Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacterial Strain
2.3. Bacterial Culture and 48-h Inoculum Preparation
2.4. Inoculation Procedure
2.5. Inactive Inoculum Control
2.6. Diluted Inoculum Group
2.7. 24-h Incubated Inoculum
2.8. Antibiotic Treatment
2.9. Infection Monitoring
2.10. Tissue Sampling and Processing
3. Results
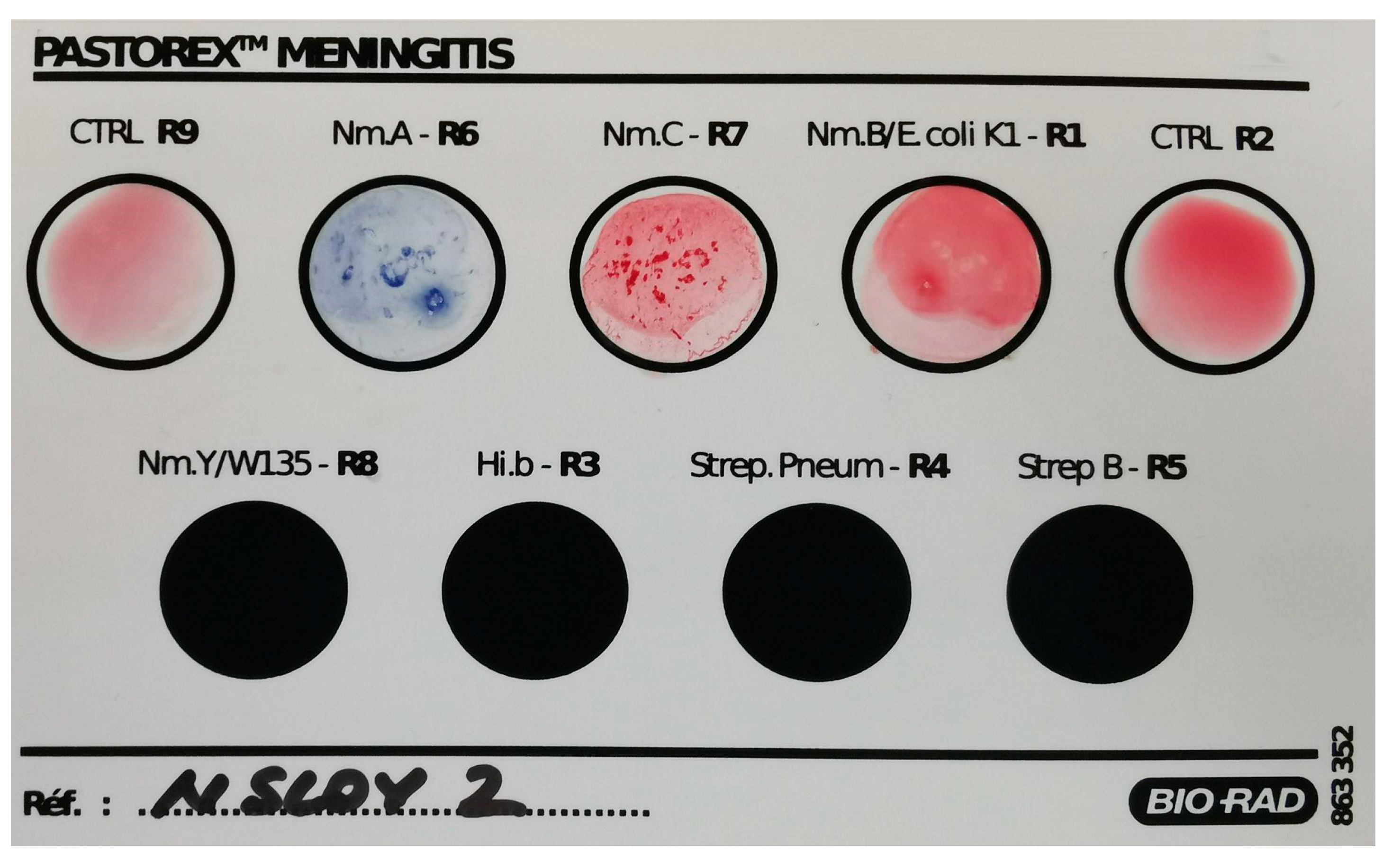
3.1. Extensive Characterisation of the Neisserial Strain
3.2. Establishing the Model
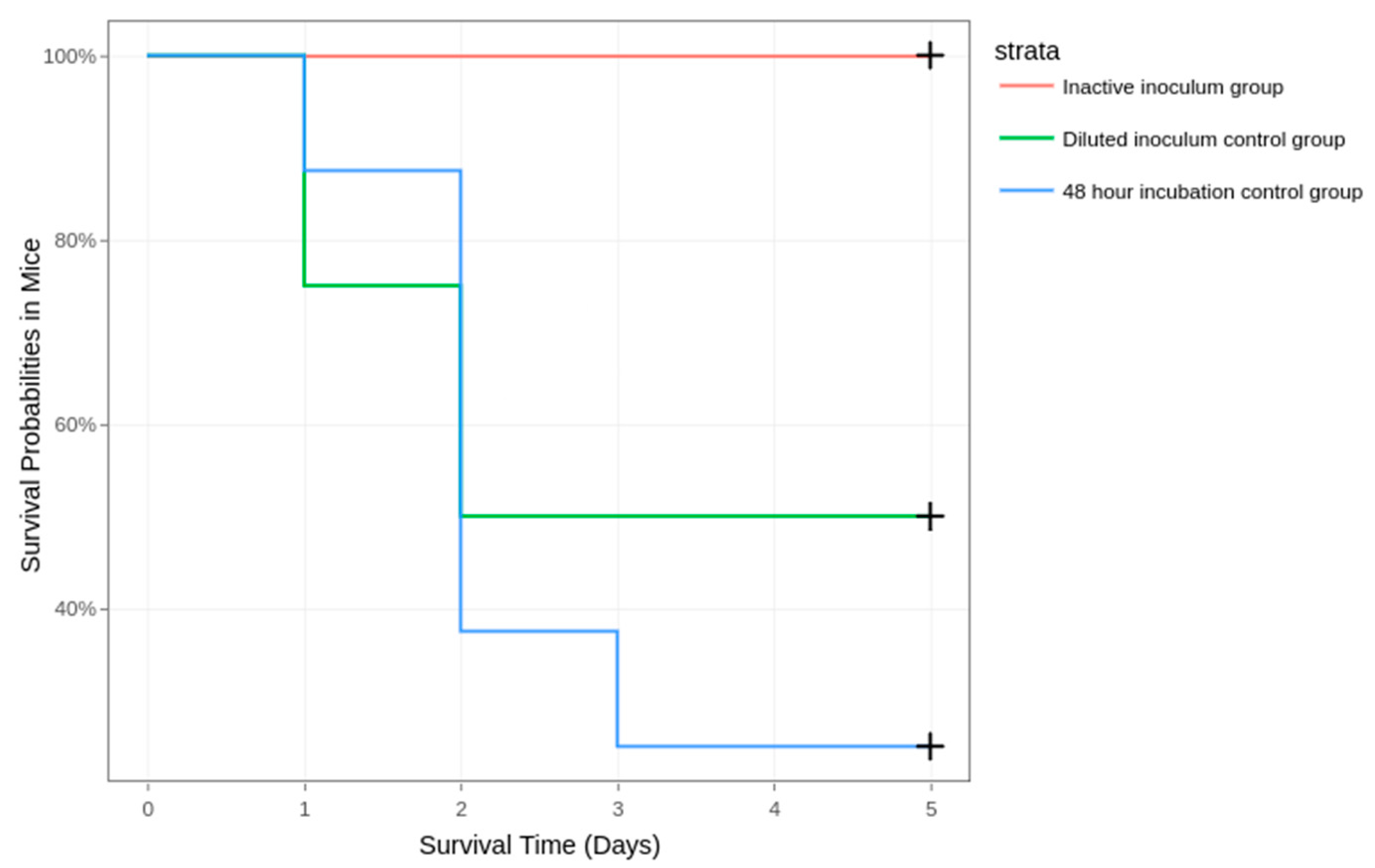
3.2.1. Stage 1: Establishing the Required Incubation Time
