The Surviving, Not Thriving, Photoreceptors in Patients with ABCA4 Stargardt Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. OCT Imaging
2.3. Preparing the OCT Images for Analysis
2.4. AO Imaging
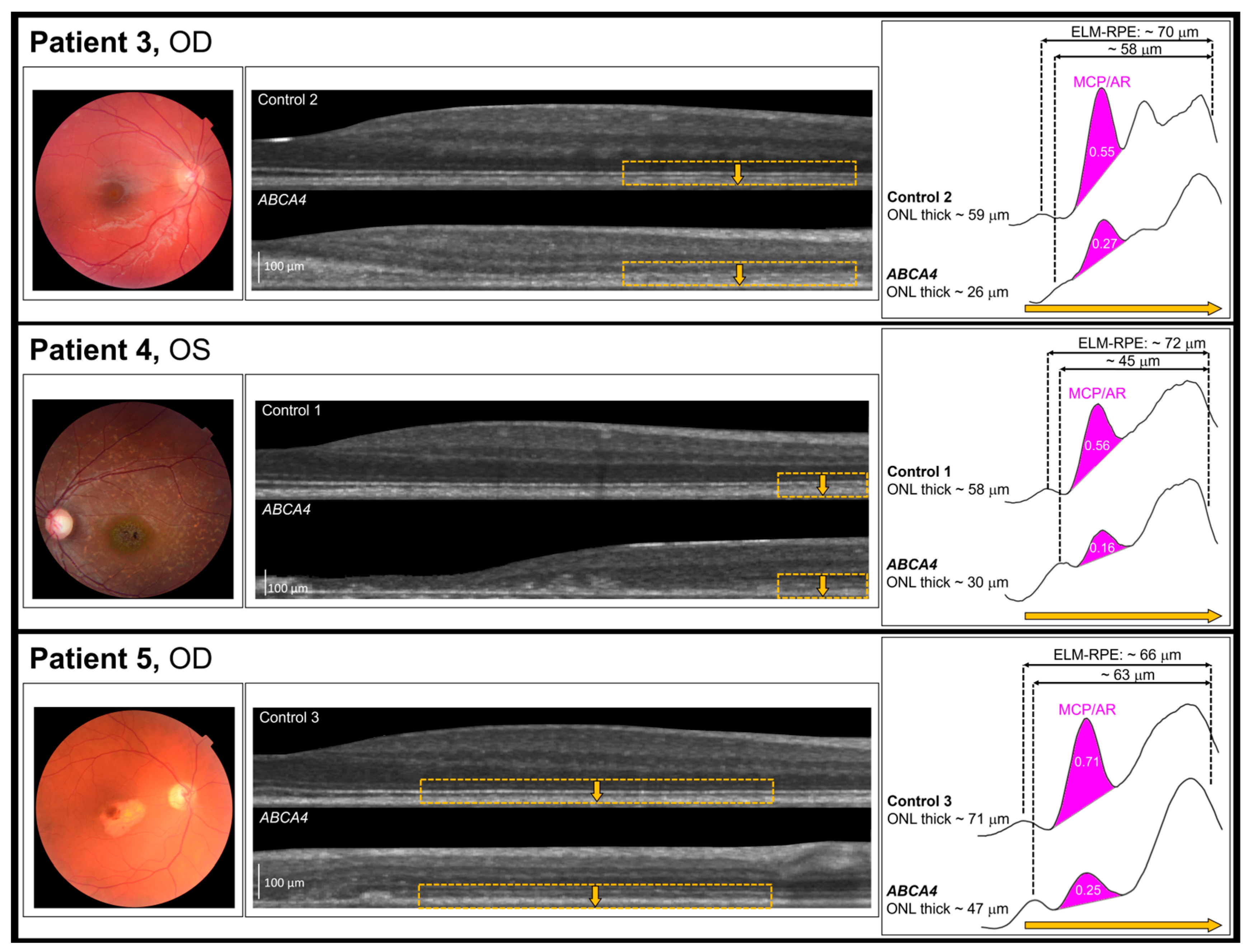
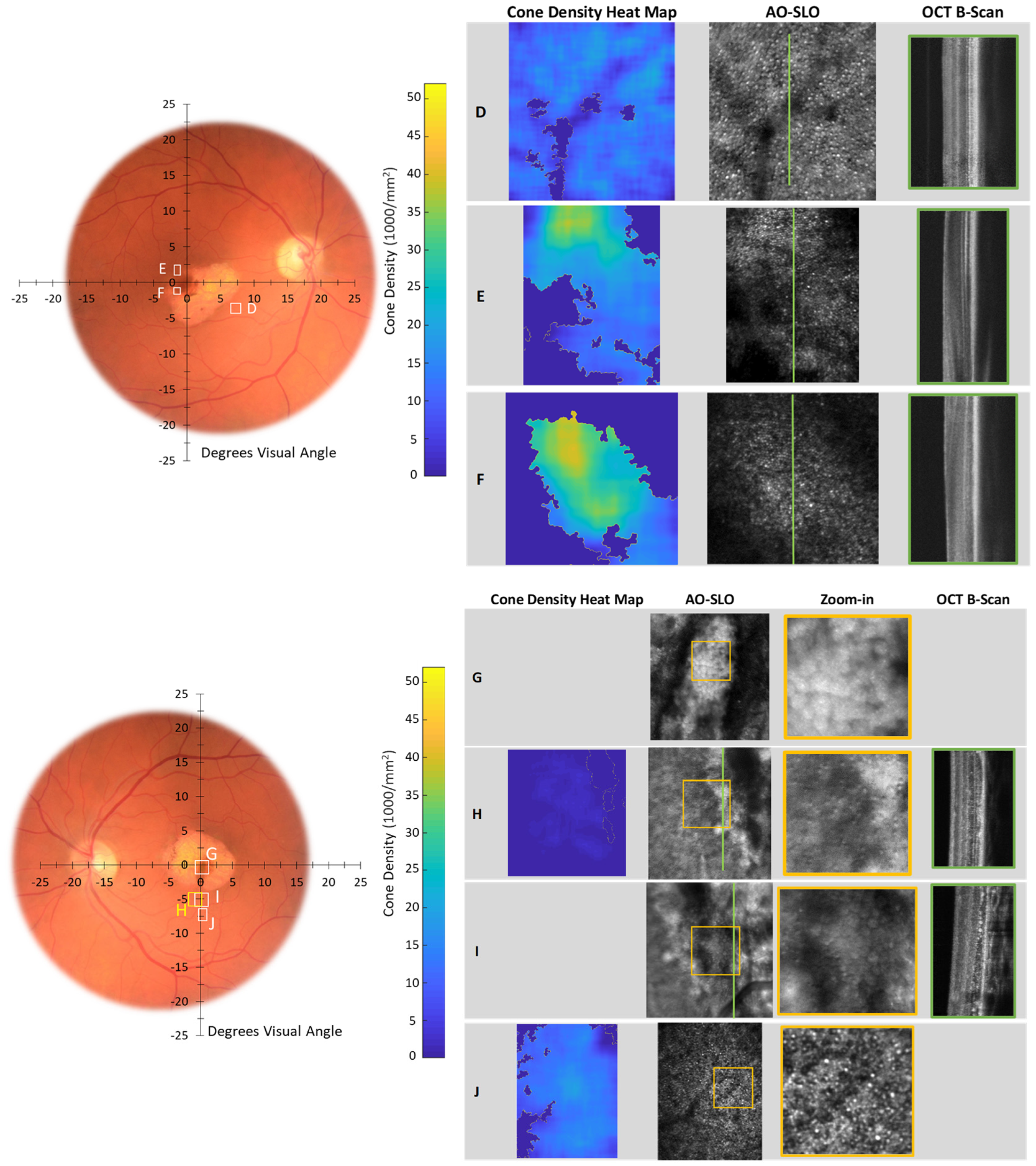
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
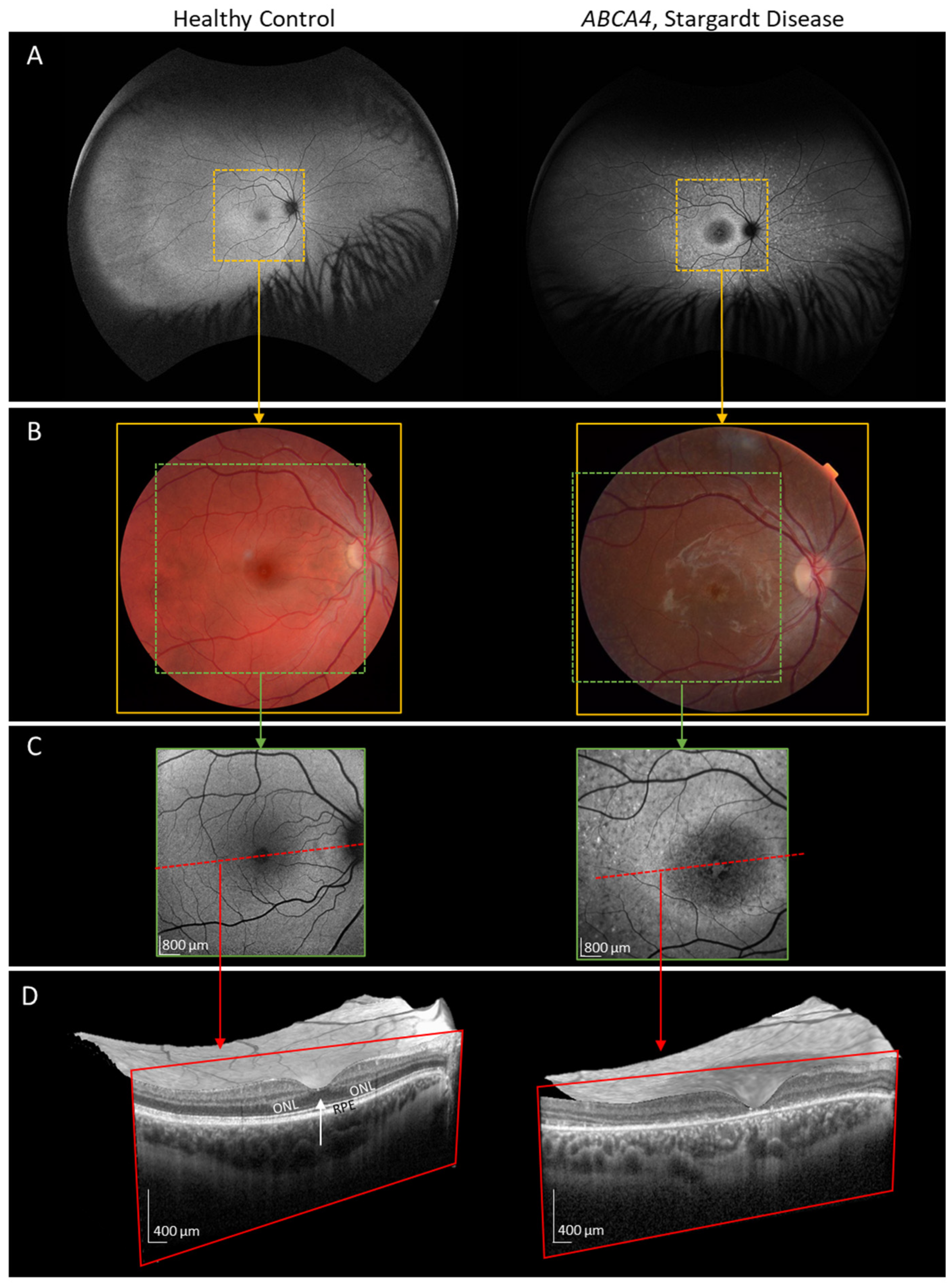
Appendix A
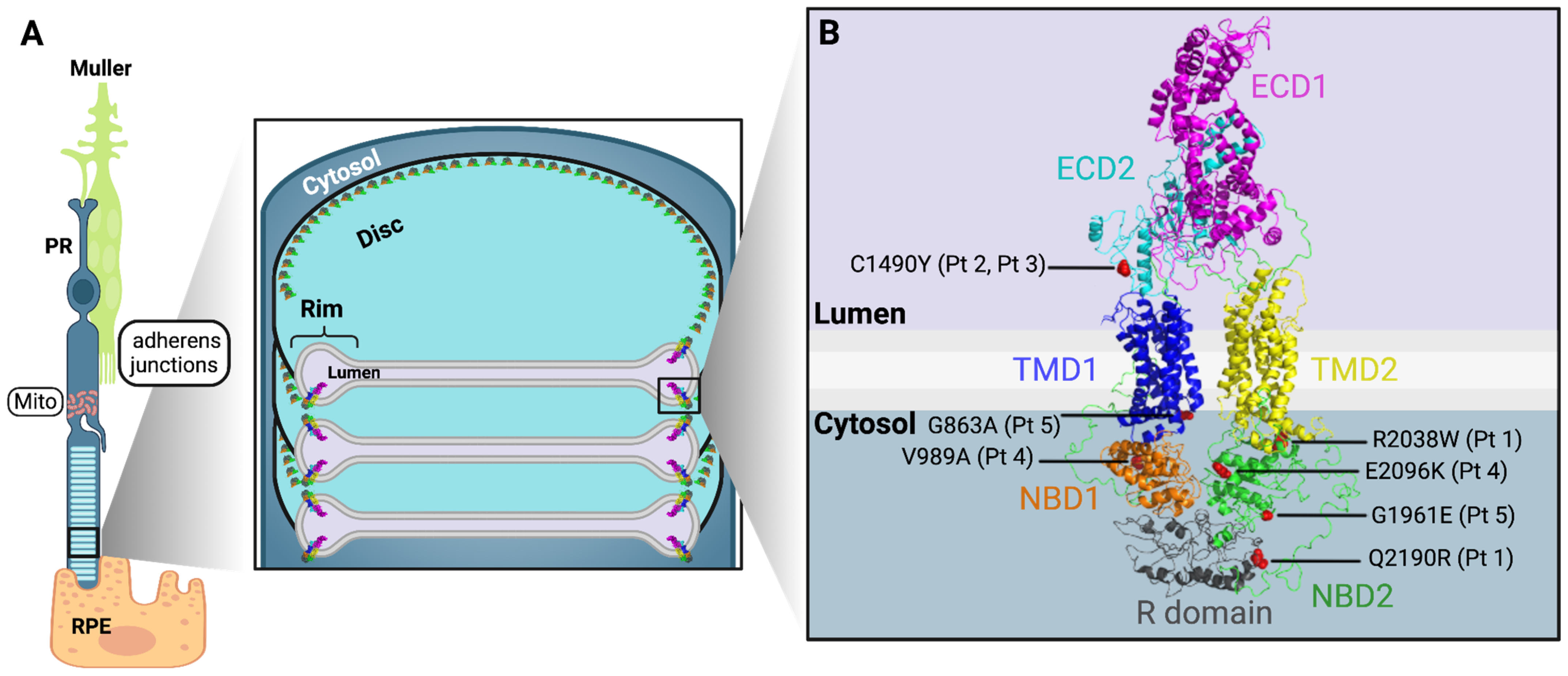
Appendix B
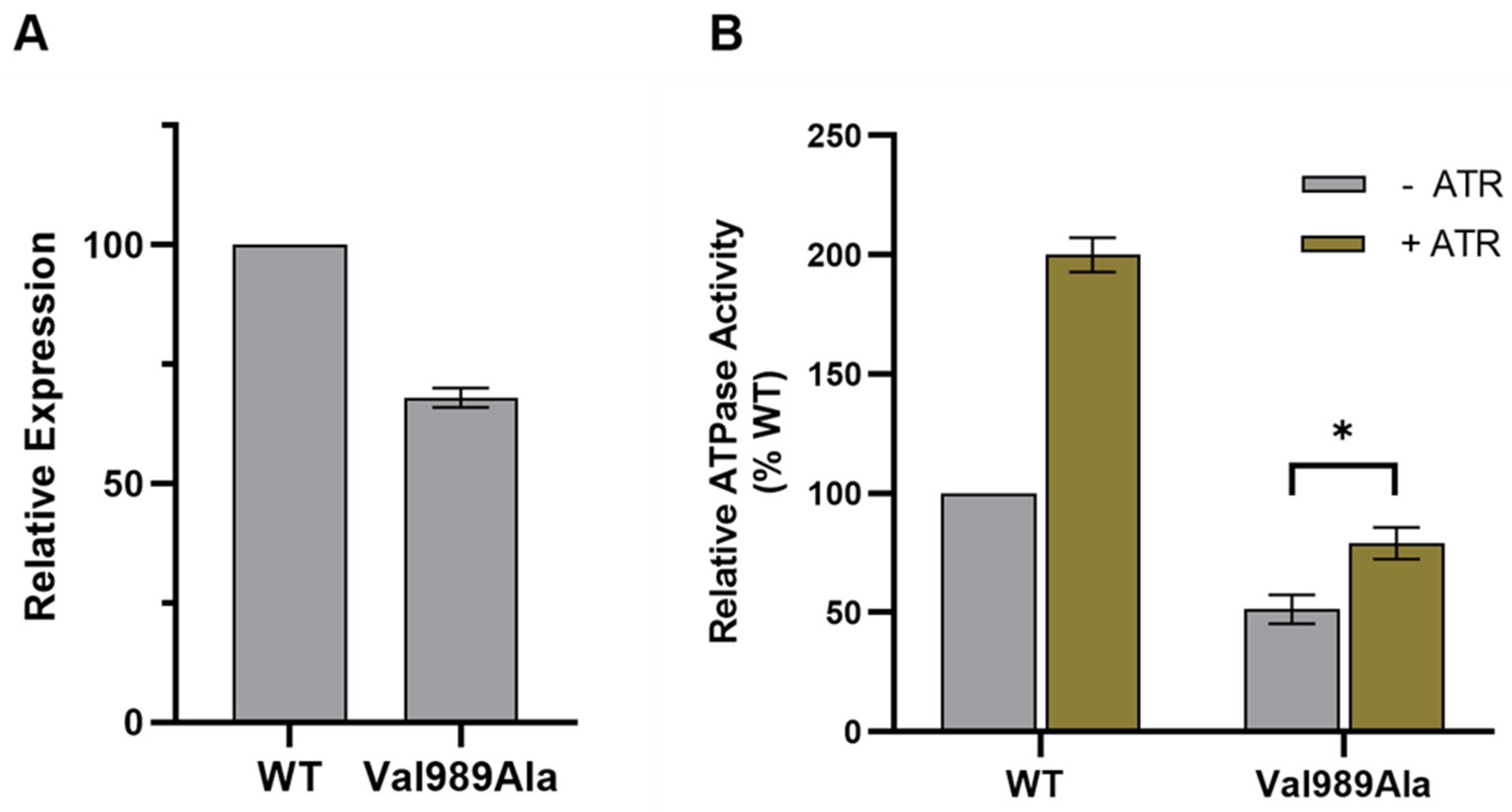
Appendix C

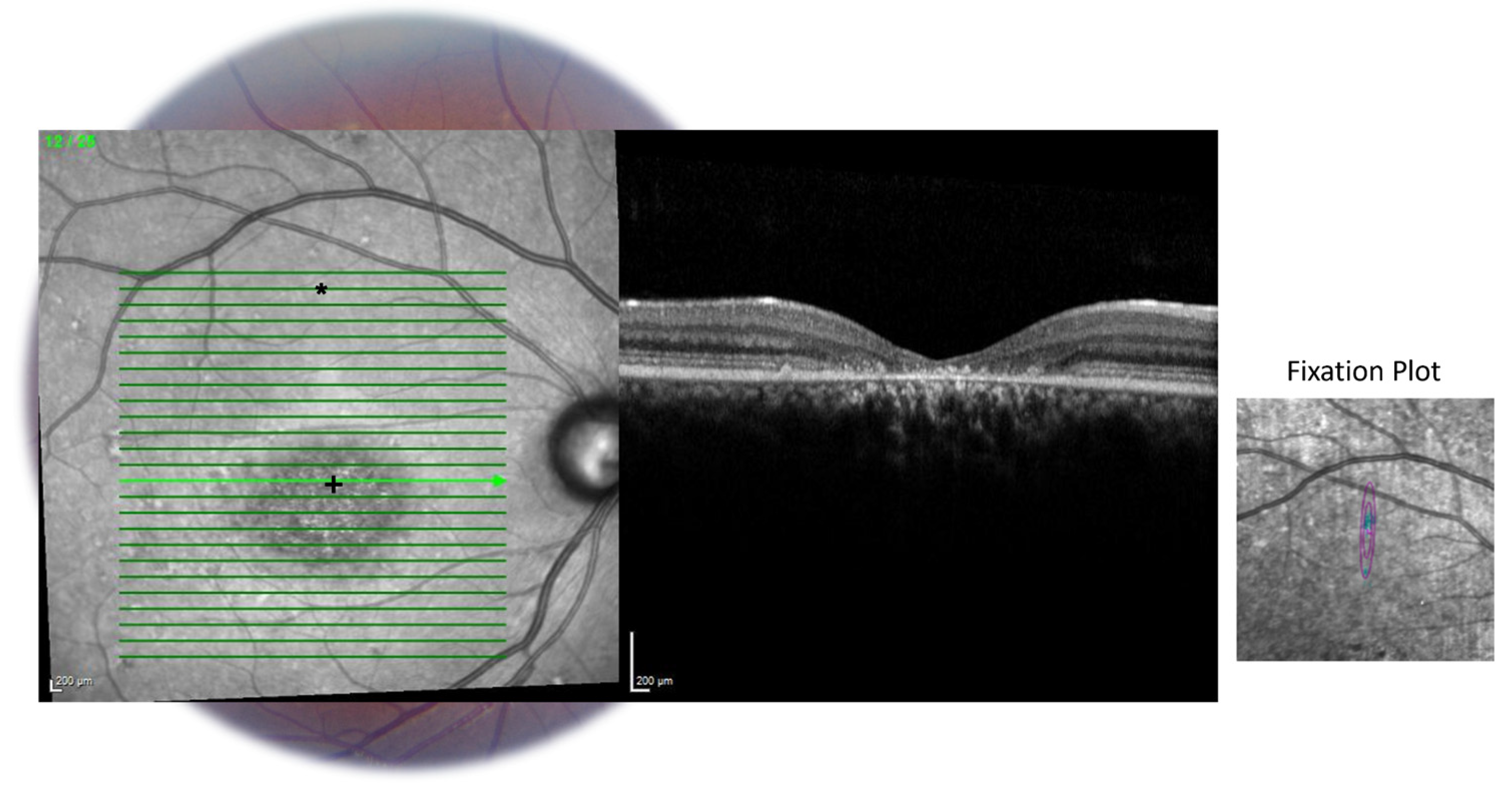

References
- Li, C.H.Z.; Pas, J.A.A.H.; Corradi, Z.; Hitti-Malin, R.J.; Hoogstede, A.; Runhart, E.H.; Dhooge, P.P.A.; Collin, R.W.J.; Cremers, F.P.M.; Hoyng, C.B. Study of Late-Onset Stargardt Type 1 Disease: Characteristics, Genetics, and Progression. Ophthalmology 2024, 131, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Waheed, N.; Laich, Y.; Yang, P.; Fujinami-Yokokawa, Y.; Higgins, J.J.; Lu, J.T.; Curtiss, D.; Clary, C.; Michaelides, M. Stargardt Macular Dystrophy and Therapeutic Approaches. Br. J. Ophthalmol. 2024, 108, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Hardcastle, A.J.; Hunt, D.M.; Moore, A.T. Progressive Cone and Cone-Rod Dystrophies: Phenotypes and Underlying Molecular Genetic Basis. Surv. Ophthalmol. 2006, 51, 232–258. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Hunt, D.M.; Moore, A.T. The Genetics of Inherited Macular Dystrophies. J. Med. Genet. 2003, 40, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt Disease: Clinical Features, Molecular Genetics, Animal Models and Therapeutic Options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S.; Georgiou, M.; Kalitzeos, A.; Moore, A.T.; Michaelides, M. Progressive Cone and Cone-Rod Dystrophies: Clinical Features, Molecular Genetics and Prospects for Therapy. Br. J. Ophthalmol. 2019, 103, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; (Yokokawa) Fujinami, Y.; Yang, L.; Arno, G.; Fujinami, K. Stargardt Disease in Asian Population. In Advances in Vision Research, Volume II: Genetic Eye Research in Asia and the Pacific; Prakash, G., Iwata, T., Eds.; Springer: Singapore, 2019; pp. 279–295. [Google Scholar] [CrossRef]
- Green, R.C.; Goddard, K.A.B.; Jarvik, G.P.; Amendola, L.M.; Appelbaum, P.S.; Berg, J.S.; Bernhardt, B.A.; Biesecker, L.G.; Biswas, S.; Blout, C.L.; et al. Clinical Sequencing Exploratory Research Consortium: Accelerating Evidence-Based Practice of Genomic Medicine. Am. J. Hum. Genet. 2016, 98, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Georgiou, M.; Khan, K.N.; Michaelides, M. Macular Dystrophies: Clinical and Imaging Features, Molecular Genetics and Therapeutic Options. Br. J. Ophthalmol. 2020, 104, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Fujinami-Yokokawa, Y.; Yang, L.; Liu, X.; Arno, G.; Pontikos, N. Stargardt Macular Dystrophy. In Inherited Retinal Disease; Yu, H.-G., Ed.; Springer Nature: Singapore, 2022; pp. 151–168. [Google Scholar] [CrossRef]
