Reference Ranges of 2-Dimensional Placental Biometry and 3-Dimensional Placental Volume between 11 and 14 Weeks of Gestation
Abstract
1. Introduction
2. Material and Methods
2.1. Maternal and Pregnancy Characteristics
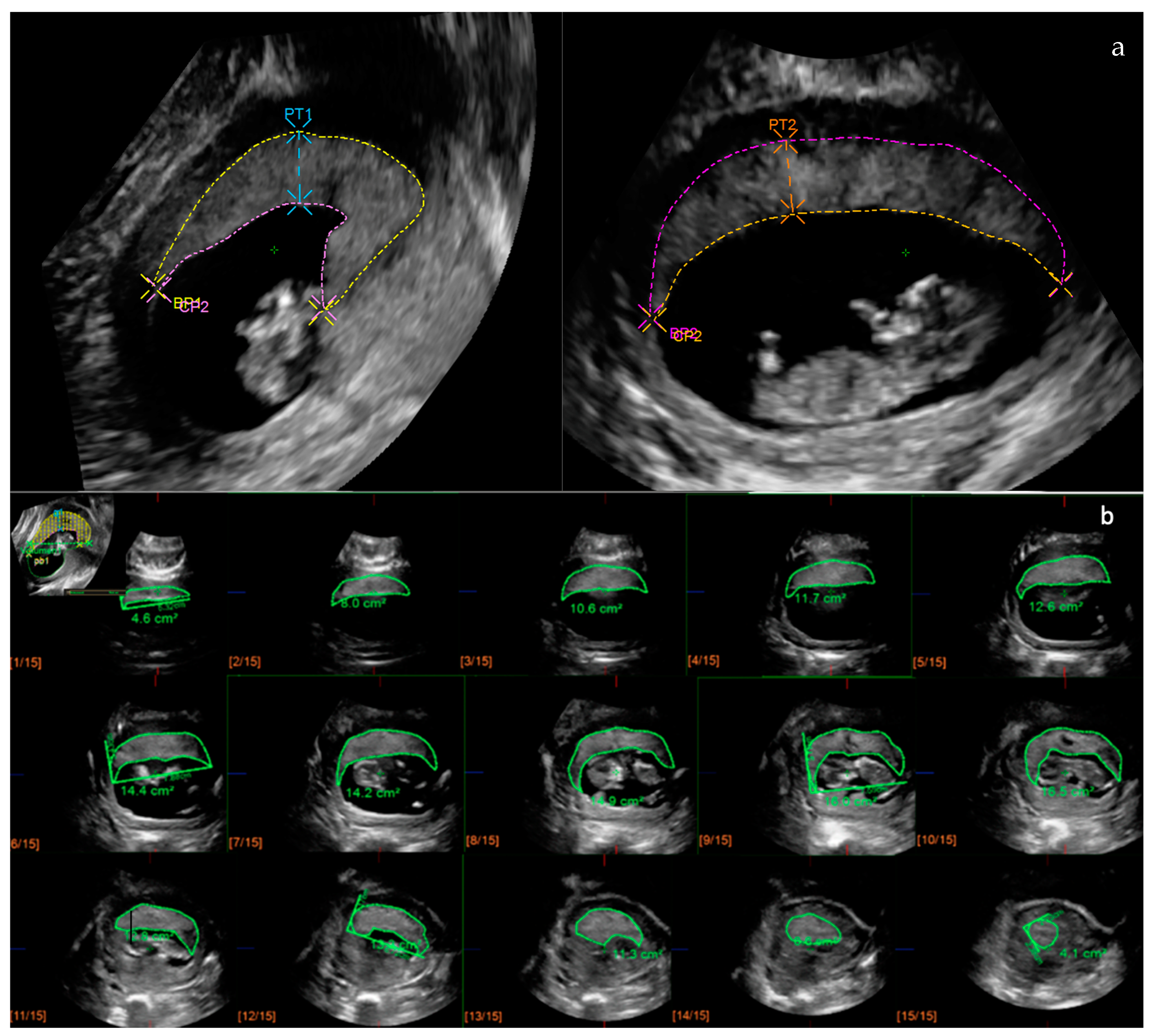
2.2. Placental Ultrasonography
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loverro, M.T.; Di Naro, E.; Nicolardi, V.; Resta, L.; Mastrolia, S.A.; Schettini, F.; Capozza, M.; Loverro, M.; Loverro, G.; Laforgia, N. Pregnancy Complications, Correlation With Placental Pathology and Neonatal Outcomes. Front. Clin. Diabetes Healthc. 2022, 2, 807192. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Saleem, S.; Goudar, S.S.; Moore, J.; Guruprasad, G.; Kulkarni, V.; Dhaded, S.M.; Tikmani, S.S.; Nausheen, S.; Masheer, S.; et al. The PURPOSe cause of death study in stillbirths and neonatal deaths in India and Pakistan: A review. Int. J. Obstet. Gynaecol. 2023, 130 (Suppl. 3), 26–35. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Sibley, C.P. Placental nutrient supply and fetal growth. Int. J. Dev. Biol. 2010, 54, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, J.S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chaddha, V.; Viero, S.; Huppertz, B.; Kingdom, J. Developmental biology of the placenta and the origins of placental insufficiency. Semin. Fetal Neonatal Med. 2004, 9, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Haram, K.; Mortensen, J.H.; Myking, O.; Roald, B.; Magann, E.F.; Morrison, J.C. Early development of the human placenta and pregnancy complications. J. Matern. Fetal Neonatal Med. 2020, 33, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Jauniaux, E.; Ramsay, B.; Campbell, S. Ultrasonographic investigation of placental morphologic characteristics and size during the second trimester of pregnancy. Am. J. Obstet. Gynecol. 1994, 170, 130–137. [Google Scholar] [CrossRef]
- Haeussner, E.; Schmitz, C.; Koch von, F.; Frank, H.G. Birth weight correlates with size but not shape of the normal human placenta. Placenta 2013, 34, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.D.; Correia-Branco, A.; Adhikari, N.; Mercan, E.; Mallidi, S.; Wallingford, M.C. New Frontiers in Placenta Tissue Imaging. EMJ Radiol. 2020, 1, 54–62. [Google Scholar] [PubMed]
