Determination of the Course of Cyan Fluorescence of Pseudomonas aeruginosa with a Handheld Bacterial Imaging Device
Abstract
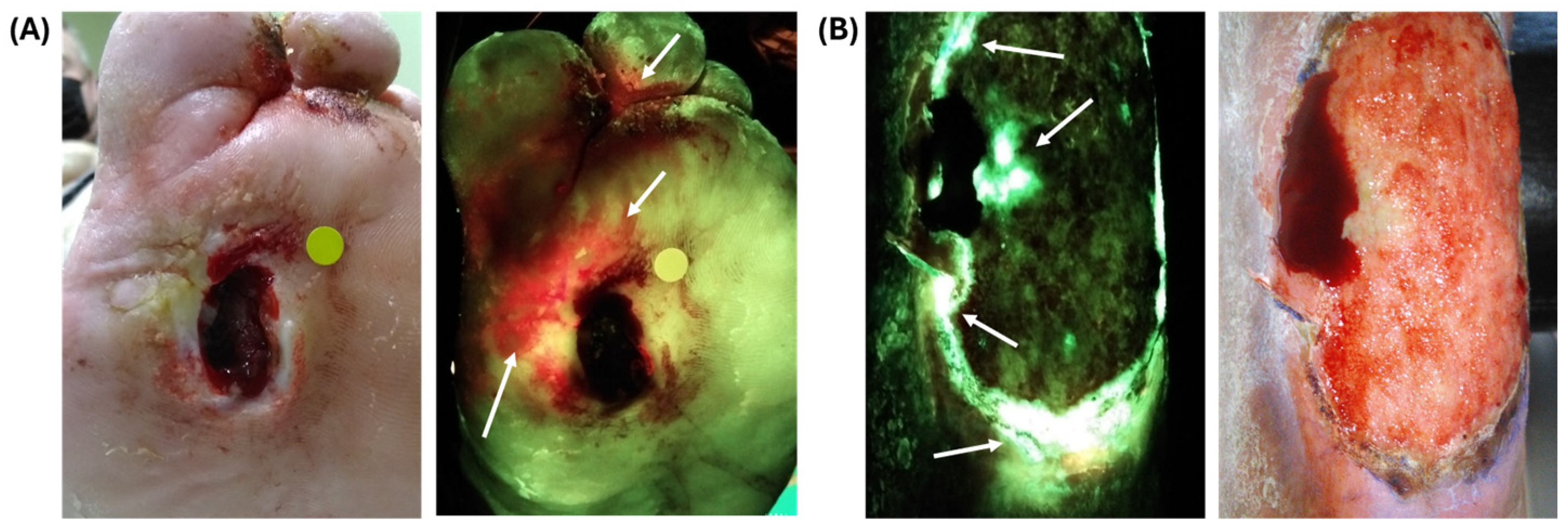
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Media
2.2. Fluorescence Imaging
2.3. Spectral Emission
2.4. Generation of Complimented Mutant Using Electroporation
2.5. Induction of pPVD31 Using IPTG and Pyoverdine Extraction
2.6. Statistical Analysis
3. Results
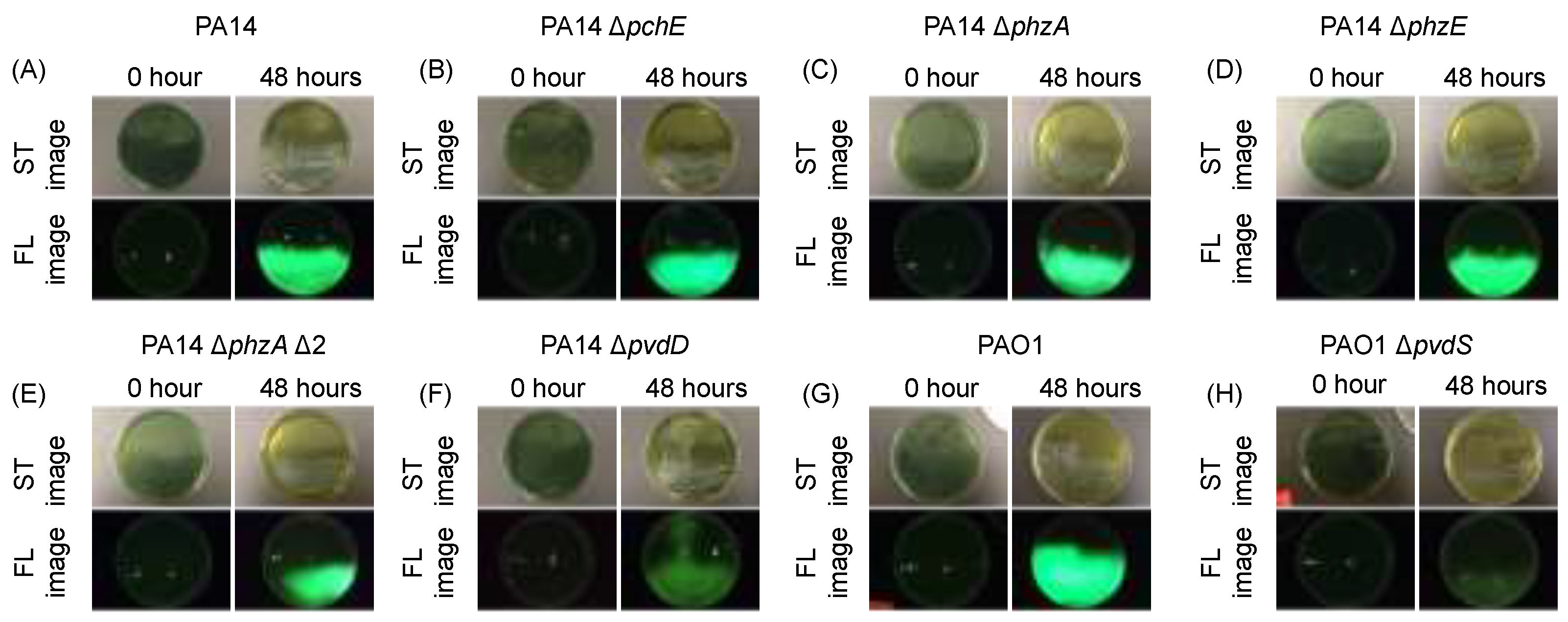
3.1. Investigating P. aeruginosa Knockout Mutants to Determine Which Exoproduct(s) Are Responsible for Cyan Fluorescence
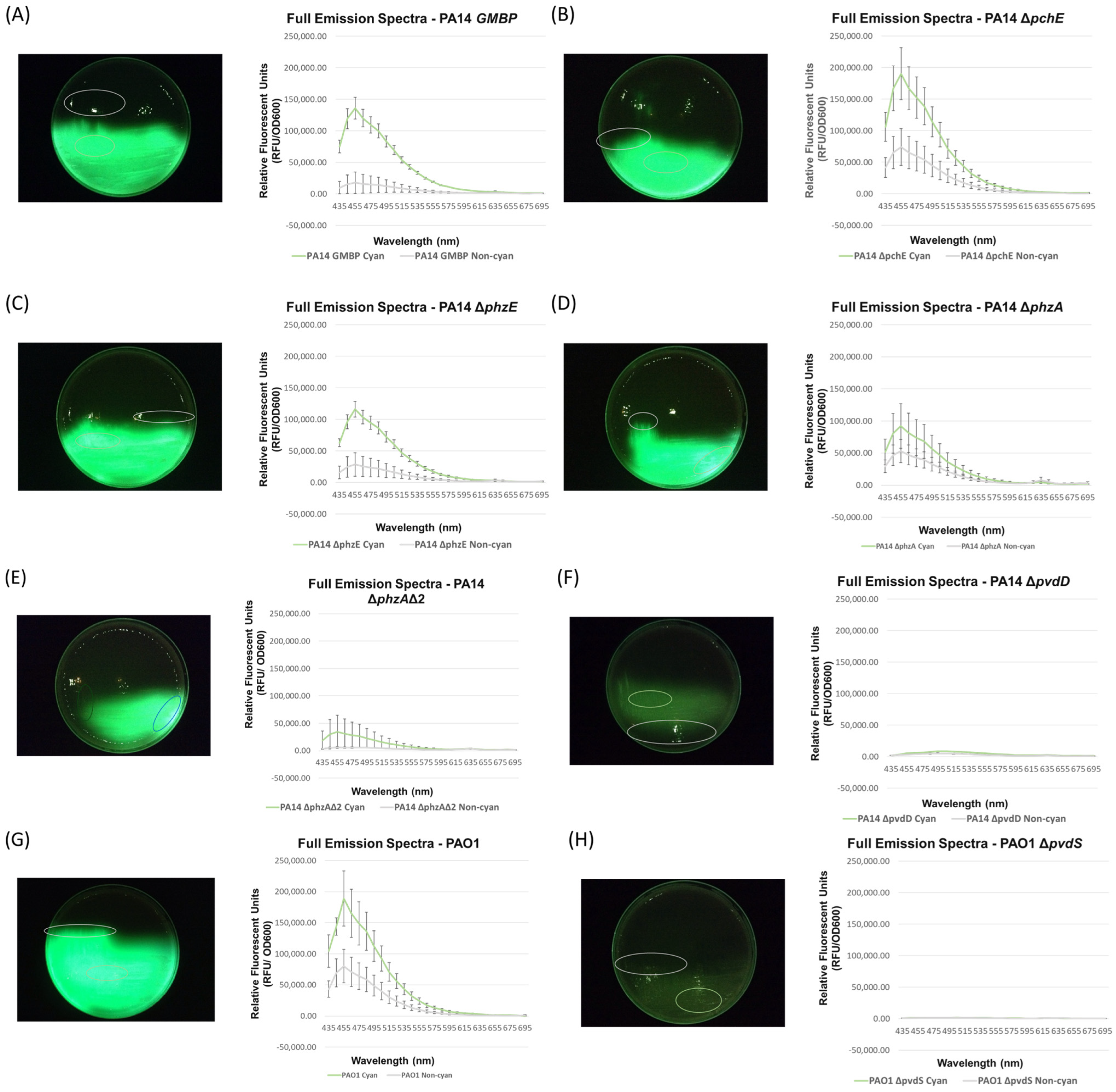
3.2. Full Spectral Analysis of Knockout Mutants Supports the Hypothesis That Pyoverdine Is Responsible for P. aeruginosa Cyan Fluorescence
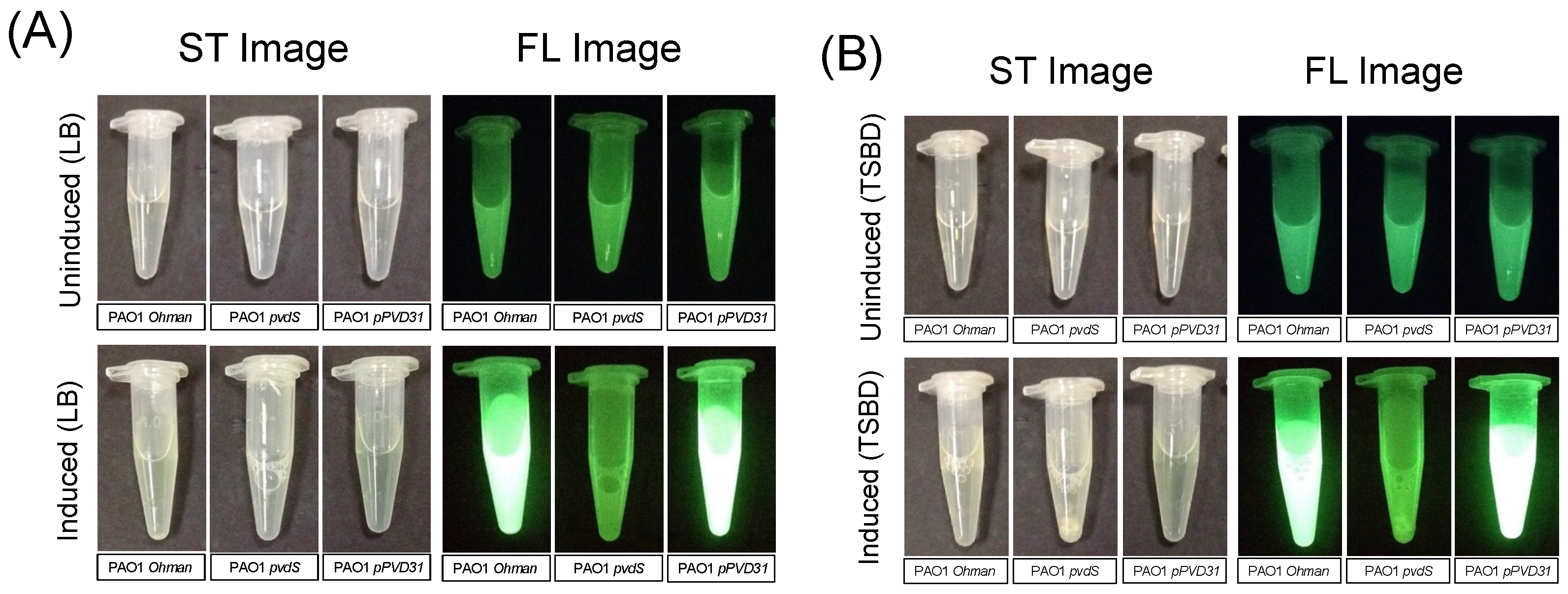
3.3. Complimenting the Pyoverdine Gene in the P. aeruginosa Knockout Mutant via Plasmid Restores Cyan Fluorescence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowler, P.G. Wound pathophysiology, infection and therapeutic options. Ann. Med. 2002, 34, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F.; White, R.J. Criteria for identifying wound infection—Revisited. Ostomy Wound Manag. 2005, 51, 28–34. [Google Scholar]
- Bianchi, T.; Wolcott, R.; Peghetti, A.; Leaper, D.; Cutting, K.; Polignano, R.; Rita, Z.R.; Moscatelli, A.; Greco, A.; Romanelli, M.; et al. Recommendations for the management of biofilm: A consensus document. J. Wound Care 2016, 25, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair. Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D. Biofilms cause chronic infections. J. Wound Care 2017, 26, 423–425. [Google Scholar] [CrossRef]
- Hoiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.-J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral. Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Xu, L.; McLennan, S.V.; Lo, L.; Natfaji, A.; Bolton, T.; Liu, Y.; Twigg, S.M.; Yue, D.K. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007, 30, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.E.; Frantz, R.A. Wound bioburden and infection-related complications in diabetic foot ulcers. Biol. Res. Nurs. 2008, 10, 44–53. [Google Scholar] [CrossRef]