- Menon, I.; Huber, T.; Sanyal, S.; Banerjee, S.; Barré, P.; Canis, S.; Warren, J.D.; Hwa, J.; Sakmar, T.P.; Menon, A.K. Opsin Is a Phospholipid Flippase. Curr. Biol. 2011, 21, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Smallwood, P.M.; Nathans, J. Identification and Characterization of All-Trans-Retinol Dehydrogenase from Photoreceptor Outer Segments, the Visual Cycle Enzyme That Reduces All-Trans-Retinal to All-Trans-Retinol. J. Biol. Chem. 2000, 275, 11034–11043. [Google Scholar] [CrossRef] [PubMed]
- Quazi, F.; Lenevich, S.; Molday, R.S. ABCA4 Is an N-Retinylidene-Phosphatidylethanolamine and Phosphatidylethanolamine Importer. Nat. Commun. 2012, 3, 925. [Google Scholar] [CrossRef] [PubMed]
- Scortecci, J.F.; Molday, L.L.; Curtis, S.B.; Garces, F.A.; Panwar, P.; Van Petegem, F.; Molday, R.S. Cryo-EM Structures of the ABCA4 Importer Reveal Mechanisms Underlying Substrate Binding and Stargardt Disease. Nat. Commun. 2021, 12, 5902. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.H.; Fisher, S.K.; Anderson, D.H. Disc Morphogenesis in Vertebrate Photoreceptors. J. Comp. Neurol. 1980, 190, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lee, J.; Chen, J. Molecular Structures of the Eukaryotic Retinal Importer ABCA4. eLife 2021, 10, e63524. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sparrow, J.R. Bisretinoid Phospholipid and Vitamin A Aldehyde: Shining a Light. J. Lipid Res. 2021, 62, 100042. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Marsiglia, M.; Allikmets, R.; Tsang, S.; Lee, W.; Duncker, T.; Zernant, J. Flecks in Recessive Stargardt Disease: Short-Wavelength Autofluorescence, Near-Infrared Autofluorescence, and Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5029–5039. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kasilian, M.; Mahroo, O.A.R.; Tanna, P.; Kalitzeos, A.; Robson, A.G.; Tsunoda, K.; Iwata, T.; Moore, A.T.; Fujinami, K.; et al. Early Patterns of Macular Degeneration in ABCA4-Associated Retinopathy. Ophthalmology 2018, 125, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Nõupuu, K.; Oll, M.; Duncker, T.; Burke, T.; Zernant, J.; Bearelly, S.; Tsang, S.H.; Sparrow, J.R.; Allikmets, R. The External Limiting Membrane in Early-Onset Stargardt Disease. Investig. Opthalmology Vis. Sci. 2014, 55, 6139. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Leitgeb, R.; Kowalczyk, A.; Bajraszewski, T.; Fercher, A.F. In Vivo Human Retinal Imaging by Fourier Domain Optical Coherence Tomography. J. Biomed. Opt. 2002, 7, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Curcio, C.A. Anatomical Correlates to the Bands Seen in the Outer Retina by Optical Coherence Tomography: Literature Review and Model. Retina 2011, 31, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Karwoski, C.J. Light-Evoked Expansion of Subretinal Space Volume in the Retina of the Frog. J. Neurosci. 1992, 12, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.D.; Gallemore, R.P.; Dmitriev, A.; Steinberg, R.H. Light-Dependent Hydration of the Space Surrounding Photoreceptors in Chick Retina. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2700–2711. [Google Scholar]
- Li, J.D.; Govardovskii, V.I.; Steinberg, R.H. Light-Dependent Hydration of the Space Surrounding Photoreceptors in the Cat Retina. Vis. Neurosci. 1994, 11, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Bissig, D.; Berkowitz, B.A. Light-Dependent Changes in Outer Retinal Water Diffusion in Rats In Vivo. Mol. Vis. 2012, 18, 2561–2577. [Google Scholar] [PubMed]
- Berkowitz, B.A.; Paruchuri, A.; Stanek, J.; Podolsky, R.H.; Childers, K.L.; Roberts, R. Acetazolamide Challenge Changes Outer Retina Bioenergy-Linked and Anatomical OCT Biomarkers Depending on Mouse Strain. Investig. Ophthalmol. Vis. Sci. 2024, 65, 21. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Banzon, T.; Jalickee, S.; Wang, N.S.; Miller, S.S. CO2-Induced Ion and Fluid Transport in Human Retinal Pigment Epithelium. J. Gen. Physiol. 2009, 133, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Khacho, M.; Tarabay, M.; Patten, D.; Khacho, P.; MacLaurin, J.G.; Guadagno, J.; Bergeron, R.; Cregan, S.P.; Harper, M.-E.; Park, D.S.; et al. Acidosis Overrides Oxygen Deprivation to Maintain Mitochondrial Function and Cell Survival. Nat. Commun. 2014, 5, 3550. [Google Scholar] [CrossRef] [PubMed]
- Seager, R.; Lee, L.; Henley, J.M.; Wilkinson, K.A. Mechanisms and Roles of Mitochondrial Localisation and Dynamics in Neuronal Function. Neuronal Signal. 2020, 4, NS20200008. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Finkel, T. Cardiac Mitochondria: A Surprise about Size. J. Mol. Cell. Cardiol. 2015, 82, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fariss, R.N.; Qian, J.W.; Cohen, E.D.; Qian, H. Light-Induced Thickening of Photoreceptor Outer Segment Layer Detected by Ultra-High Resolution OCT Imaging. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT105–OCT111. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, Y.; Bissig, D.; Cohen, E.D.; Podolsky, R.H.; Childers, K.L.; Vernon, G.; Chen, S.; Berkowitz, B.A.; Qian, H. Functional Regulation of an Outer Retina Hyporeflective Band on Optical Coherence Tomography Images. Sci. Rep. 2021, 11, 10260. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.D.; Lee, B.; Schottenhamml, J.; Maier, A.; Pugh, E.N., Jr.; Fujimoto, J.G. Photoreceptor Layer Thickness Changes During Dark Adaptation Observed With Ultrahigh-Resolution Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4632–4643. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Z.; Qi, M.; Li, Y.; Zhang, M.; Liao, D.; Gao, P. Application of Adaptive Optics in Ophthalmology. Photonics 2022, 9, 288. [Google Scholar] [CrossRef]
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive Optics: Principles and Applications in Ophthalmology. Eye 2021, 35, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Twenty-Five Years of Clinical Applications Using Adaptive Optics Ophthalmoscopy [Invited]. Available online: https://opg.optica.org/boe/fulltext.cfm?uri=boe-14-1-387&id=524451 (accessed on 30 May 2024).
- Doble, N.; Yoon, G.; Chen, L.; Bierden, P.; Singer, B.; Olivier, S.; Williams, D.R. Use of a Microelectromechanical Mirror for Adaptive Optics in the Human Eye. Opt. Lett. 2002, 27, 1537–1539. [Google Scholar] [CrossRef] [PubMed]
- Romero-Borja, F.; Venkateswaran, K.; Roorda, A.; Hebert, T. Optical Slicing of Human Retinal Tissue in Vivo with the Adaptive Optics Scanning Laser Ophthalmoscope. Appl. Opt. 2005, 44, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Roorda, A.; Romero-Borja, F.; Donnelly Iii, W.; Queener, H.; Hebert, T.; Campbell, M. Adaptive Optics Scanning Laser Ophthalmoscopy. Opt. Express 2002, 10, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, R.J.; Jones, S.M.; Olivier, S.S.; Zhao, M.; Bower, B.A.; Izatt, J.A.; Choi, S.; Laut, S.; Werner, J.S. Adaptive-Optics Optical Coherence Tomography for High-Resolution and High-Speed 3D Retinal in Vivo Imaging. Opt. Express 2005, 13, 8532–8546. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal Vision and High-Resolution Retinal Imaging through Adaptive Optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.; Chen, L.; Yoon, G.Y.; Singer, B.; Yamauchi, Y.; Williams, D.R. Improvement in Retinal Image Quality with Dynamic Correction of the Eye’s Aberrations. Opt. Express 2001, 8, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Podolsky, R.H.; Childers, K.L.; Roberts, R.; Katz, R.; Waseem, R.; Robbings, B.M.; Hass, D.T.; Hurley, J.B.; Sweet, I.R.; et al. Transducin-Deficient Rod Photoreceptors Evaluated With Optical Coherence Tomography and Oxygen Consumption Rate Energy Biomarkers. Investig. Ophthalmol. Vis. Sci. 2022, 63, 22. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Podolsky, R.H.; Childers, K.L.; Burgoyne, T.; De Rossi, G.; Qian, H.; Roberts, R.; Katz, R.; Waseem, R.; Goodman, C. Functional Changes Within the Rod Inner Segment Ellipsoid in Wildtype Mice: An Optical Coherence Tomography and Electron Microscopy Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 8. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.X.; Ferguson, R.D.; Mujat, M.; Patel, A.; Plumb, E.; Iftimia, N.; Chui, T.Y.P.; Akula, J.D.; Fulton, A.B. Multimodal Adaptive Optics Retinal Imager: Design and Performance. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2012, 29, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Ramamirtham, R.; Akula, J.D.; Soni, G.; Swanson, M.J.; Bush, J.N.; Moskowitz, A.; Swanson, E.A.; Favazza, T.L.; Tavormina, J.L.; Mujat, M.; et al. Extrafoveal Cone Packing in Eyes With a History of Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 467–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mujat, M.; Akula, J.D.; Fulton, A.B.; Ferguson, R.D.; Iftimia, N. Non-Rigid Registration for High-Resolution Retinal Imaging. Diagnostics 2023, 13, 2285. [Google Scholar] [CrossRef] [PubMed]
- Akula, J.D.; Arellano, I.A.; Swanson, E.A.; Favazza, T.L.; Bowe, T.S.; Munro, R.J.; Ferguson, R.D.; Hansen, R.M.; Moskowitz, A.; Fulton, A.B. The Fovea in Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Bissig, D.; Zhou, C.G.; Le, V.; Bernard, J.T. Optical Coherence Tomography Reveals Light-Dependent Retinal Responses in Alzheimer’s Disease. NeuroImage 2020, 219, 117022. [Google Scholar] [CrossRef] [PubMed]
- Igathinathane, C.; Pordesimo, L.O.; Columbus, E.P.; Batchelor, W.D.; Methuku, S.R. Shape Identification and Particles Size Distribution from Basic Shape Parameters Using ImageJ. Comput. Electron. Agric. 2008, 63, 168–182. [Google Scholar] [CrossRef]
- Mulchrone, K.F.; Choudhury, K.R. Fitting an Ellipse to an Arbitrary Shape: Implications for Strain Analysis. J. Struct. Geol. 2004, 26, 143–153. [Google Scholar] [CrossRef]
