Radiomic Gradient in Peritumoural Tissue of Liver Metastases: A Biomarker for Clinical Practice? Analysing Density, Entropy, and Uniformity Variations with Distance from the Tumour
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Image Acquisition
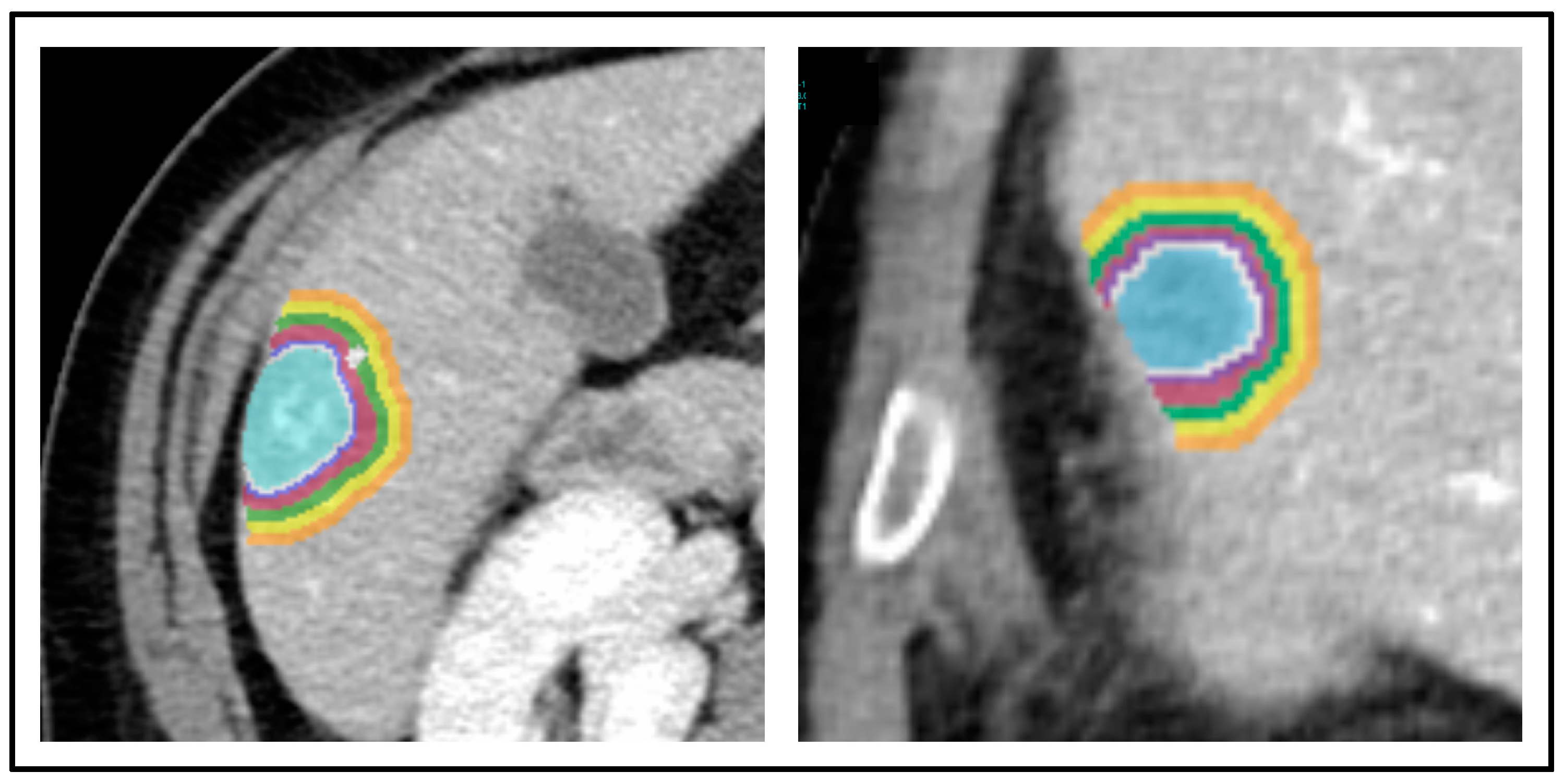
2.3. Image Segmentation
2.4. Imaging Pre-Processing and Radiomic Analysis
2.5. Clinical Parameters
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Population
3.2. Density (HU_mean)
3.3. Entropy
3.4. Uniformity
3.5. Impact of Tumour Size
3.6. Impact of Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Mastroleo, F.; Krengli, M.; Jereczek-Fossa, B.A. Quo vadis Radiomics? Bibliometric analysis of 10-year Radiomics journey. Eur. Radiol. 2023, 33, 6736–6745. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Ammirabile, A.; Zwanenburg, A. Radiomics in liver surgery: Defining the path toward clinical application. Updates Surg. 2023, 75, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Maino, C.; Vernuccio, F.; Cannella, R.; Franco, P.N.; Giannini, V.; Dezio, M.; Pisani, A.R.; Blandino, A.A.; Faletti, R.; De Bernardi, E.; et al. Radiomics and liver: Where we are and where we are headed? Eur. J. Radiol. 2024, 171, 111297. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Jiang, B.; Zhao, K.; Zhang, Y.; Xie, X. Clinical application of deep learning and radiomics in hepatic disease imaging: A systematic scoping review. Br. J. Radiol. 2022, 95, 20211136. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.C.; Santone, A.; Avella, P.; Bianco, P.; Scacchi, A.; Scaglione, M.; Bellifemine, F.; Danzi, R.; Varriano, G.; et al. Early Diagnosis of Liver Metastases from Colorectal Cancer through CT Radiomics and Formal Methods: A Pilot Study. J. Clin. Med. 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Masci, C.; Costa, G.; Sollini, M.; Chiti, A.; Ieva, F.; Torzilli, G.; Viganò, L. PET/CT-based radiomics of mass-forming intrahepatic cholangiocarcinoma improves prediction of pathology data and survival. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3387–3400. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Rossi, N.; Langella, S.; Ruzzenente, A.; Serenari, M.; Ardito, F.; Cucchetti, A.; Gallo, T.; Zamboni, G.; Mosconi, C.; et al. Radiomic Analysis of Intrahepatic Cholangiocarcinoma: Non-Invasive Prediction of Pathology Data: A Multicenter Study to Develop a Clinical-Radiomic Model. Cancers 2023, 15, 4204. [Google Scholar] [CrossRef]
- Chen, S.; Feng, S.; Wei, J.; Liu, F.; Li, B.; Li, X.; Hou, Y.; Gu, D.; Tang, M.; Xiao, H.; et al. Pretreatment prediction of immunoscore in hepatocellular cancer: A radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur. Radiol. 2019, 29, 4177–4187. [Google Scholar] [CrossRef]
