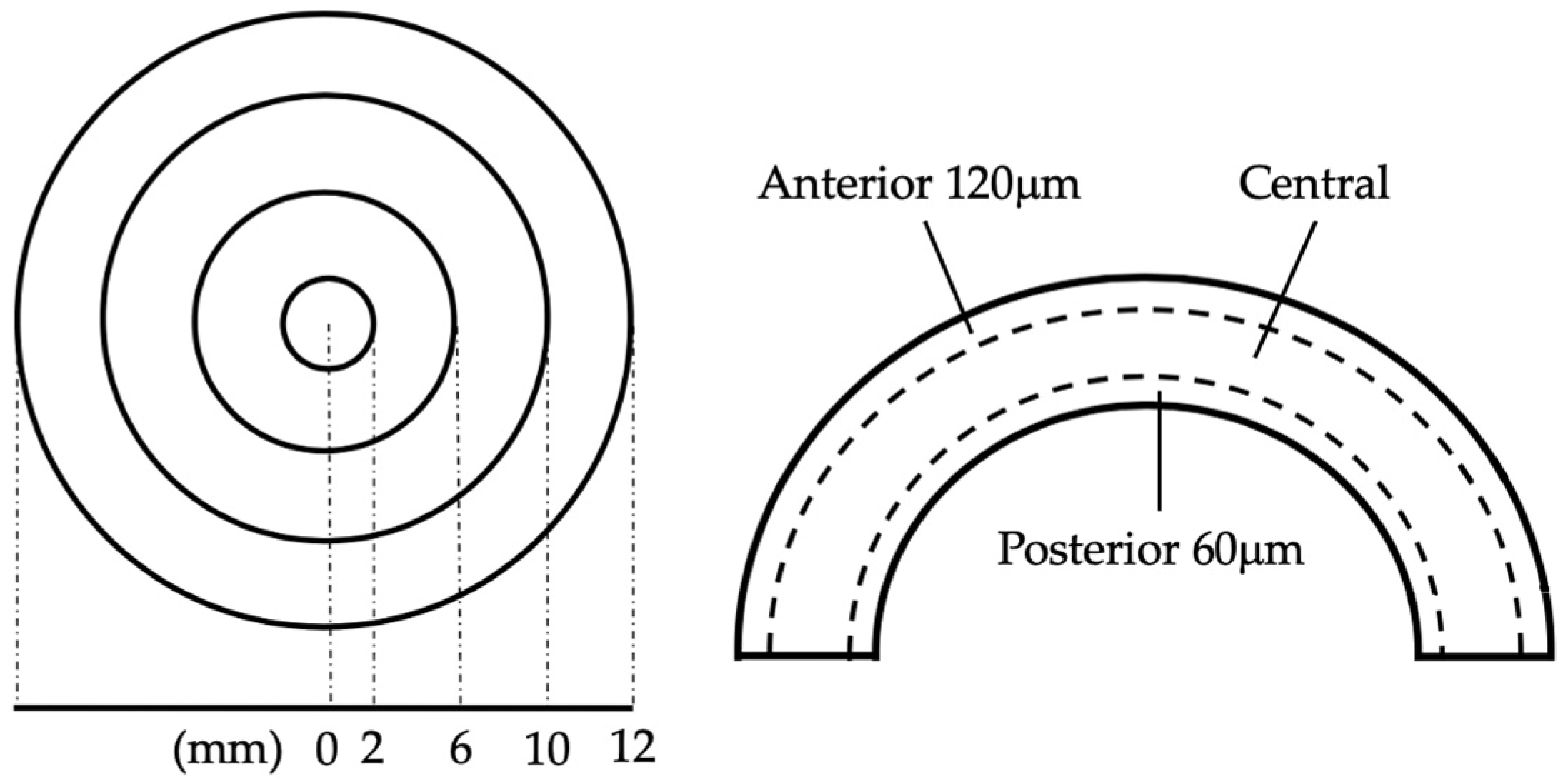
| Overall (N = 16) | |
|---|---|
| Sex (N) | |
| Male | 9 (56.25%) |
| Female | 7 (43.75%) |
| Laterality (N) | |
| OD | 7 (43.8%) |
| OS | 9 (56.3%) |
| Age (year) | 66.3 ± 7.04 |
| VA bare (logMAR) | |
| Pre | 1.45 ± 0.49 |
| Post | 0.36 ± 0.19 |
| Corvis ST Parameters a | Pre | Post | Difference | p Value b |
|---|---|---|---|---|
| Non-biomechanical | ||||
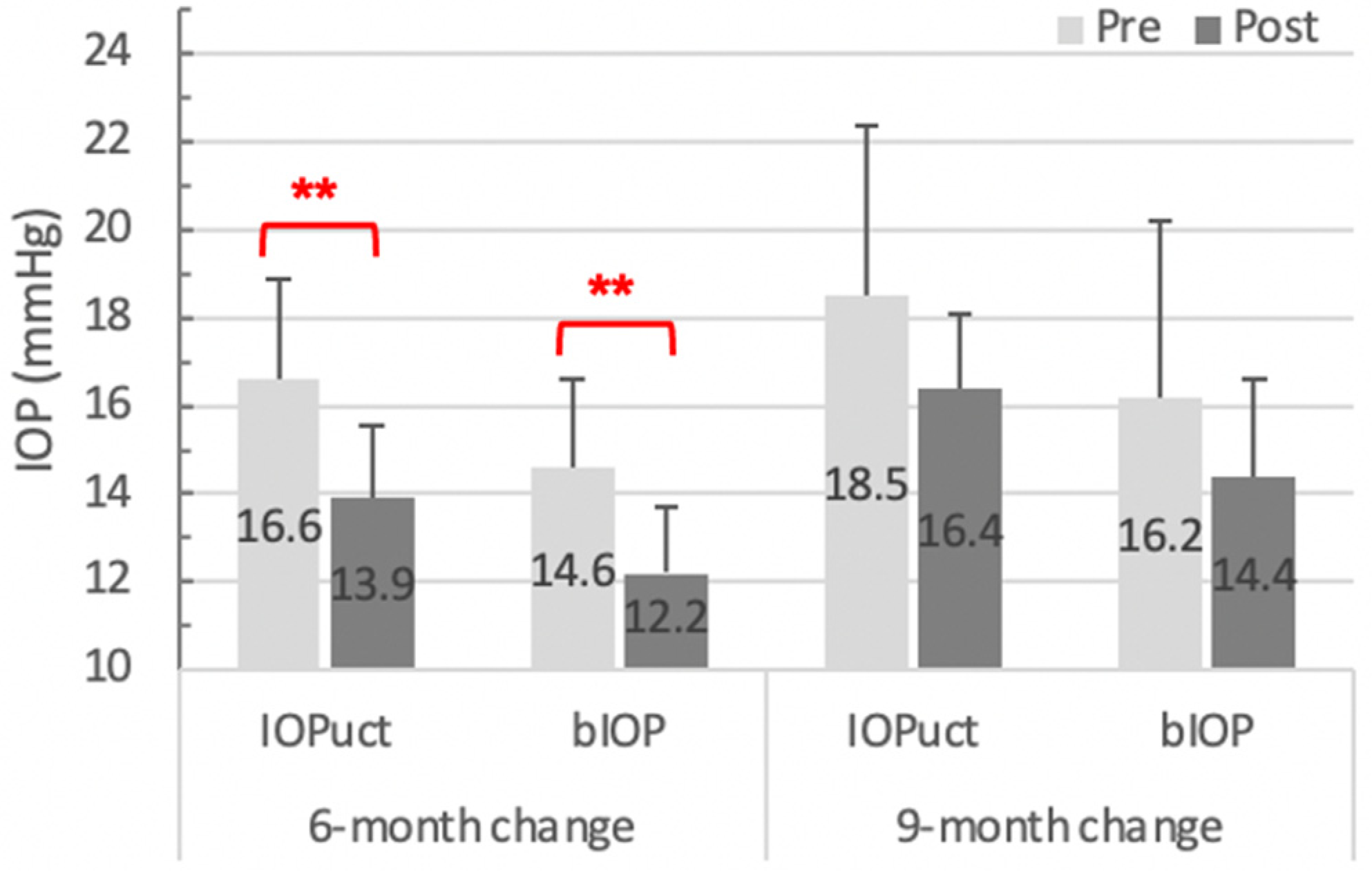
| IOPuct (mmHg) | 16.6 ± 4.23 | 13.9 ± 3.14 | −2.69 ± 2.52 | <0.001 * |
| CCT ((m) | 559 ± 37.1 | 558 ± 35.4 | −0.375 ± 16.2 | 0.93 |
| Basic parameters | ||||
| A1 indices | ||||
| A1 Time (ms) | 7.51 ± 0.556 | 7.00 ± 0.358 | −0.517 ± 0.409 | <0.001 * |
| A1 Velocity (m/s) | 0.141 ± 0.026 | 0.164 ± 0.021 | 0.024 ± 0.024 | 0.001 * |
| A1 DA (mm) | 0.157 ± 0.011 | 0.157 ± 0.007 | 0.001 ± 0.001 | 0.83 |
| A1 DA_c (mm) | 0.109 ± 0.010 | 0.144 ± 0.006 | 0.005 ± 0.008 | 0.03 * |
| A1 DL (mm) | 2.55 ± 0.147 | 2.59 ± 0.129 | 0.044 ± 0.121 | 0.16 |
| A1 dArcL (mm) | −0.024 ± 0.004 | −0.025 ± 0.003 | −0.001 ± 0.003 | 0.29 |
| A1 Darea (mm2) | 0.220 ± 0.031 | 0.219 ± 0.029 | −0.001 ± 0.031 | 0.91 |
| A2 indices | ||||
| A2 Time (ms) | 21.0 ± 0.772 | 20.8 ± 0.429 | −0.165 ± 0.720 | 0.37 |
