Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Approvals, Participants, and Eye Exam
2.2. AO-OCT Imaging
2.3. Image Processing and Analysis
2.4. Statistical Analysis
3. Results
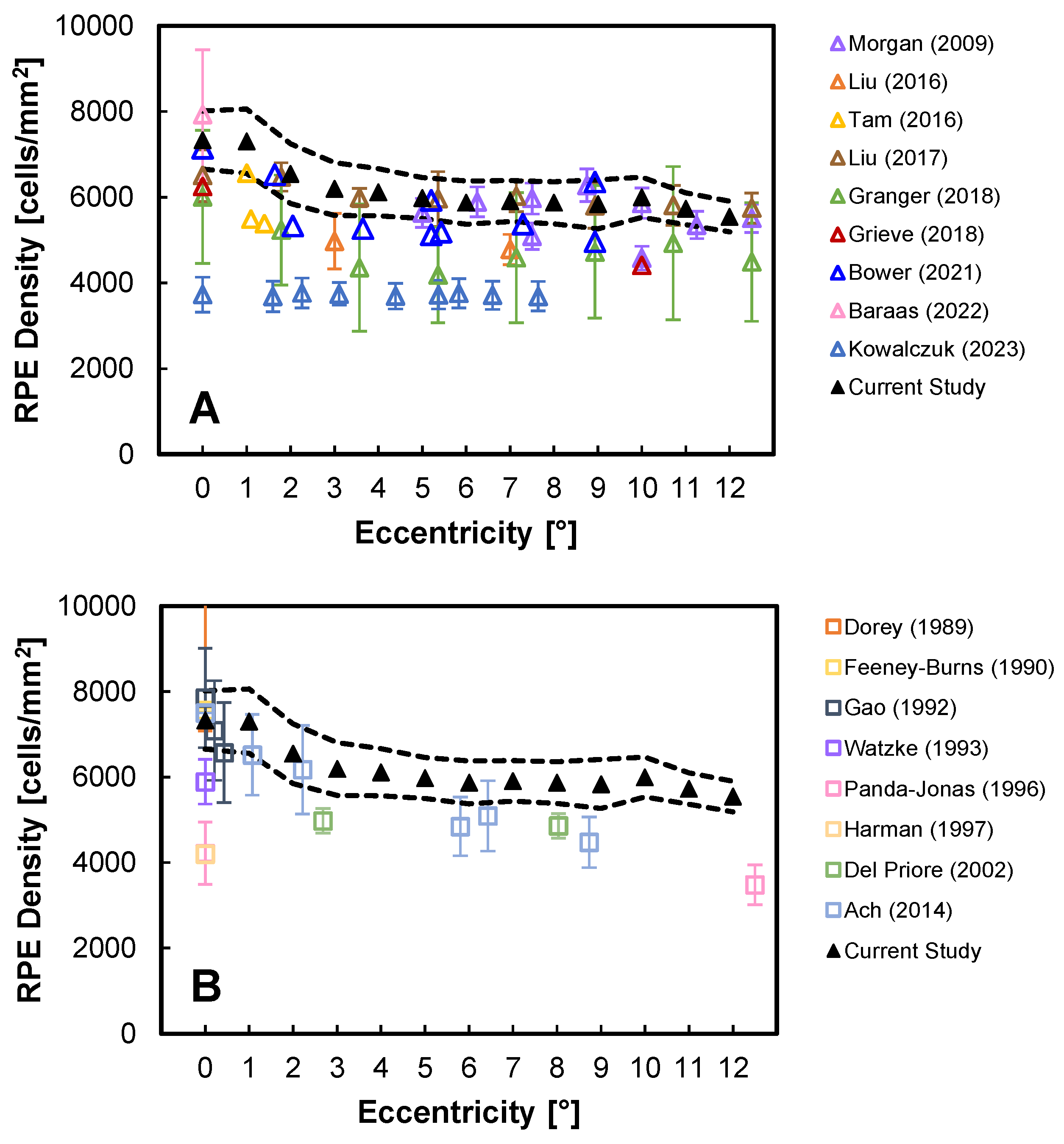
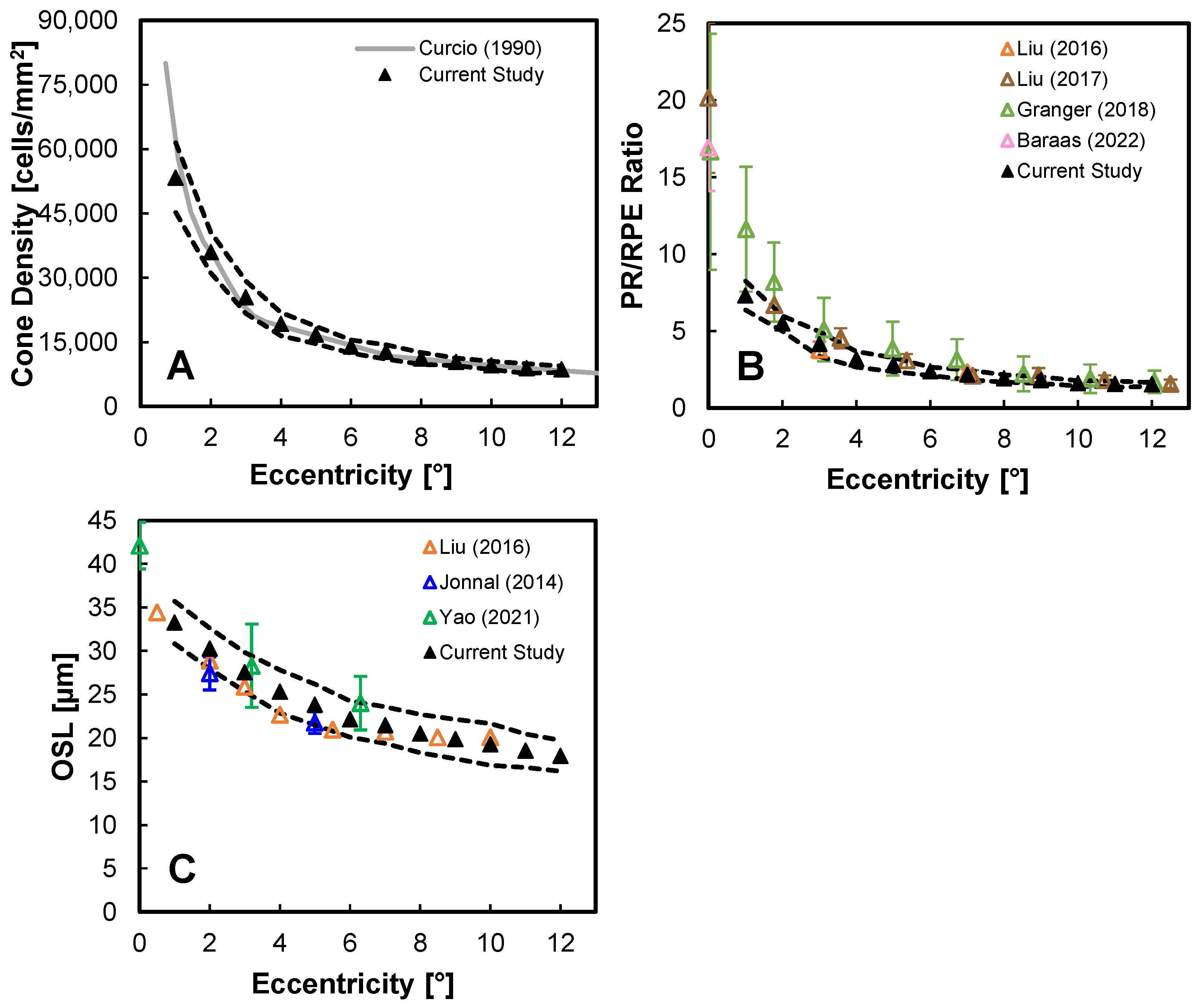
3.1. Primary PR-RPE Complex Metrics
3.2. Other PR-RPE Complex Measures
3.3. Reproducibility of PR-RPE Complex Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Commercial Disclaimer
References
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Chikafumi, C. The retinal pigment epithelium: An important player of retinal disorders and regeneration. Exp. Eye Res. 2014, 123, 107–114. [Google Scholar] [CrossRef]
- Aguilera, N.; Liu, T.; Bower, A.; Li, J.; Abouassali, S.; Lu, R.; Giannini, J.; Pfau, M.; Bender, C.; Smelkinson, M.G.; et al. Widespread subclinical cellular changes revealed across a neural-epithelial-vascular complex in choroideremia using adaptive optics. Commun. Biol. 2022, 5, 893. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, A.; Zhang, F.; Kurokawa, K.; Liu, Y.; Bernucci, M.T.; Crowell, J.A.; Miller, D.T. Cone photoreceptor dysfunction in retinitis pigmentosa revealed by optoretinography. Proc. Nat. Acad. Sci. USA 2021, 118, e2107444118. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Ach, T.; Tolstik, E.; Messinger, J.D.; Zarubina, A.V.; Heintzmann, R.; Curcio, C.A. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3242–3252. [Google Scholar] [CrossRef] [PubMed]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178. [Google Scholar] [CrossRef]
- Hee, M.R.; Izatt, J.A.; Swanson, E.A.; Huang, D.; Schuman, J.S.; Lin, C.P.; Puliafito, C.A.; Fujimoto, J.G. Optical Coherence Tomography of the Human Retina. Arch. Ophthalmol. 1995, 113, 325–332. [Google Scholar] [CrossRef]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A 1997, 14, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Roorda, A.; Williams, D.R. The arrangement of the three cone classes in the living human eye. Nature 1999, 397, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.G.; Loumou, P.; Griffin, S.; Qin, J.; Ratnam, K.; Porco, T.C.; Roorda, A.; Duncan, J.L. Relationship between Foveal Cone Structure and Visual Acuity Measured With Adaptive Optics Scanning Laser Ophthalmoscopy in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3385–3393. [Google Scholar] [CrossRef]
- Heitkotter, H.; Patterson, E.J.; Woertz, E.N.; Cava, J.A.; Gaffney, M.; Adhan, I.; Tam, J.; Cooper, R.F.; Carroll, J. Extracting spacing-derived estimates of rod density in healthy retinae. Biomed. Opt. Exp. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Roorda, A.; Zhang, Y.; Duncan, J.L. High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.I.W.; Dubra, A.; Wolfe, R.; Merigan, W.H.; Williams, D.R. In vivo autofluorescence imaging of the human and macaque retinal pigment epithelial cell mosaic. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1350–1359. [Google Scholar] [CrossRef]