3.2.2. Stage 2: Excluding Endotoxic Shock Death, Establishing the Lethal Dose for Half the Inoculated Mice (LD50), and Rising the Survivability Rate through Treatment
3.3. Clinical Parameters and Mortality
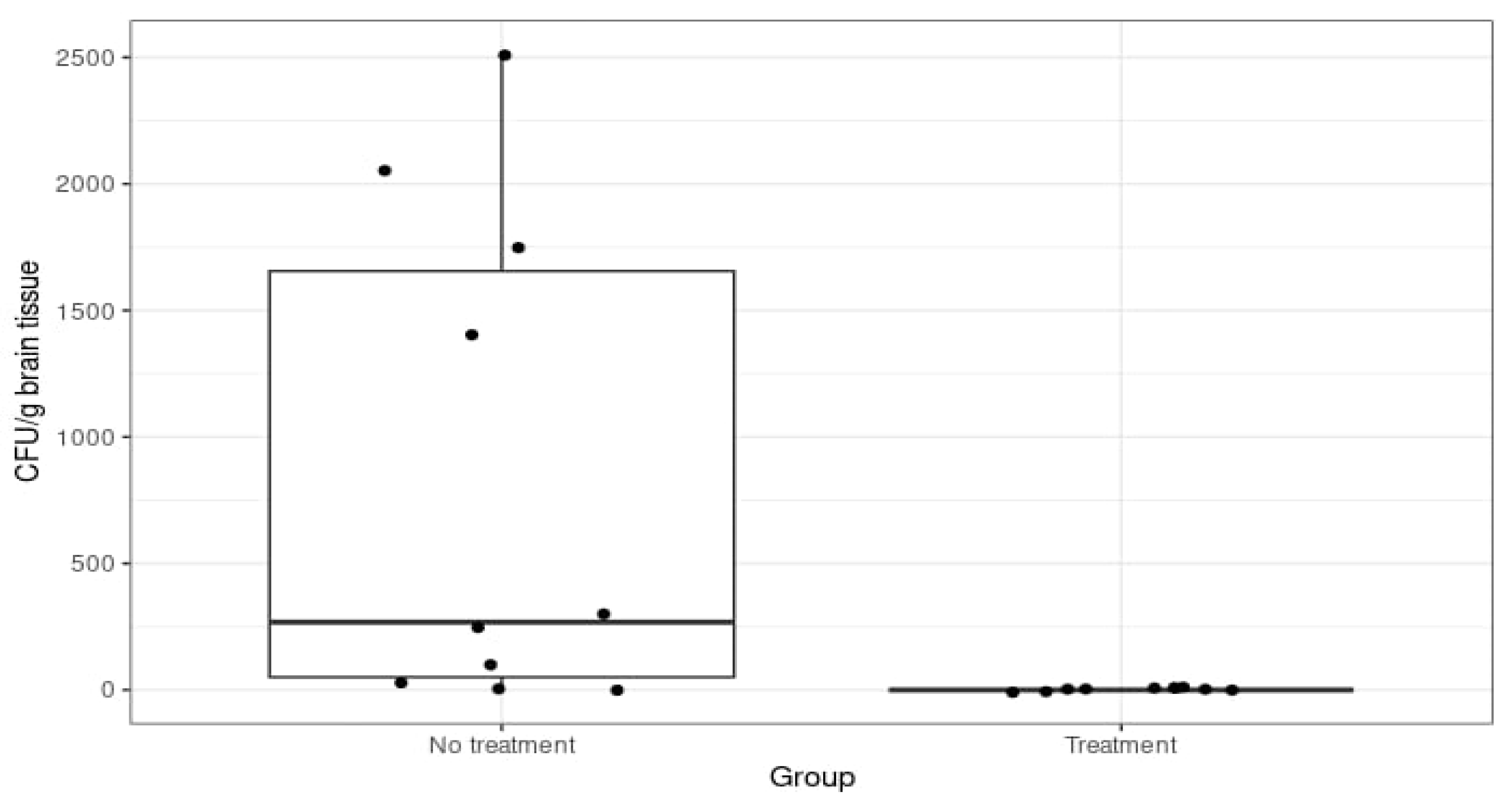
3.4. CFU Count in Tissue Samples
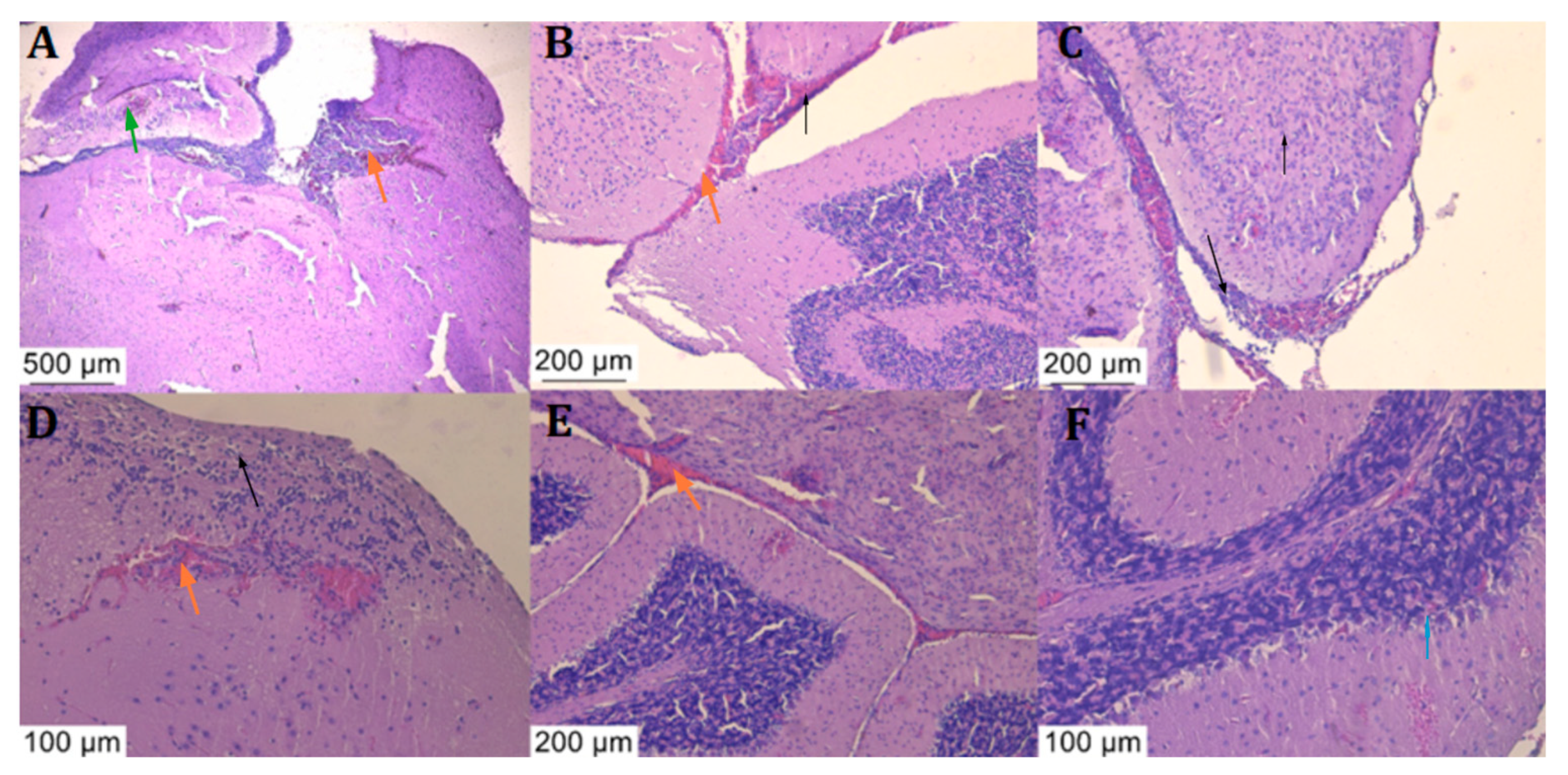
3.5. Histologic Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meningococcus—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/meningococcus (accessed on 10 March 2024).
- Joshi, R.; Saroj, S.D. Survival and Evasion of Neisseria meningitidis from Macrophages. Med. Microecol. 2023, 17, 100087. [Google Scholar] [CrossRef]