- Turtiainen, J.; Hakala, T.; Hakkarainen, T.; Karhukorpi, J. The impact of surgical wound bacterial colonization on the incidence of surgical site infection after lower limb vascular surgery: A prospective observational study. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair. Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.M.; Dunham, D.; Rennie, M.Y.; Kirman, J.; Lopez, A.J.; Keim, K.C.; Smith, A.C. In vitro detection of porphyrin-producing wound bacteria with real-time fluorescence imaging. Future Microbiol. 2020, 15, 319–332. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding Real-Time Fluorescence Signals from Bacteria and Wound Tissues Observed with the MolecuLight i:X(TM). Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.Y.; Lindvere-Teene, L.; Tapang, K.; Linden, R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: A clinical study. J. Wound Care 2017, 26, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Snyder, R.J.; Bowler, P.G. Use of fluorescence imaging to optimize location of tissue sampling in hard-to-heal wounds. Front. Cell Infect. Microbiol. 2022, 12, 1070311. [Google Scholar] [CrossRef] [PubMed]
- Ottolino-Perry, K.; Chamma, E.; Blackmore, K.M.; Lindvere-Teene, L.; Starr, D.; Tapang, K.; Rosen, C.F.; Pitcher, B.; Panzarella, T.; Linden, R.; et al. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. Int. Wound J. 2017, 14, 833–841. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Edmonds, M.E.; Serena, T.E. Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers. Int. Wound J. 2023, 20, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Sandy-Hodgetts, K.; Andersen, C.A.; Al-Jalodi, O.; Serena, L.; Teimouri, C.; Serena, T.E. Uncovering the high prevalence of bacterial burden in surgical site wounds with point-of-care fluorescence imaging. Int. Wound J. 2022, 19, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.J.; Jones, L.M.; Reynolds, L.; Diaz, R.C.; George, I.K.; Little, W.; Fleming, D.; D’Souza, A.; Rennie, M.Y.; Rumbaugh, K.P.; et al. Detection of bacterial fluorescence from in vivo wound biofilms using a point-of-care fluorescence imaging device. Int. Wound J. 2021, 18, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Moller, K.; Jensen, P.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [PubMed]