- Bower, A.J.; Liu, T.; Aguilera, N.; Li, J.; Liu, J.; Lu, R.; Giannini, J.P.; Huryn, L.A.; Dubra, A.; Liu, Z.; et al. Integrating adaptive optics-SLO and OCT for multimodal visualization of the human retinal pigment epithelial mosaic. Biomed. Opt. Exp. 2021, 12, 1449–1466. [Google Scholar] [CrossRef]
- Rossi, E.A.; Rangel-Fonseca, P.; Parkins, K.; Fischer, W.; Latchney, L.R.; Folwell, M.A.; Williams, D.R.; Dubra, A.; Chung, M.M. In vivo imaging of retinal pigment epithelium cells in age related macular degeneration. Biomed. Opt. Exp. 2013, 4, 2527–2539. [Google Scholar] [CrossRef]
- Grieve, K.; Gofas-Salas, E.; Ferguson, R.D.; Sahel, J.A.; Paques, M.; Rossi, E.A. In vivo near-infrared autofluorescence imaging of retinal pigment epithelial cells with 757 nm excitation. Biomed. Opt. Exp. 2018, 9, 5946–5961. [Google Scholar] [CrossRef]
- Granger, C.E.; Yang, Q.; Song, H.; Saito, K.; Nozato, K.; Latchney, L.R.; Leonard, B.T.; Chung, M.M.; Williams, D.R.; Rossi, E.A. Human retinal pigment epithelium: In vivo cell morphometry, multispectral autofluorescence, and relationship to cone mosaic. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jung, H.; Liu, J.; Droettboom, M.; Tam, J. Noninvasive near infrared autofluorescence imaging of retinal pigment epithelial cells in the human retina using adaptive optics. Biomed. Opt. Exp. 2017, 8, 4348–4360. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Liu, J.; Dubra, A.; Fariss, R. In vivo imaging of the human retinal pigment epithelial mosaic using adaptive optics enhanced indocyanine green ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4376–4384. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.; Sulai, Y.N.; Dubra, A. In vivo dark-field imaging of the retinal pigment epithelium cell mosaic. Biomed. Opt. Exp. 2013, 4, 1710–1723. [Google Scholar] [CrossRef]
- Laforest, T.; Künzi, M.; Kowalczuk, L.; Carpentras, D.; Behar-Cohen, F.; Moser, C. Transscleral optical phase imaging of the human retina. Nat. Photonics 2020, 14, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, L.; Dornier, R.; Kunzi, M.; Iskandar, A.; Misutkova, Z.; Gryczka, A.; Navarro, A.; Jeunet, F.; Mantel, I.; Behar-Cohen, F.; et al. In Vivo Retinal Pigment Epithelium Imaging using Transscleral Optical Imaging in Healthy Eyes. Ophthalmol. Sci. 2023, 3, 100234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kocaoglu, O.P.; Miller, D.T. 3D imaging of retinal pigment epithelial cells in the living human retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kurokawa, K.; Hammer, D.X.; Miller, D.T. In vivo measurement of organelle motility in human retinal pigment epithelial cells. Biomed. Opt. Exp. 2019, 10, 4142–4158. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Baraas, R.C.; Pedersen, H.R.; Knoblauch, K.; Gilson, S.J. Human foveal Cone and RPE cell topographies and their correspondence with foveal shape. Investig. Ophthalmol. Vis. Sci. 2022, 63, 8. [Google Scholar] [CrossRef]
- Curcio, C.A.; Kar, D.; Owsley, C.; Sloan, K.R.; Ach, T. Age-Related Macular Degeneration, a Mathematically Tractable Disease. Investig. Ophthalmol. Vis. Sci. 2024, 65, 4. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Zucca, K.; Agrawal, A.; Hammer, D.X. Ultrahigh-speed multimodal adaptive optics system for microscopic structural and functional imaging of the human retina. Biomed. Opt. Exp. 2022, 13, 5860–5878. [Google Scholar] [CrossRef]
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal Pigment Epithelial Lipofuscin and Melanin and Choriodal Melanin in Human Eyes. Investig. Ophthalmol. Vis. Sci. 1986, 27, 145–152. [Google Scholar]
- Kurokawa, K.; Crowell, J.; Do, N.; Lee, J.; Miller, D. Multi-reference global registration of individual A-lines in adaptive optics optical coherence tomography retinal images. J. Biomed. Opt. 2021, 26, 016001. [Google Scholar] [CrossRef]
- Soltanian-Zadeh, S.; Liu, Z.; Liu, Y.; Lassoued, A.; Cukras, C.A.; Miller, D.T.; Hammer, D.X.; Farsiu, S. Deep learning-enabled volumetric cone photoreceptor segmentation in adaptive optics optical coherence tomography images of normal and diseased eyes. Biomed. Opt. Exp. 2023, 14, 815–833. [Google Scholar] [CrossRef]
- Bennett, A.G.; Rudnicka, A.R.; Edgar, D.F. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefe’s Arch. Clin. Exp. Ophthalmol. 1994, 232, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.; Li, M. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2000, 15, 155–163. [Google Scholar] [CrossRef]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Dorey, C.K.; Wu, G.; Ebenstein, D.; Garsd, A.; Weiter, J.J. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1691–1699. [Google Scholar]
- Feeney-Burns, L.; Burns, R.P.; Gao, C.L. Age-related macular changes in humans over 90 years old. Am. J. Ophthalmol. 1990, 109, 265–278. [Google Scholar] [CrossRef]
- Gao, H.; Hollyfield, J. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Watzke, R.C.; Soldevilla, J.D.; Trune, D.R. Morphometric analysis of human retinal pigment epithelium: Correlation with age and location. Curr. Eye Res. 1993, 12, 133–142. [Google Scholar] [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am. J. Ophthalmol. 1996, 121, 181–189. [Google Scholar] [CrossRef]