- Harrison, L.H.; Trotter, C.L.; Ramsay, M.E. Global Epidemiology of Meningococcal Disease. Vaccine 2009, 27, B51–B63. [Google Scholar] [CrossRef]
- Johswich, K.O.; Zhou, J.; Law, D.K.S.; St. Michael, F.; McCaw, S.E.; Jamieson, F.B.; Cox, A.D.; Tsang, R.S.W.; Gray-Owen, S.D. Invasive Potential of Nonencapsulated Disease Isolates of Neisseria meningitidis. Infect. Immun. 2012, 80, 2346–2353. [Google Scholar] [CrossRef]
- Vogel, U.; Claus, H. Vaccine Development against Neisseria meningitidis. Microb. Biotechnol. 2011, 4, 20–31. [Google Scholar] [CrossRef]
- Tzeng, Y.-L.; Thomas, J.; Stephens, D.S. Regulation of Capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772. [Google Scholar] [CrossRef]
- Claus, H.; Jördens, M.S.; Kriz, P.; Musilek, M.; Jarva, H.; Pawlik, M.-C.; Meri, S.; Vogel, U. Capsule Null Locus Meningococci: Typing of Antigens Used in an Investigational Multicomponent Meningococcus Serogroup B Vaccine. Vaccine 2012, 30, 155–160. [Google Scholar] [CrossRef]
- Bacterial Meningitis|CDC. Available online: https://www.cdc.gov/meningitis/index.html (accessed on 24 April 2024).
