Utilizing Esophageal Motility Tests in Diagnosing and Evaluating Gastroesophageal Reflux Disease
Abstract
1. Introduction
2. Understanding Pathophysiologies of GERD Based on HREM
2.1. Impairment of the Anti-Reflux Barrier at the Esophagogastric Junction (EGJ)
2.1.1. Abnormal Morphology of the EGJ
2.1.2. Contractile Capacity of the EGJ
- It aids in the diagnosis of GERD; Hyoju et al. [11] identified that an EGJ-CI threshold of 29.5 mmHg cm was indicative of GERD, demonstrating a sensitivity of 77.8% and a specificity of 81.7%. Additionally, they found that an EGJ-CI value of 47 mmHg cm was optimal for GERD diagnosis, achieving a sensitivity of 54% and a specificity of 85% [24];
- It distinguishes between differential diagnoses. The EGJ-CI has also been instrumental in differentiating between patients with functional heartburn (FH) and those with rGERD. It was observed that the rGERD group had a significantly lower EGJ-CI (averaging 25.8 mmHg·cm) in comparison to the FH group, which averaged 39.2 mmHg·cm [25]. This distinction underscores the utility of EGJ-CI for differentiating between these two conditions, aiding in accurate diagnosis and subsequent treatment planning;
- It aids in the prediction of acid reflux. Emerging evidence indicates that patients with abnormal acid exposure time (AET) exhibit lower EGJ-CI values compared to those with normal AET [26,27]. A specific EGJ-CI threshold of 39.3 mmHg·cm has been identified as predictive of abnormal acid exposure, demonstrating a sensitivity of 0.65 and specificity of 0.57 [28]. Current research links diminished EGJ-CI values to increased severity of reflux, suggesting that lower EGJ-CI readings correlate with more pronounced EGJ dysfunction [15].
2.2. Transient Lower Esophageal Sphincter Relaxation (TLESR)
2.3. Motor Disorders in the Esophageal Body
3. Auxiliary Diagnosis of GERD Based on HREM
4. Differential Diagnosis of Esophageal Motility Disorders Related to GERD Based on HREM
5. Guiding Anti-Reflux Surgical Treatment of GERD Based on HREM
6. Predicting PPI Efficacy in GERD Based on HREM
7. Predicting Acid Exposure and Assessing GERD Severity Based on HREM
8. Advancement in HREM-Based Provocative Testing: The Supine Position Straight Leg Raise (SLR) Test
9. Advancements in GERD Assessment Using HREM-Based Technologies
9.1. Combined Impedance Monitoring–High-Resolution Impedance Manometry (HRIM)
9.2. 3D High-Resolution Pressure Measurement with HREM
10. Esophageal Motility Assessment Utilizing the Endolumenal Functional Lumen Imaging Probe (EndoFLIP)
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Muderris, T.; Gokcan, M.K.; Yorulmaz, I. The clinical value of pharyngeal pH monitoring using a double-probe, triple-sensor catheter in patients with laryngopharyngeal reflux. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.; Fang, X. Esophageal Motor Dysfunctions in Gastroesophageal Reflux Disease and Therapeutic Perspectives. J. Neurogastroenterol. Motil. 2019, 25, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, N.J.; Sifrim, D.; Sweis, R.; Fullard, M.; Basu, K.; McCord, M.; Booth, M.; Hayman, J.; Boeckxstaens, G.; Johnston, B.T.; et al. British Society of Gastroenterology guidelines for oesophageal manometry and oesophageal reflux monitoring. Gut 2019, 68, 1731–1750. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Gyawali, C.P.; Savarino, E.; Yadlapati, R.; Zerbib, F.; Wu, J.; Vela, M.; Tutuian, R.; Tatum, R.; Sifrim, D.; et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol. Motil. 2017, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sifrim, D.; Zerbib, F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 2012, 61, 1340–1354. [Google Scholar] [CrossRef]