- Moretto, R.; Corallo, S.; Belfiore, A.; Rossini, D.; Boccaccino, A.; Lonardi, S.; Centonze, G.; Morano, F.; Germani, M.M.; Loupakis, F.; et al. Prognostic impact of immune-microenvironment in colorectal liver metastases resected after triplets plus a biologic agent: A pooled analysis of five prospective trials. Eur. J. Cancer 2020, 135, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Baldin, P.; Van den Eynde, M.; Mlecnik, B.; Bindea, G.; Beniuga, G.; Carrasco, J.; Haicheur, N.; Marliot, F.; Lafontaine, L.; Fredriksen, T.; et al. Prognostic assessment of resected colorectal liver metastases integrating pathological features, RAS mutation and Immunoscore. J. Pathol. Clin. Res. 2021, 7, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Miki, Y.; Sugai, S.; Yanagisawa, A.; Kato, Y.; Sakamoto, Y.; Yamamoto, J.; Yamaguchi, T.; Muto, T.; Makuuchi, M. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: Minimum surgical margins for successful resection. Arch. Surg. 2002, 137, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Holdhoff, M.; Schmidt, K.; Diehl, F.; Aggrawal, N.; Angenendt, P.; Romans, K.; Edelstein, D.L.; Torbenson, M.; Kinzler, K.W.; Vogelstein, B.; et al. Detection of tumor DNA at the margins of colorectal cancer liver metastasis. Clin. Cancer Res. 2011, 17, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Branciforte, B.; Laurenti, V.; Costa, G.; Procopio, F.; Cimino, M.; Del Fabbro, D.; Di Tommaso, L.; Torzilli, G. The Histopathological Growth Pattern of Colorectal Liver Metastases Impacts Local Recurrence Risk and the Adequate Width of the Surgical Margin. Ann. Surg. Oncol. 2022, 29, 5515–5524. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Costa, G.; Gennaro, N.; la Bella, L.; Boichuk, A.; Sollini, M.; Politi, L.S.; Balzarini, L.; Torzilli, G.; Chiti, A.; et al. Contrast Administration Impacts CT-Based Radiomics of Colorectal Liver Metastases and Non-Tumoral Liver Parenchyma Revealing the “Radiological” Tumour Microenvironment. Diagnostics 2021, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Laino, M.E.; Fiz, F.; Morandini, P.; Costa, G.; Maffia, F.; Giuffrida, M.; Pecorella, I.; Gionso, M.; Wheeler, D.R.; Cambiaghi, M.; et al. A virtual biopsy of liver parenchyma to predict the outcome of liver resection. Updates Surg. 2023, 75, 1519–1531. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallieres, M.; Abdalah, M.A.; Aerts, H.