- Jockenhöfer, F.; Gollnick, H.; Herberger, K.; Isbary, G.; Renner, R.; Stücker, M.; Valesky, E.; Wollina, U.; Weichenthal, M.; Karrer, S.; et al. Bacteriological pathogen spectrum of chronic leg ulcers: Results of a multicenter trial in dermatologic wound care centers differentiated by regions. J. Dtsch. Dermatol. Ges. 2013, 11, 1057–1063. [Google Scholar] [CrossRef]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- Madsen, S.M.; Westh, H.; Danielsen, L.; Rosdahl, V.T. Bacterial colonization and healing of venous leg ulcers. Apmis 1996, 104, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Halbert, A.R.; Stacey, M.C.; Rohr, J.B.; Jopp-Mckay, A. The effect of bacterial colonization on venous ulcer healing. Australas. J. Dermatol. 1992, 33, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef]
- Janniger, C.K.; Schwartz, R.; Szepietowski, J.C.; Reich, A. Intertrigo and common secondary skin infections. Am. Fam. Physician 2005, 72, 833–838. [Google Scholar] [PubMed]
- Polk, H.C., Jr.; Ward, C.G.; Clarkson, J.G.; Taplin, D. Early detection of Pseudomonas burn infection. Clinical experience with Wood’s light fluorescence. Arch. Surg. 1969, 98, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Ponka, D.; Baddar, F. Wood lamp examination. Can. Fam. Physician 2012, 58, 976. [Google Scholar] [PubMed]
- Raizman, R.; Little, W.; Smith, A.C. Rapid Diagnosis of Pseudomonas aeruginosa in Wounds with Point-of-Care Fluorescence Imaing. Diagnostics 2021, 11, 280. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Baer, M.; Briggs, P.; Bullock, N.; Cole, W.; DiMarco, D.; Hamil, R.; Harrell, K.; Kasper, M.; Li, W.; et al. Diagnostic Accuracy of Point-of-Care Fluorescence Imaging for the Detection of Bacterial Burden in Wounds: Results from the 350-Patient Fluorescence Imaging Assessment and Guidance Trial. Adv. Wound Care 2021, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Woo, K. A Prospective Multisite Observational Study Incorporating Bacterial Fluorescence Information into the UPPER/LOWER Wound Infection Checklists. Wounds 2020, 32, 299–308. [Google Scholar] [PubMed]