- Savarino, E.; di Pietro, M.; Bredenoord, A.J.; Carlson, D.A.; Clarke, J.O.; Khan, A.; Vela, M.F.; Yadlapati, R.; Pohl, D.; Pandolfino, J.E.; et al. Use of the Functional Lumen Imaging Probe in Clinical Esophagology. Am. J. Gastroenterol. 2020, 115, 1786–1796. [Google Scholar] [CrossRef]
- Fass, R.; Boeckxstaens, G.E.; El-Serag, H.; Rosen, R.; Sifrim, D.; Vaezi, M.F. Gastro-oesophageal reflux disease. Nat. Rev. Dis. Primers 2021, 7, 55. [Google Scholar] [CrossRef]
- Xie, C.; Wang, J.; Li, Y.; Tan, N.; Cui, Y.; Chen, M.; Xiao, Y. Esophagogastric Junction Contractility Integral Reflect the Anti-reflux Barrier Dysfunction in Patients with Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2017, 23, 27–33. [Google Scholar] [CrossRef]
- Tolone, S.; De Bortoli, N.; Marabotto, E.; de Cassan, C.; Bodini, G.; Roman, S.; Furnari, M.; Savarino, V.; Docimo, L.; Savarino, E. Esophagogastric junction contractility for clinical assessment in patients with GERD: A real added value? Neurogastroenterol. Motil. 2015, 27, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.; Cho, Y.K.; Lee, H.H.; Yoon, S.B.; Lim, C.H.; Kim, J.S.; Park, J.M.; Choi, M.G. Esophagogastric junction contractile integral and morphology: Two high-resolution manometry metrics of the anti-reflux barrier. J. Gastroenterol. Hepatol. 2017, 32, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Pandolfino, J.E.; Kim, H.; Ghosh, S.K.; Clarke, J.O.; Zhang, Q.; Kahrilas, P.J. High-resolution manometry of the EGJ: An analysis of crural diaphragm function in GERD. Am. J. Gastroenterol. 2007, 102, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, A.; Gyawali, C.P. High-resolution Manometry can Characterize Esophagogastric Junction Morphology and Predict Esophageal Reflux Burden. J. Clin. Gastroenterol. 2020, 54, 22–27. [Google Scholar] [CrossRef]
- Yadlapati, R.; Kahrilas, P.J.; Fox, M.R.; Bredenoord, A.J.; Prakash Gyawali, C.; Roman, S.; Babaei, A.; Mittal, R.K.; Rommel, N.; Savarino, E.; et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0(©). Neurogastroenterol. Motil. 2021, 33, e14058. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Mittal, R.K.; Bor, S.; Kohn, G.P.; Lenglinger, J.; Mittal, S.K.; Pandolfino, J.E.; Serra, J.; Tatum, R.; Yadlapati, R. Chicago Classification update (v4.0): Technical review of high-resolution manometry metrics for EGJ barrier function. Neurogastroenterol. Motil. 2021, 33, e14113. [Google Scholar] [CrossRef] [PubMed]
- Khajanchee, Y.S.; Cassera, M.A.; Swanström, L.L.; Dunst, C.M. Diagnosis of Type-I hiatal hernia: A comparison of high-resolution manometry and endoscopy. Dis. Esophagus 2013, 26, 1–6. [Google Scholar] [CrossRef]
- Tolone, S.; Savarino, E.; Zaninotto, G.; Gyawali, C.P.; Frazzoni, M.; de Bortoli, N.; Frazzoni, L.; Del Genio, G.; Bodini, G.; Furnari, M.; et al. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: A comparison with surgical in vivo evaluation. United Eur. Gastroenterol. J. 2018, 6, 981–989. [Google Scholar] [CrossRef]
- Weijenborg, P.W.; van Hoeij, F.B.; Smout, A.J.; Bredenoord, A.J. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol. Motil. 2015, 27, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.H.; Lee, A.M.; Breithaupt, W.; Varga, G.; Babic, B.; Horgan, S. Pathophysiology of gastroesophageal reflux disease-which factors are important? Transl. Gastroenterol. Hepatol. 2021, 6, 53. [Google Scholar] [CrossRef]