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Chun, Y.S.; Vauthey, J.N.; Boonsirikamchai, P.; Maru, D.M.; Kopetz, S.; Palavecino, M.; Curley, S.A.; Abdalla, E.K.; Kaur, H.; Charnsangavej, C.; et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009, 302, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Muto, Y.; Ichida, K.; Fukui, T.; Takayama, Y.; Kakizawa, N.; Kato, T.; Hasegawa, F.; Watanabe, F.; Kaneda, Y.; et al. Morphological response contributes to patient selection for rescue liver resection in chemotherapy patients with initially un-resectable colorectal liver metastasis. Oncol. Lett. 2017, 14, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Latacz, E.; Höppener, D.; Bohlok, A.; Leduc, S.; Tabariès, S.; Fernández Moro, C.; Lugassy, C.; Nyström, H.; Bozóky, B.; Floris, G.; et al. Histopathological growth patterns of liver metastasis: Updated consensus guidelines for pattern scoring, perspectives and recent mechanistic insights. Br. J. Cancer 2022, 127, 988–1013. [Google Scholar] [CrossRef] [PubMed]
- Mentha, G.; Terraz, S.; Morel, P.; Andres, A.; Giostra, E.; Roth, A.; Rubbia-Brandt, L.; Majno, P. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br. J. Surg. 2008, 96, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Panettieri, E.; Vellone, M.; Ferrucci, M.; Coppola, A.; Silvestrini, N.; Arena, V.; Adducci, E.; Capelli, G.; Vecchio, F.M.; et al. The impact of R1 resection for colorectal liver metastases on local recurrence and overall survival in the era of modern chemotherapy: An analysis of 1428 resection areas. Surgery 2019, 165, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Procopio, F.; Cimino, M.M.; Donadon, M.; Gatti, A.; Costa, G.; Del Fabbro, D.; Torzilli, G. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann. Surg. Oncol. 2016, 23, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Sergentanis, T.N.; Ntanasis-Stathopoulos, I.; Andreatos, N.; Tzanninis, I.G.; Sasaki, K.; Psaltopoulou, T.; Wang, J.; Buettner, S.; Papalois, A.E.; et al. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann. Surg. 2018, 267, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Beckers, R.C.J.; Trebeschi, S.; Maas, M.; Schnerr, R.S.; Sijmons, J.M.L.; Beets, G.L.; Houwers, J.B.; Beets-Tan, R.G.H.; Lambregts, D.M.J. CT texture analysis in colorectal liver metastases and the surrounding liver parenchyma and its potential as an imaging biomarker of disease aggressiveness, response and survival. Eur. J. Radiol. 2018, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Hu, F.; Sun, Y.; Hu, T.; Tong, T. MR texture analysis: Potential imaging biomarker for predicting the chemotherapeutic response of patients with colorectal liver metastases. Abdom. Radiol. 2019, 44, 65–71. [Google Scholar] [CrossRef]