- Yadav, S.; Rammohan, G. Meningococcal Meningitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Meningococcal Disease|CDC. Available online: https://www.cdc.gov/meningococcal/index.html (accessed on 5 November 2023).
- UHS Health Topic—Meningitis. Available online: https://healthyhorns.utexas.edu/HT/HT_meningitis.html (accessed on 10 March 2024).
- Caracoti, V.I.; Caracoti, C.S.; Muntean, A.A.; Coman, C.; Popa, M.I. Proposing a murine meningococcal meningitis animal model based on extensive review of literature. ROAMI 2023, 1, 35–41. [Google Scholar]
- Pagliuca, C.; Scaglione, E.; Carraturo, F.; Mantova, G.; Marino, M.M.; Pishbin, M.V.; Pagliarulo, C.; Colicchio, R.; Salvatore, P. Inducing Meningococcal Meningitis Serogroup C in Mice via Intracisternal Delivery. J. Vis. Exp. JoVE 2019, 153, e60047. [Google Scholar] [CrossRef]
- Kánová, E.; Pulzová, L.; Kováč, A.; Bhide, M. Deciphering the Interactome of Neisseria meningitidis With Human Brain Microvascular Endothelial Cells. Front. Microbiol. 2018, 9, 2294. [Google Scholar] [CrossRef]
- Káňová, E.; Tkáčová, Z.; Bhide, K.; Kulkarni, A.; Jiménez-Munguía, I.; Mertinková, P.; Drážovská, M.; Tyagi, P.; Bhide, M. Transcriptome Analysis of Human Brain Microvascular Endothelial Cells Response to Neisseria meningitidis and Its Antigen MafA Using RNA-Seq. Sci. Rep. 2019, 9, 18763. [Google Scholar] [CrossRef]
- Kulkarni, A.; Mochnáčová, E.; Majerova, P.; Čurlík, J.; Bhide, K.; Mertinková, P.; Bhide, M. Single Domain Antibodies Targeting Receptor Binding Pockets of NadA Restrain Adhesion of Neisseria meningitidis to Human Brain Microvascular Endothelial Cells. Front. Mol. Biosci. 2020, 7, 573281. [Google Scholar] [CrossRef]
- Kulkarni, A.; Jozefiaková, J.; Bhide, K.; Mochnaćová, E.; Bhide, M. Differential Transcriptome Response of Blood Brain Barrier Spheroids to Neuroinvasive Neisseria and Borrelia. Front. Cell. Infect. Microbiol. 2023, 13, 1326578. [Google Scholar] [CrossRef]
- Uadiale, K.; Bestman, A.; Kamau, C.; Caugant, D.A.; Greig, J. Evaluation of Pastorex Meningitis Kit Performance for the Rapid Identification of Neisseria meningitidis Serogroup C in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 381–385. [Google Scholar] [CrossRef]
- Eucast: AST of Bacteria. Available online: https://www.eucast.org/ast_of_bacteria (accessed on 10 March 2024).