| A2 Velocity (m/s) | −0.324 ± 0.072 | −0.412 ± 0.052 | −0.088 ± 0.086 | <0.001 * |
| A2 DA (mm) | 0.403 ± 0.066 | 0.434 ± 0.080 | 0.031 ± 0.079 | 0.14 |
| A2 DA_c (mm) | 0.126 ± 0.020 | 0.138 ± 0.027 | 0.012 ± 0.024 | 0.07 |
| A2 DL (mm) | 3.27 ± 0.958 | 3.35 ± 1.13 | 0.081 ± 0.667 | 0.64 |
| A2 dArcL (mm) | −0.030 ± 0.008 | −0.032 ± 0.010 | −0.002 ± 0.0076 | 0.34 |
| A2 Darea (mm2) | 0.303 ± 0.083 | 0.331 ± 0.124 | 0.029 ± 0.079 | 0.17 |
| HC indices | ||||
| HC Time (ms) | 17.1 ± 0.675 | 16.9 ± 0.884 | −0.144 ± 0.960 | 0.56 |
| HC DA (mm) c | 1.03 ± 0.135 | 1.17 ± 0.127 | 0.141 ± 0.101 | <0.001 * |
| HC DA_c (mm) | 0.850 ± 0.148 | 0.984 ± 0.138 | 0.134 ± 0.114 | <0.001 * |
| HC DL (mm) | 6.36 ± 0.768 | 6.76 ± 0.690 | 0.391 ± 0.547 | 0.01 * |
| HC dArcL (mm) | −0.154 ± 0.042 | −0.175 ± 0.038 | −0.021 ± 0.039 | 0.053 |
| HC Darea (mm2) | 3.02 ± 0.758 | 3.68 ± 0.812 | 0.663 ± 0.641 | <0.001 * |
| Limit indices | ||||
| WEM (mm) | 0.286 ± 0.062 | 0.309 ± 0.075 | 0.022 ± 0.080 | 0.29 |
| WEM time (ms) | 21.7 ± 0.916 | 21.6 ± 0.808 | −0.160 ± 1.06 | 0.55 |
| Max DA (mm) c | 1.03 ± 0.135 | 1.17 ± 0.127 | 0.141 ± 0.101 | <0.001 * |
| Max DA_c (mm) | 0.872 ± 0.139 | 0.997 ± 0.139 | 0.125 ± 0.112 | <0.001 * |