- Higgins, L.E.; Simcox, L.; Sibley, C.P.; Heazell, A.E.; Johnstone, E.D. Third trimester placental volume and biometry measurement: A method-development study. Placenta 2016, 42, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Florido, J.; Ocón, O.; Luna del Castillo Jde, D.; Vega-Cañadas, J.; Manrique-Espinoza, N.; Navarrete, L. Analysis of measurement process of placental volume in early pregnancy: An interobserver reliability study. J. Perinat. Med. 2014, 42, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.B.; Leung, K.Y.; Li, T.K.; Chan, H.Y.; Lee, Y.P.; Tang, M.H. Comparison of inter- and intraobserver agreement and reliability between three diferent types of placental volume measurement technique (XI VOCAL, VOCAL and multiplanar) and validity in the in-vitro setting. Ultrasound Obstet. Gynecol. 2010, 36, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Mandel, D.; Shlakhter, O.; Coletta, J.; Pessel, C.; Timor-Tritsch, I.E.; Salafia, C.M. Placental morphologic features and chorionic surface vasculature at term are highly correlated with 3-dimensional sonographic measurements at 11 to 14 weeks. J. Ultrasound Med. 2011, 30, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, W.; Akolekar, R.; Dagklis, T.; Veduta, A.; Nicolaides, K.H. Placental Volume at 11–13 Weeks’ Gestation in the Prediction of Birth Weight Percentile. Fetal Diagn. Ther. 2011, 30, 23–28. [Google Scholar] [PubMed]
- Moran, M.; McAuliffe, F.M. Imaging and assessment of placental function. J. Clin. Ultrasound 2011, 39, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, J.S.; Sheiner, E. Ultrasound of the Placenta: A Systematic Approach. Part. I Imaging. Placenta. 2008, 29, 225–240. [Google Scholar]
- Soongsatitanon, A.; Phupong, V. First trimester 3D ultrasound placental volume for predicting preeclampsia and/or intrauterine growth restriction. J. Obstet. Gynaecol. 2019, 39, 474–479. [Google Scholar] [CrossRef]