- Chui, T.Y.P.; VanNasdale, D.A.; Burns, S.A. The Use of Forward Scatter to Improve Retinal Vascular Imaging with an Adaptive Optics Scanning Laser Ophthalmoscope. Biomed. Opt. Express 2012, 3, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.; Sulai, Y.N.; Langlo, C.S.; Fishman, G.A.; Curcio, C.A.; Carroll, J.; Dubra, A. In Vivo Imaging of Human Cone Photoreceptor Inner Segments. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.A.; Granger, C.E.; Sharma, R.; Yang, Q.; Saito, K.; Schwarz, C.; Walters, S.; Nozato, K.; Zhang, J.; Kawakami, T.; et al. Imaging Individual Neurons in the Retinal Ganglion Cell Layer of the Living Eye. Proc. Natl. Acad. Sci. USA 2017, 114, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human Photoreceptor Topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef] [PubMed]
- Razeen, M.M.; Cooper, R.F.; Langlo, C.S.; Goldberg, M.R.; Wilk, M.A.; Han, D.P.; Connor, T.B.; Fishman, G.A.; Collison, F.T.; Sulai, Y.N.; et al. Correlating Photoreceptor Mosaic Structure to Clinical Findings in Stargardt Disease. Transl. Vis. Sci. Technol. 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Rossi, E.A.; Latchney, L.; Bessette, A.; Stone, E.; Hunter, J.J.; Williams, D.R.; Chung, M. Cone and Rod Loss in Stargardt Disease Revealed by Adaptive Optics Scanning Light Ophthalmoscopy. JAMA Ophthalmol. 2015, 133, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Roorda, A.; Duncan, J.L. Advances in Imaging of Stargardt Disease. Adv. Exp. Med. Biol. 2010, 664, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S.; Kiilgaard, J.F.; la Cour, M.; Prause, J.U.; Zeuthen, T. Cotransport of H+, Lactate, and H2O in Porcine Retinal Pigment Epithelial Cells. Exp. Eye Res. 2003, 76, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Qian, H. OCT Imaging of Rod Mitochondrial Respiration In Vivo. Exp. Biol. Med. 2021, 246, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, B.A.; Podolsky, R.H.; Lins-Childers, K.M.; Li, Y.; Qian, H. Outer Retinal Oxidative Stress Measured In Vivo Using QUEnch-assiSTed (QUEST) OCT. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Liu, J.; Finkel, T. Mitohormesis. Cell Metab. 2023, 35, 1872–1886. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F. Nonsense-Mediated mRNA Decay, a Finely Regulated Mechanism. Biomedicines 2022, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Biswas-Fiss, E.E. Functional Analysis of Genetic Mutations in Nucleotide Binding Domain 2 of the Human Retina Specific ABC Transporter. Biochemistry 2003, 42, 10683–10696. [Google Scholar] [CrossRef] [PubMed]