- Raizman, R.; Dunham, D.; Lindvere-Teene, L.; Jones, L.M.; Tapang, K.; Linden, R.; Rennie, M.Y. Use of a bacterial fluorescence imaging device: Wound measurement, bacterial detection and targeted debridement. J. Wound Care 2019, 28, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Harrell, K.; Serena, L.; Yaakov, R.A. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J. Wound Care 2019, 28, 346–357. [Google Scholar] [CrossRef]
- Pijpe, A.; Ozdemir, Y.; Sinnige, J.C.; Kwa, K.A.; Middelkoop, E.; Vries, A.M.-D. Detection of bacteria in burn wounds with a novel handheld autofluorescence wound imaging device: A pilot study. J. Wound Care 2019, 28, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Farhan, N.; Jeffery, S. Utility of MolecuLight i:X for Managing Bacterial Burden in Pediatric Burns. J. Burn. Care Res. 2020, 41, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hurley, C.M.; McClusky, P.; Sugrue, R.M.; Clover, J.A.; Kelly, J.E. Efficacy of a bacterial fluorescence imaging device in an outpatient wound care clinic: A pilot study. J. Wound Care 2019, 28, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, E.L.; Jeffery, S.L.A. Efficacy of an imaging device at identifying the presence of bacteria in wounds at a plastic surgery outpatients clinic. J. Wound Care 2018, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.M.; Abdallah, M.A. The Fluorescent Pigment of Pseudomonas fluorescens: Biosynthesis, Purification and Physicochemical Properties. Microbiology 1978, 107, 319–328. [Google Scholar] [CrossRef]
- Schalk, I.J.; Guillon, L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: Implications for metal homeostasis. Env. Microbiol. 2013, 15, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kirienko, N.V. High-Throughput Genetic Screen Reveals that Early Attachment and Biofilm Formation Are Necessary for Full Pyoverdine Production by Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 1707. [Google Scholar] [CrossRef]
- Meyer, J.M. Pyoverdines: Pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch. Microbiol. 2000, 174, 135–142. [Google Scholar] [CrossRef]