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-Depth Characterization and Visualization of Bacterial Genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus Sequence Typing of Total-Genome-Sequenced Bacteria. J. Clin. Microbiol. 2020, 50, 1355–1361. [Google Scholar] [CrossRef]
- Maiden, M.C.; Bygraves, J.A.; Feil, E.; Morelli, G.; Russell, J.E.; Urwin, R.; Zhang, Q.; Zhou, J.; Zurth, K.; Caugant, D.A.; et al. Multilocus Sequence Typing: A Portable Approach to the Identification of Clones within Populations of Pathogenic Microorganisms. Proc. Natl. Acad. Sci. USA 1998, 95, 3140–3145. [Google Scholar] [CrossRef]
- Bennett, J.S.; Jolley, K.A.; Sparling, P.F.; Saunders, N.J.; Hart, C.A.; Feavers, I.M.; Maiden, M.C. Species Status of Neisseria Gonorrhoeae: Evolutionary and Epidemiological Inferences from Multilocus Sequence Typing. BMC Biol. 2007, 5, 35. [Google Scholar] [CrossRef]
- Theilen, U.; Wilson, L.; Wilson, G.; Beattie, J.O.; Qureshi, S.; Simpson, D. Management of Invasive Meningococcal Disease in Children and Young People: Summary of SIGN Guidelines. BMJ 2008, 336, 1367–1370. [Google Scholar] [CrossRef]
- Hoffman, O.; Weber, R.J. Pathophysiology and Treatment of Bacterial Meningitis. Ther. Adv. Neurol. Disord. 2009, 2, 401–412. [Google Scholar] [CrossRef]
- Coman, C.; Ancuta, D.L.; Ionita, F.; Muntean, A.A.; Caracoti, C.S.; Caracoti, I.V.; Dragomirescu, C.; Popa, M.I. Preliminary results in inducing meningitis in balb/c mice using a human strain of Neisseria meningitidis. Sci. Work. Ser. C Vet. Med. 2022, 1, 199–205. [Google Scholar]
- Schork, S.; Schlüter, A.; Blom, J.; Schneiker-Bekel, S.; Pühler, A.; Goesmann, A.; Frosch, M.; Schoen, C. Genome Sequence of a Neisseria meningitidis Capsule Null Locus Strain from the Clonal Complex of Sequence Type 198. J. Bacteriol. 2012, 194, 5144–5145. [Google Scholar] [CrossRef]
- Rouphael, N.G.; Stephens, D.S. Neisseria meningitidis: Biology, Microbiology, and Epidemiology. In Neisseria meningitidis: Advanced Methods and Protocols; Christodoulides, M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 1–20. ISBN 978-1-61779-346-2. [Google Scholar]
- Holbein, B.E. Iron-Controlled Infection with Neisseria meningitidis in Mice. Infect. Immun. 1980, 29, 886–891. [Google Scholar] [CrossRef]
- Holbein, B.E.; Jericho, K.W.; Likes, G.C. Neisseria meningitidis Infection in Mice: Influence of Iron, Variations in Virulence among Strains, and Pathology. Infect. Immun. 1979, 24, 545–551. [Google Scholar] [CrossRef]
- Colicchio, R.; Ricci, S.; Lamberti, F.; Pagliarulo, C.; Pagliuca, C.; Braione, V.; Braccini, T.; Talà, A.; Montanaro, D.; Tripodi, S.; et al. The Meningococcal ABC-Type L-Glutamate Transporter GltT Is Necessary for the Development of Experimental Meningitis in Mice. Infect. Immun. 2009, 77, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Hoang, L.M.N.; Thomas, E.; Tyler, S.; Pollard, A.J.; Stephens, G.; Gustafson, L.; McNabb, A.; Pocock, I.; Tsang, R.; Tan, R. Rapid and Fatal Meningococcal Disease Due to a Strain of Neisseria meningitidis Containing the Capsule Null Locus. Clin. Infect. Dis. 2005, 40, e38–e42. [Google Scholar] [CrossRef]
- Ganesh, K.; Allam, M.; Wolter, N.; Bratcher, H.B.; Harrison, O.B.; Lucidarme, J.; Borrow, R.; de Gouveia, L.; Meiring, S.; Birkhead, M.; et al. Molecular Characterization of Invasive Capsule Null Neisseria meningitidis in South Africa. BMC Microbiol. 2017, 17, 40. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, B.; Xu, L.; Gao, Y.; Shao, Z. First Case of Neisseria meningitidis Capsule Null Locus Infection in China. Infect. Dis. 2015, 47, 591–592. [Google Scholar] [CrossRef]