- Vachon-Marceau, C.; Demers, S.; Markey, S.; Okun, N.; Girard, M.; Kingdom, J.; Bujold, E. First-trimester placental thickness and the risk of preeclampsia or SGA. Placenta 2017, 57, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.; Wang, E.; Parry, S. Two-dimensional sonographic placental measurements in the prediction of small-for-gestational-ageinfants. Ultrasound Obstet. Gynecol. 2012, 40, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Trilla, C.; Medina, M.C.; Parra, J.; Llurba, E.; Espinós, J.J. Inter- and Intraobserver Agreement in First Trimester Ultrasound Evaluation of Placental Biometry. Fetal Diagn. Ther. 2020, 47, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Wright, E.M. How to construct ‘normal ranges’ for fetal variables. Ultrasound Obstet. Gynecol. 1998, 11, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Guyomard, A.; Macé, G.; Ferdynus, C.; Franceschini, C.; Rousseau, T.; Sagot, P. Reference ranges and distribution of placental volume by 3-dimensional virtual organ computer-aided analysis between 11 weeks and 13 weeks 6 days. J. Ultrasound Med. 2013, 32, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Meengeonthong, D.; Luewan, S.; Sirichotiyakul, S.; Tongsong, T. Reference ranges of placental volume measured by virtual organ computer-aided analysis between 10 and 14 weeks of gestation. J. Clin. Ultrasound 2017, 45, 185–191. [Google Scholar] [CrossRef] [PubMed]
- de Paula, C.F.; Ruano, R.; Campos, J.A.; Zugaib, M. Placental volumes measured by 3-dimensional ultrasonography in normal pregnancies from 12 to 40 weeks’ gestation. J. Ultrasound Med. 2008, 27, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Greenberg, I.D. Reference range determination: The problem of small sample sizes. J. Nucl. Med. 1991, 32, 2306–2310. [Google Scholar] [PubMed]
- Walter, S.R.; Dunsmuir, W.T.M.; Westbrook, J.I. Inter-observer agreement and reliability assessment for observational studies of clinical work. J. Biomed. Inform. 2019, 100, 103317. [Google Scholar] [CrossRef] [PubMed]
- Steckler, A.; McLeroy, K.R. The importance of external validity. Am. J. Public Health 2008, 98, 9–10. [Google Scholar] [CrossRef]
| Characteristics | N = 1142 |
|---|---|