- Van Hoeij, F.B.; Smout, A.J.; Bredenoord, A.J. Predictive value of routine esophageal high-resolution manometry for gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2015, 27, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Tolone, S.; de Cassan, C.; de Bortoli, N.; Roman, S.; Galeazzi, F.; Salvador, R.; Marabotto, E.; Furnari, M.; Zentilin, P.; Marchi, S.; et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol. Motil. 2015, 27, 1175–1182. [Google Scholar] [CrossRef]
- Wallner, B.; Björ, O.; Andreasson, A.; Hellström, P.M.; Forsberg, A.M.; Talley, N.J.; Agreus, L. Identifying clinically relevant sliding hiatal hernias: A population-based endoscopy study. Scand. J. Gastroenterol. 2018, 53, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Sundaram, A.; Mittal, S.K. Role of the lower esophageal sphincter on acid exposure revisited with high-resolution manometry. J. Am. Coll. Surg. 2011, 213, 743–750. [Google Scholar] [CrossRef]
- Jasper, D.; Freitas-Queiroz, N.; Hollenstein, M.; Misselwitz, B.; Layer, P.; Navarro-Rodriguez, T.; Fox, M.; Keller, J. Prolonged measurement improves the assessment of the barrier function of the esophago-gastric junction by high-resolution manometry. Neurogastroenterol. Motil. 2017, 29, e12925. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Shang, Y.; Wang, N.; Liu, X.; Xin, C.; Yan, X.; Zhai, Y.; Yin, J.; Zhang, J.; Zhang, Z. The role of abnormal contraction index of gastroesophageal junction in 82 patients with refractory heartburn and reflux disease. Chin. J. Dig. 2021, 41, 88–93. [Google Scholar]
- Rengarajan, A.; Bolkhir, A.; Gor, P.; Wang, D.; Munigala, S.; Gyawali, C.P. Esophagogastric junction and esophageal body contraction metrics on high-resolution manometry predict esophageal acid burden. Neurogastroenterol. Motil. 2018, 30, e13267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, L.; Ye, B.; Lin, L. Value of adjunctive evidence from MII-pH monitoring and high-resolution manometry in inconclusive GERD patients with AET 4-6. Ther. Adv. Gastroenterol. 2021, 14, 17562848211013484. [Google Scholar] [CrossRef]
- Gor, P.; Li, Y.; Munigala, S.; Patel, A.; Bolkhir, A.; Gyawali, C.P. Interrogation of esophagogastric junction barrier function using the esophagogastric junction contractile integral: An observational cohort study. Dis. Esophagus 2016, 29, 820–828. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Roman, S.; Bredenoord, A.J.; Fox, M.; Keller, J.; Pandolfino, J.E.; Sifrim, D.; Tatum, R.; Yadlapati, R.; Savarino, E. Classification of esophageal motor findings in gastro-esophageal reflux disease: Conclusions from an international consensus group. Neurogastroenterol. Motil. 2017, 29, e13104. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.D.; Rengarajan, A.; Abrahao, L.; Bhatia, S.; Bor, S.; Carlson, D.A.; Cisternas, D.; Gonlachanvit, S.; Hani, A.; Hayat, J.; et al. Esophagogastric junction morphology and contractile integral on high-resolution manometry in asymptomatic healthy volunteers: An international multicenter study. Neurogastroenterol. Motil. 2021, 33, e14009. [Google Scholar] [CrossRef]
- Dervin, H.; Bassett, P.; Sweis, R. Esophagogastric junction contractile integral (EGJ-CI) complements reflux disease severity and provides insight into the pathophysiology of reflux disease. Neurogastroenterol. Motil. 2023, 35, e14597. [Google Scholar] [CrossRef] [PubMed]