| Max DA_c time (ms) | 16.1 ± 0.854 | 16.3 ± 0.984 | 0.187 ± 1.43 | 0.61 |
| Max dArcL (mm) | −0.180 ± 0.049 | −0.206 ± 0.042 | −0.025 ± 0.054 | 0.08 |
| Max ICR (mm−1) | 0.177 ± 0.016 | 0.184 ± 0.026 | 0.008 ± 0.021 | 0.16 |
| Radius (mm) | 7.41 ± 1.14 | 6.95 ± 1.21 | −0.456 ± 0.742 | 0.03 * |
| Peak Distance (mm) | 4.72 ± 0.455 | 5.03 ± 0.418 | 0.305 ± 0.291 | <0.001 * |
| Integrated parameters | ||||
| bIOP (mmHg) | 14.6 ± 3.77 | 12.2 ± 2.83 | −2.39 ± 2.32 | <0.001 * |
| SSI (mmHg/mm) | 1.33 ± 0.240 | 1.15 ± 0.270 | −0.179 ± 0.150 | <0.001 * |
| PachySlope (a.u.) | 37.8 ± 11.5 | 41.4 ± 17.0 | 3.66 ± 10.1 | 0.17 |
| Max DAR_1 mm (a.u.) | 1.53 ± 0.039 | 1.55 ± 0.028 | 0.011 ± 0.033 | 0.20 |
| Max DAR_2 mm (a.u.) | 4.12 ± 0.379 | 4.31 ± 0.291 | 0.191 ± 0.244 | 0.007 * |
| ARTh (mm) | 646 ± 165 | 550 ± 187 | −96.7 ± 180 | 0.005 * |
| Integrated Radius (mm) | 7.69 ± 0.890 | 8.16 ± 0.761 | 0.469 ± 0.634 | 0.01 * |
| SP-A1 (mmHg/mm) | 110 ± 26.4 | 92.7 ± 20.1 | −16.8 ± 15.7 | <0.001 * |
| CBI (a.u.) d | 0.177 ± 0.239 | 0.299 ± 0.255 | 0.122 ± 0.256 | 0.16 d |
| Corvis ST Parameters a | Pre | Post | Difference | p Value b |
|---|---|---|---|---|
| Non-biomechanical | ||||
| IOPuct (mmHg) | 18.5 ± 4.15 | 16.4 ± 1.81 | −2.07 ± 2.73 | 0.09 |