- Ravel, J.; Cornelis, P. Genomics of pyoverdine-mediated iron uptake in pseudomonads. Trends Microbiol. 2003, 11, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Fleuchot, B.; Lauciello, L.; Jafari, P.; Applegate, L.A.; Raffoul, W.; Que, Y.-A.; Perron, K. Effect of Human Burn Wound Exudate on Pseudomonas aeruginosa Virulence. mSphere 2016, 1, e00111-15. [Google Scholar] [CrossRef] [PubMed]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Natl. Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef]
- Holloway, B.W.; Krishnapillai, V.; Morgan, A.F. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 1979, 43, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Q.; Vasil, M.L.; Johnson, Z.; Ochsner, U.A.; Bayer, A.S. The oxygen- and iron-dependent sigma factor pvdS of Pseudomonas aeruginosa is an important virulence factor in experimental infective endocarditis. J. Infect. Dis. 2000, 181, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Gaines, J.M.; Carty, N.L.; Tiburzi, F.; Davinic, M.; Visca, P.; Colmer-Hamood, J.A.; Hamood, A.N. Regulation of the Pseudomonas aeruginosa toxA, regA and ptxR genes by the iron-starvation sigma factor PvdS under reduced levels of oxygen. Microbiology 2007, 153 Pt 12, 4219–4233. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, H.E.; Merriman, T.R.; Lamont, I.L. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J. Bacteriol. 1995, 177, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Cueva, A.R.; Pham, O.; Diaby, A.; Fleming, D.; Rumbaugh, K.P.; Fernandes, G.E. Pyoverdine Assay for Rapid and Early Detection of Pseudomonas aeruginosa in Burn Wounds. ACS Appl. Bio Mater. 2020, 3, 5350–5356. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [PubMed]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: New York, NY, USA, 1972. [Google Scholar]
- Ochsner, U.A.; Johnson, Z.; Lamont, I.L.; Cunliffe, H.E.; Vasil, M.L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol. Microbiol. 1996, 21, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Iglewski, B.H. Transformation of Pseudomonas aeruginosa by electroporation. Nucleic Acids Res. 1989, 17, 10509. [Google Scholar] [CrossRef] [PubMed]