- Fiz, F.; Vigano, L.; Gennaro, N.; Costa, G.; La Bella, L.; Boichuk, A.; Cavinato, L.; Sollini, M.; Politi, L.S.; Chiti, A.; et al. Radiomics of Liver Metastases: A Systematic Review. Cancers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Eveno, C.; Karoui, M.; Gayat, E.; Luciani, A.; Auriault, M.L.; Kluger, M.D.; Baumgaertner, I.; Baranes, L.; Laurent, A.; Tayar, C.; et al. Liver resection for colorectal liver metastases with peri-operative chemotherapy: Oncological results of R1 resections. HPB 2013, 15, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Knitter, S.; Schmelzle, M.; Kradolfer, D.; Maurer, M.H.; Auer, T.A.; Fehrenbach, U.; Lachenmayer, A.; Banz, V.; Schöning, W.; et al. Recurrence at surgical margin following hepatectomy for colorectal liver metastases is not associated with R1 resection and does not impact survival. Surgery 2021, 169, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Ayez, N.; Lalmahomed, Z.S.; Eggermont, A.M.; Ijzermans, J.N.; de Jonge, J.; van Montfort, K.; Verhoef, C. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann. Surg. Oncol. 2012, 19, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Shindoh, J.; Yoshioka, R.; Gonoi, W.; Abe, H.; Okura, N.; Yoshida, S.; Sakamoto, Y.; Hasegawa, K.; Fukayama, M.; et al. Clinical Impact of Preoperative Chemotherapy on Microscopic Cancer Spread Surrounding Colorectal Liver Metastases. Ann. Surg. Oncol. 2017, 24, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Nierop, P.M.; Höppener, D.J.; Buisman, F.E.; van der Stok, E.P.; Galjart, B.; Balachandran, V.P.; Jarnagin, W.R.; Kingham, T.P.; Shia, J.; Mauer, M.; et al. Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases. J. Pathol. Clin. Res. 2022, 8, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Starmans, M.P.A.; Buisman, F.E.; Renckens, M.; Willemssen, F.; van der Voort, S.R.; Groot Koerkamp, B.; Grünhagen, D.J.; Niessen, W.J.; Vermeulen, P.B.; Verhoef, C.; et al. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: A pilot study. Clin. Exp. Metastasis 2021, 38, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, X.; Sun, J.; Dong, L.; Wei, F.; Bao, C.; Zhong, J.; Li, Y. A CT-based radiomics nomogram for predicting histopathologic growth patterns of colorectal liver metastases. J. Cancer Res. Clin. Oncol. 2023, 149, 9543–9555. [Google Scholar] [CrossRef] [PubMed]
- Brouquet, A.; Blot, C.; Allard, M.A.; Lazure, T.; Sebbagh, M.; Gayet, M.; Lewin, M.; Adam, R.; Penna, C.; Sa Cunha, A.; et al. What is the Prognostic Value of a Discordant Radiologic and Pathologic Response in Patients Undergoing Resection of Colorectal Liver Metastases After Preoperative Chemotherapy? Ann. Surg. Oncol. 2020, 27, 2877–2885. [Google Scholar] [CrossRef]