| Basic parameters | ||||
| A1 indices | ||||
| A1 Time | 7.66 ± 0.56 | 7.30 ± 0.23 | −0.36 ± 0.40 | 0.054 |
| A1 Velocity | 0.13 ± 0.03 | 0.15 ± 0.02 | 0.02 ± 0.02 | 0.09 |
| A1 DA_c | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.00 ± 0.01 | 0.40 |
| A2 indices | ||||
| A2 velocity | −0.35 ± 0.08 | −0.38 ± 0.05 | −0.04 ± 0.06 | 0.14 |
| HC indices | ||||
| HC DA (= Max DA) | 0.98 ± 0.15 | 1.07 ± 0.10 | 0.09 ± 0.09 | 0.03 * |
| HC DA_c | 0.78 ± 0.16 | 0.88 ± 0.10 | 0.10 ± 0.08 | 0.02 * |
| HC DL | 6.22 ± 0.97 | 6.40 ± 0.73 | 0.18 ± 0.42 | 0.31 |
| HC Darea | 2.70 ± 0.81 | 3.09 ± 0.56 | 0.40 ± 0.38 | 0.03 * |
| Limit indices | ||||
| Max DA_c | 0.81 ± 0.16 | 0.89 ± 0.10 | 0.08 ± 0.08 | 0.045 * |
| Radius | 7.50 ± 1.37 | 6.93 ± 1.69 | −0.57 ± 0.85 | 0.12 |
| Peak distanceDistance | 4.49 ± 0.51 | 4.76 ± 0.33 | 0.27 ± 0.26 | 0.03 * |
| Integrated parameters | ||||
| bIOP | 16.2 ± 4.32 | 14.4 ± 2.39 | −1.77 ± 2.62 | 0.12 |
| SSI | 1.47 ± 0.25 | 1.30 ± 0.24 | −0.17 ± 0.16 | 0.03 * |
| Max DAR_2 mm | 3.85 ± 0.26 | 4.09 ± 0.25 | 0.24 ± 0.10 | <0.001 * |
| ARTh | 644 ± 153 | 542 ± 166 | −102 ± 89.61 | 0.02 * |
| Integrated Radius | 7.10 ± 0.70 | 7.78 ± 0.97 | 0.68 ± 0.61 | 0.03 * |