| Age (years) | 33.4 (5.03) |
| Ethnicity | |
| Caucasian | 791 (69.3) |
| Latin-American | 250 (21.9) |
| African American | 23 (2.0) |
| Asian | 28 (2.5) |
| South-Asian | 19 (1.7) |
| North-African | 31 (2.7) |
| BMI (Kg/m2) | 24.0 (4.3) |
| Smoking habit | 48 (4.2) |
| Medical history | |
| Chronic hypertension | 10 (0.9) |
| Diabetes mellitus | 7 (0.6) |
| Renal disease | 3 (0.3) |
| Autoimmune disease | 10 (0.9) |
| Thrombophilia | 13 (1.1) |
| Neurologic condition | 10 (0.9) |
| Thyroid disorders | 97 (8.5) |
| Others | 17 (1.5) |
| Assisted reproductive technology | 103 (9.0) |
| Obstetric history | |
| Nulliparous | 657 (57.5) |
| Parous | 485 (42.5) |
| Placental location | |
| Anterior | 529 (46.3) |
| Posterior | 473 (41.4) |
| Lateral | 93 (8.1) |
| Fundal | 32 (2.8) |
| Others | 3 (0.3) |
| First-trimester ultrasound | |
| Gestational age (weeks) | 12.9 (0.6) |
| CRL (mm) | 65.8 (7.9) |
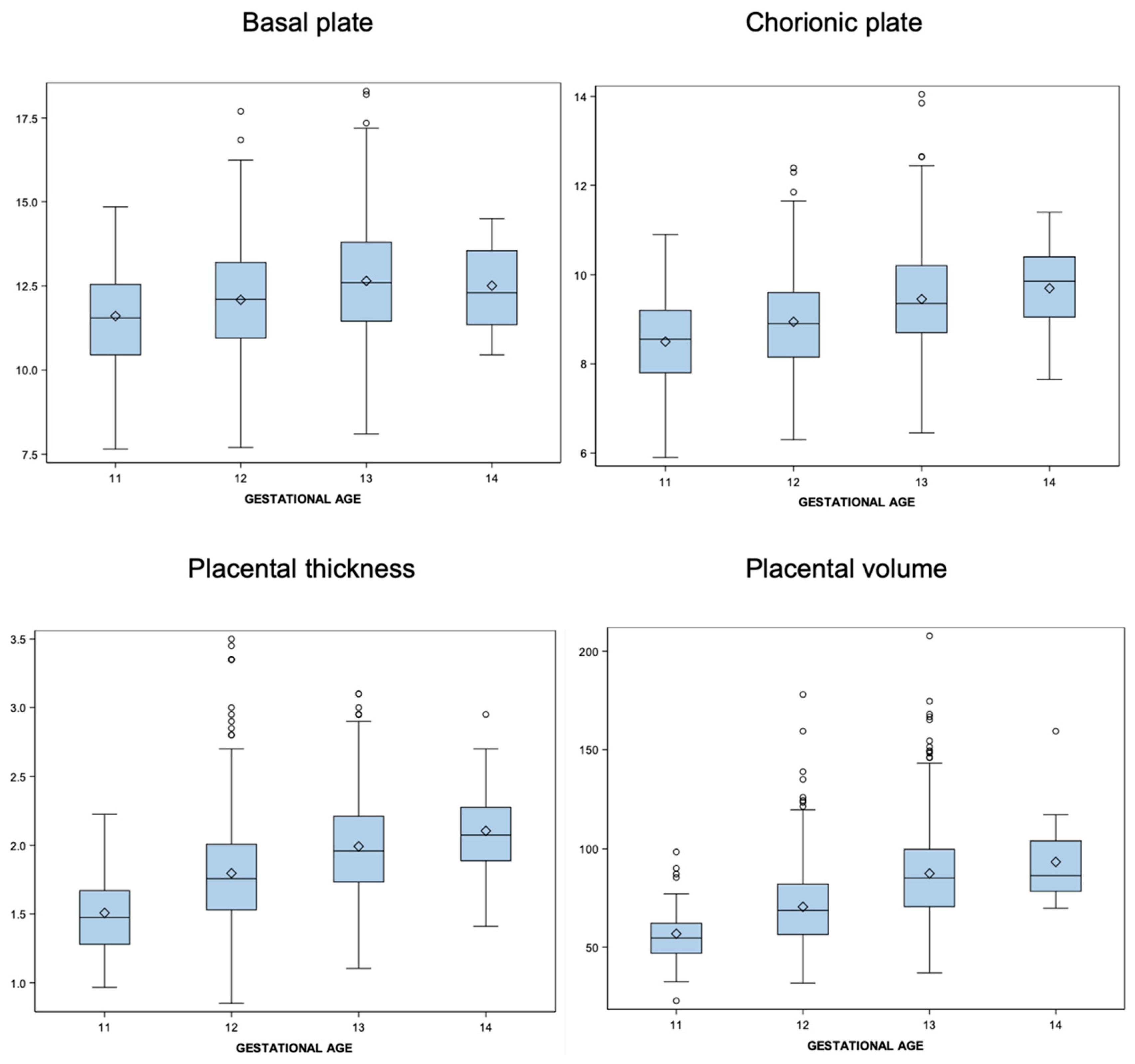
| Basal plate | ||||||
| Gestational age | N | Mean | Median | SD | Lower Quartile | Upper Quartile |
| 11 | 71 | 11.61 | 11.55 | 1.461 | 10.450 | 12.550 |
| 12 | 551 | 12.09 | 12.10 | 1.655 | 10.950 | 13.200 |
| 13 | 493 | 12.65 | 12.60 | 1.707 | 11.450 | 13.800 |
| 14 | 27 | 12.51 | 12.30 | 1.313 | 11.350 | 13.550 |
| Chorionic plate | ||||||
| Gestational age | p5 | p10 | p50 | p90 | p95 | |
| 11 | 71 | 8.498 | 8.550 | 1.003 | 7.800 | 9.200 |
| 12 | 551 | 8.943 | 8.900 | 1.057 | 8.150 | 9.600 |
| 13 | 493 | 9.451 | 9.350 | 1.104 | 8.700 | 10.200 |
| 14 | 27 | 9.694 | 9.850 | 1.007 | 9.050 | 10.400 |
| Placental thickness | ||||||
| Gestational age | p5 | p10 | p50 | p90 | p95 | |
| 11 | 71 | 1.508 | 1.475 | 0.272 | 1.280 | 1.670 |
| 12 | 551 | 1.799 | 1.760 | 0.373 | 1.530 | 2.010 |
| 13 | 493 | 1.994 | 1.960 | 0.355 | 1.735 | 2.210 |
| 14 | 27 | 2.106 | 2.075 | 0.337 | 1.890 | 2.275 |
| Placental volume | ||||||
| Gestational age | p5 | p10 | p50 | p90 | p95 | |
| 11 | 71 | 56.772 | 54.600 | 13.270 | 46.900 | 62.100 |
| 12 | 551 | 70.407 | 68.600 | 19.712 | 56.400 | 82.100 |
| 13 | 491 | 87.478 | 85.200 | 23.547 | 70.500 | 99.700 |
| 14 | 27 | 93.319 | 86.300 | 19.511 | 78.300 | 104.000 |