- Costa, G.; Cavinato, L.; Fiz, F.; Sollini, M.; Chiti, A.; Torzilli, G.; Ieva, F.; Viganò, L. Mapping Tumor Heterogeneity via Local Entropy Assessment: Making Biomarkers Visible. J. Digit. Imaging 2023, 36, 1038–1048. [Google Scholar] [CrossRef]





| Per-Patient Data | |
|---|---|
| Number of patients | 51 |
| Sex M:F | 37 (73%):14 (27%) |
| Age, years | 64 (40–76) |
| Number of metastases | 1 (1–3) |
| Metastases size, mm | 33 (11–110) |
| >50 mm | 6 (12%) |
| Pre-treatment chemotherapy | 29 (57%) |
| >1 line | 2 |
| Oxaliplatin | 18 |
| Irinotecan | 10 |
| Oxaliplatin + Irinotecan | 1 |
| Associated anti-VEGF targeted therapy | 15 |
| Associated anti-EGFR targeted therapy | 8 |
| Number of cycles | 8 (4–18) |
| Partial response/Stable disease | 22/7 |
| Per-Lesion Data | |
| Number of tumours | 63 |
| Metastases size, mm | 30 (10–110) |
| >50 mm | 6 (10%) |
| Volume, voxel | 6.07 × 103 (0.33 × 103–69.87 × 103) |
| Pre-treatment chemotherapy | 37 (59%) |
| Partial response/Stable disease * | 29/8 |
| Absolute Value | Delta Value with Virtual Biopsy | Delta % with Virtual Biopsy | |
|---|---|---|---|
| Hu-mean | |||
| Tumour | 74.8 ± 17.3 | −28.2 (−44.5–−20.5) | −27.7% (−38.8%–−21.5%) |
| 1 mm | 97.8 ± 15.4 | −9.5 ± 10.2 | −9.1% (−14.2%–−2.1%) |
| 2 mm | 106.1 ± 17.4 | −2.4 (−7.3–+4.5) | −2.2% (−7.4%–+4.2%) |
| 3–4 mm | 110.1 ± 17.6 | +1.7 (−2.2–+7.6) | +1.5% (−1.6%–+7.0%) |
| 5–6 mm | 110.5 ± 18.2 | +3.2 ± 7.4 | +2.0% (−1.8%–+7.3%) |
| 7–8 mm | 109.9 ± 18.6 | +2.6 ± 6.4 | +1.9% (−2.5%–+6.4%) |
| 9–10 mm | 109.2 ± 18.6 | +2.0 ± 5.8 | +2.0% ± 5.8% |
| Virtual biopsy | 107.3 ± 18.1 | - | - |
| Entropy (log2) | |||
| Tumour | 3.11 ± 0.33 | +0.50 (+0.30–+0.85) | +19.1% (+12.1%–36.5%) |
| 1 mm | 3.02 ± 0.36 | +0.49 (+0.27–+0.68) | +19.5% (+11.1%–+25.8%) |
| 2 mm | 2.90 ± 0.41 | +0.38 ± 0.32 | +14.3% (+5.0%–+23.7%) |
| 3–4 mm | 2.80 ± 0.40 | +0.28 ± 0.28 | +10.4% (+2.4%–+19.1%) |
| 5–6 mm | 2.76 ± 0.41 | +0.24 ± 0.27 | +10.2% ± 11.2% |
| 7–8 mm | 2.72 ± 0.40 | +0.19 ± 0.24 | +8.2% ± 10.0% |
| 9–10 mm | 2.71 ± 0.36 | +0.18 ± 0.21 | +8.0% ± 9.4% |
| Virtual biopsy | 2.54 (2.32–2.80) | - | - |
| Uniformity | |||
| Tumour | 0.139 ± 0.033 | −0.059 (−0.098–−0.037) | −32.4% ± 16.0% |
| 1 mm | 0.149 ± 0.036 | −0.059 (−0.073–−0.035) | −28.0% ± 14.3% |
| 2 mm | 0.167 ± 0.044 | −0.040 (−0.067–−0.015) | −20.1% ± 16.7% |
| 3–4 mm | 0.176 (0.144–0.202) | −0.033 ± 0.037 | −14.0% ± 15.5% |
| 5–6 mm | 0.183 (0.151–0.201) | −0.027 ± 0.034 | −11.7% ± 15.3% |
| 7–8 mm | 0.185 (0.151–0.216) | −0.023 ± 0.032 | −10.1% ± 14.5% |
| 9–10 mm | 0.187 (0.157–0.204) | −0.024 ± 0.030 | −9.9% ± 12.8% |
| Virtual biopsy | 0.199 (0.176–0.234) | - | - |
| p-Value vs. Previous VOI | p-Value vs. Tumour | p-Value vs. Virtual Biopsy | |