- Sorhouet-Pereira, C.; Efron, A.; Gagetti, P.; Faccone, D.; Regueira, M.; Corso, A.; Group, A.S.I.W.; Gabastou, J.-M.; Ibarz-Pavón, A.B. Phenotypic and Genotypic Characteristics of Neisseria meningitidis Disease-Causing Strains in Argentina, 2010. PLoS ONE 2013, 8, e58065. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Scheifele, D.W.; Halperin, S.A.; Vaudry, W.; Le Saux, N.; Tsang, R.; Bettinger, J.A.; Bridger, N.; Morris, R.; Top, K.; et al. Outcomes of Invasive Meningococcal Disease in Adults and Children in Canada Between 2002 and 2011: A Prospective Cohort Study. Clin. Infect. Dis. 2015, 60, e27–e35. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Lécuyer, H.; Join-Lambert, O.; Bourdoulous, S.; Marullo, S.; Nassif, X.; Coureuil, M. Neisseria meningitidis Colonization of the Brain Endothelium and Cerebrospinal Fluid Invasion. Cell. Microbiol. 2013, 15, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Barman, T.K.; Kumar, M.; Chaira, T.; Gangadharan, R.; Singhal, S.; Rao, M.; Mathur, T.; Bhateja, P.; Pandya, M.; Ramadass, V.; et al. Potential of the Fluoroketolide RBx 14255 against Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae in an Experimental Murine Meningitis Model. J. Antimicrob. Chemother. 2019, 74, 1962–1970. [Google Scholar] [CrossRef]



| Locus | Identity | Coverage | Alignment Length | Allele Length | Gaps | Allele |
|---|---|---|---|---|---|---|
| abcZ | 100 | 100 | 433 | 433 | 0 | abcZ_5 |
| adk | 100 | 100 | 465 | 465 | 0 | adk_4 |
| aroE | 100 | 100 | 490 | 490 | 0 | aroE_17 |
| fumC | 100 | 100 | 465 | 465 | 0 | fumC_15 |
| gdh | 100 | 100 | 501 | 501 | 0 | gdh_30 |
| pdhC | 100 | 100 | 480 | 480 | 0 | pdhC_7 |
| pgm | 100 | 100 | 450 | 450 | 0 | pgm_12 |
| VFclass | Virulence Factor | Gene | Start | Stop | Contig and Orientation |
|---|---|---|---|---|---|
| Adherence | Adhesion and penetration protein | app | 163 | 4539 | 92− |
| LOS sialylation | lst | 7320 | 7982 | 22+ | |
| LOS synthesis | kdtA/waaA | 7985 | 8419 | 22+ | |
| LOS synthesis | lgtA | 3884 | 5038 | 51− | |
| LOS synthesis | lgtB | 2334 | 3860 | 51− | |
| LOS synthesis | lgtE | 161 | 1156 | 27+ | |
| LOS synthesis | lgtF | 1212 | 2741 | 27+ | |
| LOS synthesis | lgtG | 2762 | 3820 | 27+ | |
| LOS synthesis | rfaC | 88 | 2229 | 142− | |
| LOS synthesis | rfaF | 9003 | 9827 | 42− | |
| LOS synthesis | rfaK | 230 | 1156 | 96+ | |
| Neisseria adhesion A | nadA | 1187 | 3613 | 96+ | |
| Phosphoethanolamine modification | lptA | 10,777 | 16,284 | 39− | |
| Type IV pili | pilC | 256 | 1770 | 138− | |
| Type IV pili | pilD | 22,475 | 23,746 | 19− | |
| Type IV pili | pilF | 244 | 3081 | 104− | |
| Type IV pili | pilG | 3078 | 5291 | 104− | |
| Type IV pili | pilH | 4889 | 5977 | 23− | |
| Type IV pili | pilI | 4050 | 4889 | 23− | |
| Type IV pili | pilJ | 3059 | 3901 | 23− | |
| Type IV pili | pilK | 3861 | 4619 | 101+ | |
| Type IV pili | pilM | 2718 | 3770 | 43− | |
| Type IV pili | pilN | 14,645 | 16,279 | 35+ | |
| Type IV pili | pilO | 4323 | 5459 | 83+ | |
| Type IV pili | pilP | 23,691 | 24,446 | 18+ | |
| Type IV pili | pilQ | 24,470 | 25,345 | 18+ | |
| Type IV pili | pilT | 25,422 | 26,348 | 18+ | |
| Type IV pili | pilT2 | 5708 | 7276 | 49+ | |
| Type IV pili | pilU | 4741 | 5979 | 99− | |
| Type IV pili | pilV | 1526 | 4729 | 99− | |
| Type IV pili | pilW | 68 | 1471 | 99− | |
| Type IV pili | pilX | 5198 | 6286 | 9+ | |