- Harman, A.M.; Fleming, P.A.; Hoskins, R.V.; Moore, S.R. Development and aging of cell topography in the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2016–2026. [Google Scholar]
- Del Priore, L.V.; Ku, Y.H.; Tezel, T.H. Age-related changes in human RPE cell density and apoptosis proportion in situ. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3312–3318. [Google Scholar]
- Ach, T.; Huisingh, C.; McGwin, G. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef]
- Jonnal, R.S.; Kocaoglu, O.P.; Zawadzki, R.J.; Lee, S.-H.; Werner, J.S.; Miller, D.T. The cellular origins of the outer retinal bands in optical coherence tomography images. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7904–7918. [Google Scholar] [CrossRef]
- Yao, X.; Son, T.; Kim, T.; Le, D. Interpretation of anatomic correlates of outer retinal bands in optical coherence tomography. Exp. Biol. Med. 2021, 246, 2140–2150. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography. Retina 2011, 31, 1609–1619. [Google Scholar] [CrossRef]
- Hendrickson, A.; Drucker, D. The development of parafoveal and mid-peripheral human retina. Behav. Brain Res. 1992, 49, 21–31. [Google Scholar] [CrossRef]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. International Nomenclature for Optical Coherence Tomography (IN-OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef]
- Spaide, R.F. Outer retinal Bands. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2505–2506. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sparrow, J.R.; Bonilha, V.L.; Pollreisz, A.; Lujan, B.J. Re: Cuenca et al.: Cellular characterization of OCT and outer retinal bands using specific immunohistochemistry markers and clinical implications (Ophthalmology. 2018;125;407–422), and authors response. Ophthalmology 2018, 125, e47–e49. [Google Scholar] [CrossRef]
- Lu, R.W.; Curcio, C.A.; Zhang, Y.; Zhang, Q.X.; Pittler, S.J.; Deretic, D.; Yao, X.C. Investigation of the hyper-reflective inner/outer segment band in optical coherence tomography of living frog retina. J. Biomed. Opt. 2012, 17, 060504. [Google Scholar] [CrossRef]
- Domdei, N.; Ameln, J.; Gutnikov, A.; Witten, J.L.; Holz, F.G.; Wahl, S.; Harmening, W.M. Cone density is correlated to outer segment length and retinal thickness in the human foveola. Investig. Ophthalmol. Vis. Sci. 2023, 64, 11. [Google Scholar] [CrossRef]
- Srinivasan, V.J.; Monson, B.K.; Wojtkowski, M.; Bilonick, R.A.; Gorczynska, I.; Chen, R.; Duker, J.S.; Schuman, J.S.; Fujimoto, J.G. Characterization of outer retinal morphology with high-speed, ultrahigh resolution optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1571–1579. [Google Scholar] [CrossRef]