| SP A1 | 116 ± 20.9 | 105 ± 12.1 | −11.7 ± 15.7 | 0.10 |
| CBI c | 0.09 ± 0.07 | 0.25 ± 0.26 | 0.15 ± 0.25 | 0.15 c |
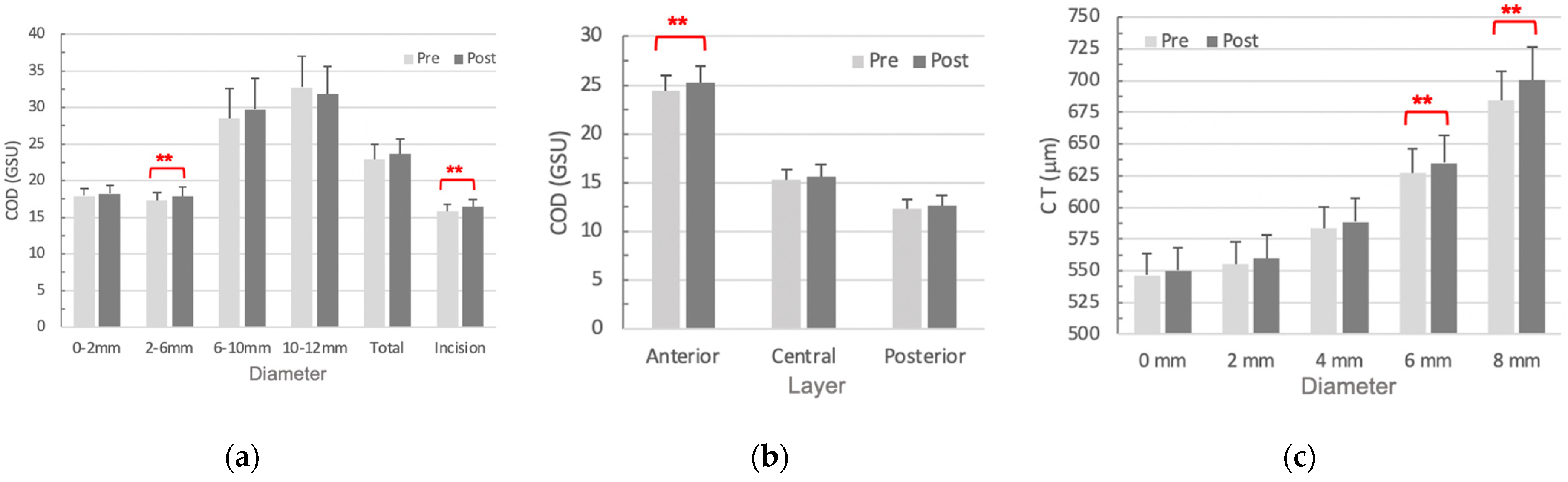
| 2–6 mm Annuli | Incision Site | |||||
|---|---|---|---|---|---|---|
| Anterior 120 μm | Total Thickness | |||||
| R a (%) | p Value | R a (%) | p Value | R a (%) | p Value | |
| A1 Time | 42.8 | 0.098 | 34.2 | 0.195 | 17.5 | 0.517 |
| A1 Velocity | −31.2 | 0.239 | −21.0 | 0.435 | −13.3 | 0.623 |
| A1 DA_c | 13.6 | 0.615 | −0.3 | 0.994 | 12.2 | 0.653 |
| A2 Velocity | 2.2 | 0.935 | 8.8 | 0.747 | 7.1 | 0.795 |
| HC DA_c | −47.4 | 0.064 | −39.1 | 0.134 | −7.5 | 0.783 |
| Max DA | −49.9 | 0.049 * | −48.2 | 0.059 | −2.4 | 0.933 |
| Max DA_c | −54.3 | 0.03 * | −48.2 | 0.059 | 24.1 | 0.369 |
| Peak Distance | −43.5 | 0.092 | −31.2 | 0.239 | −33.4 | 0.208 |
| Radius | −16.5 | 0.541 | −22.0 | 0.413 | 7.4 | 0.785 |
| bIOP | 45.6 | 0.076 | 33.4 | 0.207 | −33.8 | 0.200 |
| SSI | 21.4 | 0.425 | 14.3 | 0.598 | −11.6 | 0.672 |
| Max DAR_2 mm | −67.7 | 0.004 * | −36.6 | 0.165 | 24.1 | 0.369 |
| ARTh | 3.3 | 0.901 | −22.4 | 0.404 | 10.2 | 0.707 |
| Integrated Radius | −30.6 | 0.251 | −13.8 | 0.610 | 2.3 | 0.934 |
| SP A1 | 50.7 | 0.045 * | 40.8 | 0.116 | −20.2 | 0.453 |