| Type IV pili | pilZ | 435 | 959 | 105+ | |
| Immune modulator | Factor H binding protein | fHbp | 2729 | 3397 | 130− |
| Neisserial surface protein A | nspA | 2085 | 2732 | 130− | |
| Invasion | Class 5 outer membrane protein | opc | 1147 | 2088 | 130− |
| PorA | porA | 572 | 1168 | 130− | |
| PorB | porB | 11,222 | 12,337 | 40+ | |
| Iron uptake | ABC transporter | fbpA | 12,340 | 12,939 | 40+ |
| ABC transporter | fbpB | 12,940 | 13,587 | 40+ | |
| ABC transporter | fbpC | 13,605 | 14,150 | 40+ | |
| Ferric enterobactin transport protein A/ferric-repressed protein B | fetA/frpB | 14,169 | 16,454 | 40+ | |
| Heme uptake | hpuA | 3959 | 5002 | 86− | |
| Heme uptake | hpuB | 5462 | 6592 | 34+ | |
| Lactoferrin-binding protein | lbpA | 2570 | 3796 | 86− | |
| Lactoferrin-binding protein | lbpB | 8035 | 8424 | 12+ | |
| Ton system | exbB | 3049 | 3810 | 4+ | |
| Ton system | exbD | 94 | 567 | 130− | |
| Ton system | tonB | 7607 | 7957 | 34+ | |
| Protease | IgA protease | iga | 458 | 1627 | 151− |
| Stress adaptation | Catalase | katA | 375 | 1370 | 160+ |
| Manganese transport system | mntA | 8226 | 9899 | 66+ | |
| Manganese transport system | mntB | 5061 | 6041 | 19− | |
| Manganese transport system | mntC | 17,713 | 18,723 | 28− | |
| Methionine sulphoxide reductase | msrA/B (pilB) | 4620 | 5684 | 101+ | |
| Recombinational repair protein | recN | 6412 | 7254 | 22+ |
| Stage | Mouse Group | Mice no. | Inoculum | Deaths | Survival Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Period | CFU/Mouse | D0 | D1 | D2 | D3 | D4 | D5 | ||||
| Stage 1 | 24-h incubated inoculum group | 8 | 24 h | 1.5 × 107 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 |
| 48-h incubated inoculum group | 8 | 48 h | 1.5 × 107 | 0 | 2 | 4 | 1 | 0 | 0 | 12.50 | |
| Stage 2 | Inactivated inoculum group | 8 | 48 h | 1.5 × 107 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 |
| Diluted inoculum control group | 8 | 48 h | 1.5 × 106 | 0 | 2 | 2 | 0 | 0 | 0 | 50.00 | |
| 48-h incubated control group | 8 | 48 h | 1.5 × 107 | 0 | 1 | 4 | 1 | 0 | 0 | 25.00 | |
| Ceftriaxone treatment group | 16 | 48 h | 1.5 × 107 | 0 | 1 | 1 | 0 | 2 | 0 | 75.00 | |
| Ciprofloxacin treatment group | 16 | 48 h | 1.5 × 107 | 0 | 1 | 0 | 2 | 0 | 0 | 81.25 | |
| Summed 48-h incubated control group * | 16 | 48 h | 1.5 × 107 | 0 | 3 | 8 | 2 | 0 | 0 | 18.75 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caracoti, V.-I.; Caracoti, C.-Ș.; Ancuța, D.L.; Ioniță, F.; Muntean, A.-A.; Bhide, M.; Popa, G.L.; Popa, M.I.; Coman, C. Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain. Diagnostics 2024, 14, 1116. https://doi.org/10.3390/diagnostics14111116
Caracoti V-I, Caracoti C-Ș, Ancuța DL, Ioniță F, Muntean A-A, Bhide M, Popa GL, Popa MI, Coman C. Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain. Diagnostics. 2024; 14(11):1116. https://doi.org/10.3390/diagnostics14111116
Chicago/Turabian StyleCaracoti, Viorela-I., Costin-Ș. Caracoti, Diana L. Ancuța, Fabiola Ioniță, Andrei-A. Muntean, Mangesh Bhide, Gabriela L. Popa, Mircea I. Popa, and Cristin Coman. 2024. "Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain" Diagnostics 14, no. 11: 1116. https://doi.org/10.3390/diagnostics14111116
APA StyleCaracoti, V.-I., Caracoti, C.-Ș., Ancuța, D. L., Ioniță, F., Muntean, A.-A., Bhide, M., Popa, G. L., Popa, M. I., & Coman, C. (2024). Developing a Novel Murine Meningococcal Meningitis Model Using a Capsule-Null Bacterial Strain. Diagnostics, 14(11), 1116. https://doi.org/10.3390/diagnostics14111116