- Das, V.; Zhang, F.; Bower, A.J.; Li, J.; Liu, T.; Aguilera, N.; Alvisio, B.; Liu, Z.; Hammer, D.X.; Tam, J. Revealing speckle obscured living human retinal cells with artificial intelligence assisted adaptive optics optical coherence tomography. Commun. Med. 2024, 4, 68. [Google Scholar] [CrossRef]
- Lu, R.; Aguilera, N.; Liu, T.; Liu, J.; Giannini, J.P.; Li, J.; Bower, A.J.; Dubra, A.; Tam, J. In-vivo sub-diffraction adaptive optics imaging of photoreceptors in the human eye with annular pupil illumination and sub-Airy detection. Optica 2021, 8, 333–343. [Google Scholar] [CrossRef]




| ID | Age | Eye 1 | Sex 2 | Race 3 | AL [mm] | Lateral Pixel Size [µm/pixel] |
|---|---|---|---|---|---|---|
| 2875 | 32.8 | OS | M | W | 24.13 | 1.138 |
| 1610 | 32.0 | OD | M | W | 25.02 | 1.184 |
| 7743 | 42.9 | OS | F | A | 25.28 | 1.197 |
| 8195 | 33.3 | OD | M | W | 24.11 | 1.164 |
| 0420 | 27.7 | OD | F | B | 25.29 | 1.197 |
| 0571 | 42.2 | OS | M | A | 26.35 | 1.281 |
| 5291 | 36.6 | OD | M | W | 25.21 | 1.193 |
| 5810 | 36.5 | OS | F | W | 22.29 | 1.044 |
| 7473 | 36.6 | OD | M | W | 23.96 | 1.129 |
| 3339 | 36.6 | OS | M | W | 24.27 | 1.145 |
| 0201 | 32.9 | OS | F | W | 22.14 | 1.062 |
| Mean ± SD | 35.5 ± 4.4 | 6/5 | 4/7 | 1/2/8 | 24.37 ± 1.28 | 1.158 ± 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Aghayee, S.; Soltanian-Zadeh, S.; Kovalick, K.; Agrawal, A.; Saeedi, O.; Cukras, C.; Chew, E.Y.; Farsiu, S.; Hammer, D.X. Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography. Diagnostics 2024, 14, 1518. https://doi.org/10.3390/diagnostics14141518
Liu Z, Aghayee S, Soltanian-Zadeh S, Kovalick K, Agrawal A, Saeedi O, Cukras C, Chew EY, Farsiu S, Hammer DX. Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography. Diagnostics. 2024; 14(14):1518. https://doi.org/10.3390/diagnostics14141518
Chicago/Turabian StyleLiu, Zhuolin, Samira Aghayee, Somayyeh Soltanian-Zadeh, Katherine Kovalick, Anant Agrawal, Osamah Saeedi, Catherine Cukras, Emily Y. Chew, Sina Farsiu, and Daniel X. Hammer. 2024. "Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography" Diagnostics 14, no. 14: 1518. https://doi.org/10.3390/diagnostics14141518
APA StyleLiu, Z., Aghayee, S., Soltanian-Zadeh, S., Kovalick, K., Agrawal, A., Saeedi, O., Cukras, C., Chew, E. Y., Farsiu, S., & Hammer, D. X. (2024). Quantification of Human Photoreceptor–Retinal Pigment Epithelium Macular Topography with Adaptive Optics–Optical Coherence Tomography. Diagnostics, 14(14), 1518. https://doi.org/10.3390/diagnostics14141518