- Garces, F.; Jiang, K.; Molday, L.L.; Stöhr, H.; Weber, B.H.; Lyons, C.J.; Maberley, D.; Molday, R.S. Correlating the Expression and Functional Activity of ABCA4 Disease Variants With the Phenotype of Patients With Stargardt Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, R.; Bax, N.M.; Bauwens, M.; van den Born, L.I.; De Baere, E.; Garanto, A.; Collin, R.W.J.; Goercharn-Ramlal, A.S.A.; den Engelsman-van Dijk, A.H.A.; Rohrschneider, K.; et al. Photoreceptor Progenitor mRNA Analysis Reveals Exon Skipping Resulting from the ABCA4 c.5461-10T→C Mutation in Stargardt Disease. Ophthalmology 2016, 123, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Aukrust, I.; Jansson, R.W.; Bredrup, C.; Rusaas, H.E.; Berland, S.; Jørgensen, A.; Haug, M.G.; Rødahl, E.; Houge, G.; Knappskog, P.M. The Intronic ABCA4 c.5461-10T>C Variant, Frequently Seen in Patients with Stargardt Disease, Causes Splice Defects and Reduced ABCA4 Protein Level. Acta Ophthalmol. 2017, 95, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.L.; Grassmann, F.; Kellner, U.; Spital, G.; Rüther, K.; Jägle, H.; Hufendiek, K.; Rating, P.; Huchzermeyer, C.; Baier, M.J.; et al. Mutation Spectrum of the ABCA4 Gene in 335 Stargardt Disease Patients From a Multicenter German Cohort-Impact of Selected Deep Intronic Variants and Common SNPs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Smallwood, P.M.; Nathans, J. Biochemical Defects in ABCR Protein Variants Associated with Human Retinopathies. Nat. Genet. 2000, 26, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.B.; Molday, L.L.; Garces, F.A.; Molday, R.S. Functional Analysis and Classification of Homozygous and Hypomorphic ABCA4 Variants Associated with Stargardt Macular Degeneration. Hum. Mutat. 2020, 41, 1944–1956. [Google Scholar] [CrossRef]



| Participant | Age at Imaging (Years) | Sex | Allele 1 (cDNA, Protein) | Allele 2 (cDNA, Protein) | BCVA (logMAR) OD, OS | Spherical Equivalent (Diopter) OD, OS | Axial Length (mm) OD, OS | ||
|---|---|---|---|---|---|---|---|---|---|
| OCT | AO | ||||||||
| Patients | 1 | 15 | - | F | c.4036_4037del (p.T1346Gfs*75) | c.6112C>T (p.R2038W) c. 6569A>G (p.Q2190R) | 0.875, 0.796 | −0.75, −0.75 | - |
| 2 | 3 | - | M | c.4469G>A (p.C1490Y) | c.5461-10T>C (splice) | 0.301, 0.301 | Plano, plano | - | |
| 3 | 5 | - | F | c.4469G>A (p.C1490Y) | c.5461-10T>C (splice) | 0.176, 0.097 | 1.00, 1.00 | - | |
| 4 | 25 | 17 | F | c.2966T>C (p.V989A) | c.6286G>A (p.E2096K) | 0.602, 0.602 | −0.50, −0.50 | 23.40, 23.18 | |
| 5 | 61 | 58 | M | c.2588G>C (p.G863A) | c.5882G>A (p.G1961E) | 0.000, 0.176 | −3.50, −3.13 | 25.06, 24.84 | |
| Controls | 1 | 25 | 24 | F | - | - | −0.04, −0.10 | −0.38, −1.75 | 24.52, - |
| 2 | 5 | - | F | - | - | 0.18 0.18 | +4.50, +4.36 | - | |
| 3 | 61 | - | M | - | - | 0.00, 0.00 | - | - | |
| Participant | Scan | Eye | Aperture | Scan Size | Zoom-in | Eccentricity | Cone Density [1000/mm2] | Figure Number(s) in This Paper | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [°] | [µm × µm] | [°] | [µm × µm] | [°] | [mm] | Mean | Max | Normal Range [58] | |||||