| Basal plate | |||||
| Gestational age | p5 | p10 | p50 | p90 | p95 |
| 11 | 9.20 | 9.74 | 11.61 | 13.48 | 14.01 |
| 12 | 9.37 | 9.97 | 12.09 | 14.21 | 14.81 |
| 13 | 9.84 | 10.47 | 12.65 | 14.83 | 15.46 |
| 14 | 10.35 | 10.83 | 12.51 | 14.19 | 14.67 |
| Chorionic plate | |||||
| Gestational age | p5 | p10 | p50 | p90 | p95 |
| 11 | 6.89 | 7.21 | 8.50 | 9.78 | 10.15 |
| 12 | 7.20 | 7.59 | 8.94 | 10.30 | 10.68 |
| 13 | 7.63 | 8.04 | 9.45 | 10.86 | 11.27 |
| 14 | 8.04 | 8.41 | 9.69 | 10.98 | 11.35 |
| Placental thickness | |||||
| Gestational age | p5 | p10 | p50 | p90 | p95 |
| 11 | 1.06 | 1.16 | 1.51 | 1.86 | 1.96 |
| 12 | 1.19 | 1.32 | 1.80 | 2.28 | 2.41 |
| 13 | 1.41 | 1.54 | 1.99 | 2.45 | 2.58 |
| 14 | 1.55 | 1.67 | 2.11 | 2.54 | 2.66 |
| Placental volume | |||||
| Gestational age | p5 | p10 | p50 | p90 | p95 |
| 11 | 34.94 | 39.79 | 56.78 | 73.76 | 78.60 |
| 12 | 37.98 | 45.18 | 70.41 | 95.64 | 102.83 |
| 13 | 48.74 | 57.34 | 87.48 | 117.62 | 126.21 |
| 14 | 61.22 | 68.34 | 93.32 | 118.29 | 125.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trilla Solà, C.; Parra Roca, J.; Llurba Olivé, E. Reference Ranges of 2-Dimensional Placental Biometry and 3-Dimensional Placental Volume between 11 and 14 Weeks of Gestation. Diagnostics 2024, 14, 1556. https://doi.org/10.3390/diagnostics14141556
Trilla Solà C, Parra Roca J, Llurba Olivé E. Reference Ranges of 2-Dimensional Placental Biometry and 3-Dimensional Placental Volume between 11 and 14 Weeks of Gestation. Diagnostics. 2024; 14(14):1556. https://doi.org/10.3390/diagnostics14141556
Chicago/Turabian StyleTrilla Solà, Cristina, Juan Parra Roca, and Elisa Llurba Olivé. 2024. "Reference Ranges of 2-Dimensional Placental Biometry and 3-Dimensional Placental Volume between 11 and 14 Weeks of Gestation" Diagnostics 14, no. 14: 1556. https://doi.org/10.3390/diagnostics14141556
APA StyleTrilla Solà, C., Parra Roca, J., & Llurba Olivé, E. (2024). Reference Ranges of 2-Dimensional Placental Biometry and 3-Dimensional Placental Volume between 11 and 14 Weeks of Gestation. Diagnostics, 14(14), 1556. https://doi.org/10.3390/diagnostics14141556