| CBI b | −33.0 | 0.211 | −3.3 | 0.905 | −18.6 | 0.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.-Y.; Ho, R.-W.; Yu, H.-J.; Yang, I.-H.; Fang, P.-C.; Kuo, M.-T. Impacts and Correlations on Corneal Biomechanics, Corneal Optical Density and Intraocular Pressure after Cataract Surgery. Diagnostics 2024, 14, 1557. https://doi.org/10.3390/diagnostics14141557
Lin F-Y, Ho R-W, Yu H-J, Yang I-H, Fang P-C, Kuo M-T. Impacts and Correlations on Corneal Biomechanics, Corneal Optical Density and Intraocular Pressure after Cataract Surgery. Diagnostics. 2024; 14(14):1557. https://doi.org/10.3390/diagnostics14141557
Chicago/Turabian StyleLin, Fang-Yang, Ren-Wen Ho, Hun-Ju Yu, I-Hui Yang, Po-Chiung Fang, and Ming-Tse Kuo. 2024. "Impacts and Correlations on Corneal Biomechanics, Corneal Optical Density and Intraocular Pressure after Cataract Surgery" Diagnostics 14, no. 14: 1557. https://doi.org/10.3390/diagnostics14141557
APA StyleLin, F.-Y., Ho, R.-W., Yu, H.-J., Yang, I.-H., Fang, P.-C., & Kuo, M.-T. (2024). Impacts and Correlations on Corneal Biomechanics, Corneal Optical Density and Intraocular Pressure after Cataract Surgery. Diagnostics, 14(14), 1557. https://doi.org/10.3390/diagnostics14141557