|---|---|---|---|
| Hu-mean | |||
| Tumour | - | - | <0.001 |
| 1 mm | <0.001 | <0.001 | 0.002 |
| 2 mm | 0.006 | <0.001 | 0.709 |
| 3–4 mm | 0.203 | <0.001 | 0.378 |
| 5–6 mm | 0.894 | <0.001 | 0.320 |
| 7–8 mm | 0.847 | <0.001 | 0.427 |
| 9–10 mm | 0.846 | <0.001 | 0.550 |
| Virtual biopsy | 0.550 | <0.001 | - |
| Entropy (log2) | |||
| Tumour | - | - | <0.001 * |
| 1 mm | 0.153 | 0.153 | <0.001 * |
| 2 mm | 0.078 | 0.002 | <0.001 * |
| 3–4 mm | 0.176 | <0.001 | <0.001 * |
| 5–6 mm | 0.603 | <0.001 | <0.001 * |
| 7–8 mm | 0.520 | <0.001 | 0.006 * |
| 9–10 mm | 0.874 | <0.001 | 0.003 * |
| Virtual biopsy | 0.003 * | <0.001 * | - |
| Uniformity | |||
| Tumour | - | - | <0.001 * |
| 1 mm | 0.091 | 0.091 | <0.001 * |
| 2 mm | 0.017 | <0.001 | <0.001 * |
| 3–4 mm | 0.159 * | <0.001 * | <0.001 * |
| 5–6 mm | 0.553 * | <0.001 * | 0.002 * |
| 7–8 mm | 0.705 * | <0.001 * | 0.010 * |
| 9–10 mm | 0.938 * | <0.001 * | 0.007 * |
| Virtual biopsy | 0.007 * | <0.001 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiz, F.; Ragaini, E.M.; Sirchia, S.; Masala, C.; Viganò, S.; Francone, M.; Cavinato, L.; Lanzarone, E.; Ammirabile, A.; Viganò, L. Radiomic Gradient in Peritumoural Tissue of Liver Metastases: A Biomarker for Clinical Practice? Analysing Density, Entropy, and Uniformity Variations with Distance from the Tumour. Diagnostics 2024, 14, 1552. https://doi.org/10.3390/diagnostics14141552
Fiz F, Ragaini EM, Sirchia S, Masala C, Viganò S, Francone M, Cavinato L, Lanzarone E, Ammirabile A, Viganò L. Radiomic Gradient in Peritumoural Tissue of Liver Metastases: A Biomarker for Clinical Practice? Analysing Density, Entropy, and Uniformity Variations with Distance from the Tumour. Diagnostics. 2024; 14(14):1552. https://doi.org/10.3390/diagnostics14141552
Chicago/Turabian StyleFiz, Francesco, Elisa Maria Ragaini, Sara Sirchia, Chiara Masala, Samuele Viganò, Marco Francone, Lara Cavinato, Ettore Lanzarone, Angela Ammirabile, and Luca Viganò. 2024. "Radiomic Gradient in Peritumoural Tissue of Liver Metastases: A Biomarker for Clinical Practice? Analysing Density, Entropy, and Uniformity Variations with Distance from the Tumour" Diagnostics 14, no. 14: 1552. https://doi.org/10.3390/diagnostics14141552
APA StyleFiz, F., Ragaini, E. M., Sirchia, S., Masala, C., Viganò, S., Francone, M., Cavinato, L., Lanzarone, E., Ammirabile, A., & Viganò, L. (2024). Radiomic Gradient in Peritumoural Tissue of Liver Metastases: A Biomarker for Clinical Practice? Analysing Density, Entropy, and Uniformity Variations with Distance from the Tumour. Diagnostics, 14(14), 1552. https://doi.org/10.3390/diagnostics14141552