| Control 1 | 1 | OD | confocal | 1.4 × 0.9 | 420 × 260 | 1 | 0.3 | 39 | 52 | 50–52 | Figure 5 and Figure A2 | ||
| 2 | OD | confocal | 1.4 × 1.4 | 420 × 420 | 10 | 3.0 | 17 | 24 | 8–9 | ||||
| Patient 4 | A | OD | confocal | 1.1 × 1.0 | 310 × 280 | 0.40 × 0.30 | 100 × 75 | 2 | 0.6 | 19 | 38 | 30–50 | Figure 6 and Figure A3 |
| B | OD | offset | 1.0 × 1.0 | 280 × 280 | - | - | 3 | 0.7 | 3 | 14 | 25–40 | ||
| C | OD | offset | 1.0 × 1.0 | 280 × 280 | 0.60 × 0.30 | 170 × 84 | 4 | 1.1 | 4 | 9 | 12–25 | ||
| Patient 5 | D | OD | offset | 1.0 × 1.0 | 310 × 310 | - | - | 8 | 2.5 | 11 | 25 | 8–9 | Figure 7 and Figure A4 |
| E | OD | confocal | 0.9 × 1.1 | 280 × 340 | - | - | 1 | 0.4 | 14 | 39 | 30–40 | ||
| F | OD | confocal | 0.9 × 0.8 | 280 × 250 | - | - | 1 | 0.4 | 16 | 45 | 30–40 | ||
| G | OS | offset | 2.0 × 2.0 | 610 × 610 | 0.60 × 0.60 | 180 × 180 | ~0 | - | - | - | - | ||
| H | OS | offset | 2.0 × 2.0 | 610 × 610 | 0.80 × 0.80 | 230 × 230 | 5 | 1.5 | 2 | 4 | 12–13 | ||
| I | OS | offset | 2.0 × 2.0 | 610 × 610 | 0.80 × 0.80 | 230 × 230 | 5 | 1.5 | - | - | - | ||
| J | OS | confocal | 1.3 × 1.6 | 400 × 490 | 0.50 × 0.50 | 150 × 150 | 7 | 2.1 | 11 | 22 | 9–10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bruyn, H.; Johnson, M.; Moretti, M.; Ahmed, S.; Mujat, M.; Akula, J.D.; Glavan, T.; Mihalek, I.; Aslaksen, S.; Molday, L.L.; et al. The Surviving, Not Thriving, Photoreceptors in Patients with ABCA4 Stargardt Disease. Diagnostics 2024, 14, 1545. https://doi.org/10.3390/diagnostics14141545
De Bruyn H, Johnson M, Moretti M, Ahmed S, Mujat M, Akula JD, Glavan T, Mihalek I, Aslaksen S, Molday LL, et al. The Surviving, Not Thriving, Photoreceptors in Patients with ABCA4 Stargardt Disease. Diagnostics. 2024; 14(14):1545. https://doi.org/10.3390/diagnostics14141545
Chicago/Turabian StyleDe Bruyn, Hanna, Megan Johnson, Madelyn Moretti, Saleh Ahmed, Mircea Mujat, James D. Akula, Tomislav Glavan, Ivana Mihalek, Sigrid Aslaksen, Laurie L. Molday, and et al. 2024. "The Surviving, Not Thriving, Photoreceptors in Patients with ABCA4 Stargardt Disease" Diagnostics 14, no. 14: 1545. https://doi.org/10.3390/diagnostics14141545
APA StyleDe Bruyn, H., Johnson, M., Moretti, M., Ahmed, S., Mujat, M., Akula, J. D., Glavan, T., Mihalek, I., Aslaksen, S., Molday, L. L., Molday, R. S., Berkowitz, B. A., & Fulton, A. B. (2024). The Surviving, Not Thriving, Photoreceptors in Patients with ABCA4 Stargardt Disease. Diagnostics, 14(14), 1545. https://doi.org/10.3390/diagnostics14141545