- Ohman, D.E.; Sadoff, J.C.; Iglewski, B.H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: Isolation and characterization. Infect. Immun. 1980, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.H.; Knudsen, S.; Sorensen, S.J. The effect of the lacY gene on the induction of IPTG inducible promoters, studied in Escherichia coli and Pseudomonas fluorescens. Curr. Microbiol. 1998, 36, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Evans, K.; Meyer, J.M.; Poole, K. Quorum-sensing and siderophore biosynthesis in Pseudomonas aeruginosa: lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol. Lett. 1998, 166, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.; Ringel, T.B. The biosynthesis of pyoverdines. Microb. Cell 2018, 5, 424–437. [Google Scholar]
- Cézard, C.; Farvacques, N.; Sonnet, P. Chemistry and biology of pyoverdines, Pseudomonas primary siderophores. Curr. Med. Chem. 2015, 22, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections Caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa: Infections, Animal Modeling, and Therapeutics. Cells 2023, 12, 199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, E.; Reynolds-Reber, L.; Navarro, S.; Hamood, A.; Jones-Donaldson, L.M.; Smith, A.C. Determination of the Course of Cyan Fluorescence of Pseudomonas aeruginosa with a Handheld Bacterial Imaging Device. Diagnostics 2024, 14, 1474. https://doi.org/10.3390/diagnostics14141474
Pham E, Reynolds-Reber L, Navarro S, Hamood A, Jones-Donaldson LM, Smith AC. Determination of the Course of Cyan Fluorescence of Pseudomonas aeruginosa with a Handheld Bacterial Imaging Device. Diagnostics. 2024; 14(14):1474. https://doi.org/10.3390/diagnostics14141474
Chicago/Turabian StylePham, Emily, Landrye Reynolds-Reber, Stephany Navarro, Abdul Hamood, Laura M. Jones-Donaldson, and Allie Clinton Smith. 2024. "Determination of the Course of Cyan Fluorescence of Pseudomonas aeruginosa with a Handheld Bacterial Imaging Device" Diagnostics 14, no. 14: 1474. https://doi.org/10.3390/diagnostics14141474
APA StylePham, E., Reynolds-Reber, L., Navarro, S., Hamood, A., Jones-Donaldson, L. M., & Smith, A. C. (2024). Determination of the Course of Cyan Fluorescence of Pseudomonas aeruginosa with a Handheld Bacterial Imaging Device. Diagnostics, 14(14), 1474. https://doi.org/10.3390/diagnostics14141474