- Nicodème, F.; Pipa-Muniz, M.; Khanna, K.; Kahrilas, P.J.; Pandolfino, J.E. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: Normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol. Motil. 2014, 26, 353–360. [Google Scholar] [CrossRef]
- Wang, D.; Patel, A.; Mello, M.; Shriver, A.; Gyawali, C.P. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol. Motil. 2016, 28, 639–646. [Google Scholar] [CrossRef]
- Tack, J.; Pandolfino, J.E. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology 2018, 154, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Holloway, R.; Keller, J.; Herbella, F.; Zerbib, F.; Xiao, Y.; Bernard, L.; Bredenoord, A.J.; Bruley des Varannes, S.; Chen, M.; et al. Validation of criteria for the definition of transient lower esophageal sphincter relaxations using high-resolution manometry. Neurogastroenterol. Motil. 2017, 29, e12920. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Hong, S.J.; Han, J.P.; Seo, J.Y.; Hwang, K.H.; Maeng, H.J.; Lee, T.H.; Lee, J.S. Specific movement of esophagus during transient lower esophageal sphincter relaxation in gastroesophageal reflux disease. J. Neurogastroenterol. Motil. 2013, 19, 332–337. [Google Scholar] [CrossRef]
- Trudgill, N.J.; Riley, S.A. Transient lower esophageal sphincter relaxations are no more frequent in patients with gastroesophageal reflux disease than in asymptomatic volunteers. Am. J. Gastroenterol. 2001, 96, 2569–2574. [Google Scholar] [CrossRef]
- Iovino, P.; Mohammed, I.; Anggiansah, A.; Anggiansah, R.; Cherkas, L.F.; Spector, T.D.; Trudgill, N.J. A study of pathophysiological factors associated with gastro-esophageal reflux disease in twins discordant for gastro-esophageal reflux symptoms. Neurogastroenterol. Motil. 2013, 25, 650–656. [Google Scholar] [CrossRef]
- Schneider, J.H.; Küper, M.A.; Königsrainer, A.; Brücher, B.L. Transient lower esophageal sphincter relaxation and esophageal motor response. J. Surg. Res. 2010, 159, 714–719. [Google Scholar] [CrossRef]
- Li, S.; Shi, S.; Chen, F.; Lin, J. The effects of baclofen for the treatment of gastroesophageal reflux disease: A meta-analysis of randomized controlled trials. Gastroenterol. Res. Pract. 2014, 2014, 307805. [Google Scholar] [CrossRef]
- Yadlapati, R.; Pandolfino, J.E.; Fox, M.R.; Bredenoord, A.J.; Kahrilas, P.J. What is new in Chicago Classification version 4.0? Neurogastroenterol. Motil. 2021, 33, e14053. [Google Scholar] [CrossRef] [PubMed]
- Triadafilopoulos, G.; Tandon, A.; Shetler, K.P.; Clarke, J. Clinical and pH study characteristics in reflux patients with and without ineffective oesophageal motility (IEM). BMJ Open Gastroenterol. 2016, 3, e000126. [Google Scholar] [CrossRef] [PubMed]
- De Padua, F.; Herbella, F.A.M.; Patti, M.G. The prevalence of gastroesophageal reflux disease in named manometric patterns of dysmotility according to the Chicago Classification 4.0. Dis. Esophagus 2022, 35, doac023. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.V.; Matsumura, T.; Kaneko, T.; Takahashi, S.; Tokunaga, M.; Oura, H.; Ishikawa, K.; Akizue, N.; Kikuchi, A.; Fujie, M.; et al. Impact of ineffective esophageal motility on chemical clearance in patients with gastroesophageal reflux symptoms. Dis. Esophagus 2020, 33, doaa026. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Baker, J.R.; Lau, J.; Chen, J.W. High-Resolution Manometry Diagnosis of Ineffective Esophageal Motility Is Associated with Higher Reflux Burden. Dig. Dis. Sci. 2019, 64, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Yadlapati, R.; Fass, R.; Katzka, D.; Pandolfino, J.; Savarino, E.; Sifrim, D.; Spechler, S.; Zerbib, F.; Fox, M.R.; et al. Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut 2023, 73, 361–371. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Sifrim, D.; Carlson, D.A.; Hawn, M.; Katzka, D.A.; Pandolfino, J.E.; Penagini, R.; Roman, S.; Savarino, E.; Tatum, R.; et al. Ineffective esophageal motility: Concepts, future directions, and conclusions from the Stanford 2018 symposium. Neurogastroenterol. Motil. 2019, 31, e13584. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.D.; Rengarajan, A.; Mauro, A.; Ghisa, M.; De Bortoli, N.; Cicala, M.; Ribolsi, M.; Penagini, R.; Savarino, E.; Gyawali, C.P. Fragmented and failed swallows on esophageal high-resolution manometry associate with abnormal reflux burden better than weak swallows. Neurogastroenterol. Motil. 2020, 32, e13736. [Google Scholar] [CrossRef]
- Heinrich, H.; Sweis, R. The role of oesophageal physiological testing in the assessment of noncardiac chest pain. Ther. Adv. Chronic Dis. 2018, 9, 257–267. [Google Scholar] [CrossRef]
- Yodice, M.; Mignucci, A.; Shah, V.; Ashley, C.; Tadros, M. Preoperative physiological esophageal assessment for anti-reflux surgery: A guide for surgeons on high-resolution manometry and pH testing. World J. Gastroenterol. 2021, 27, 1751–1769. [Google Scholar] [CrossRef]
- Campagna, R.A.J.; Cirera, A.; Holmstrom, A.L.; Triggs, J.R.; Teitelbaum, E.N.; Carlson, D.A.; Pandolfino, J.E.; Hungness, E.S. Outcomes of 100 Patients More Than 4 Years After POEM for Achalasia. Ann. Surg. 2021, 273, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P. The Role of High-Resolution Manometry in Gastroesophageal Reflux Disease. Gastroenterol. Hepatol. 2019, 15, 442–444. [Google Scholar]
- Jain, M.; Agrawal, V. Role of esophageal manometry and 24-h pH testing in patients with refractory reflux symptoms. Indian J. Gastroenterol. 2020, 39, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Maret-Ouda, J.; Markar, S.R.; Lagergren, J. Gastroesophageal Reflux Disease: A Review. JAMA 2020, 324, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Fass, R. Gastroesophageal Reflux Disease. N. Engl. J. Med. 2022, 387, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, D.; Hope, W.W.; Kohn, G.P.; Reardon, P.R.; Richardson, W.S.; Fanelli, R.D. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg. Endosc. 2010, 24, 2647–2669. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, A.; Boecxstaens, V.; Andrews, C.N.; Attwood, S.E.; Berrisford, R.; Bisschops, R.; Boeckxstaens, G.E.; Bor, S.; Bredenoord, A.J.; Cicala, M.; et al. How to select patients for antireflux surgery? The ICARUS guidelines (international consensus regarding preoperative examinations and clinical characteristics assessment to select adult patients for antireflux surgery). Gut 2019, 68, 1928–1941. [Google Scholar] [CrossRef]
- Shaker, A.; Stoikes, N.; Drapekin, J.; Kushnir, V.; Brunt, L.M.; Gyawali, C.P. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am. J. Gastroenterol. 2013, 108, 1706–1712. [Google Scholar] [CrossRef]
- Sans, A.; Frey, S.; De Montrichard, M.; Takoudju, C.; Coron, E.; Blanchard, C. Impact on sleeve gastrectomy in patients with esophageal motor disorder. Surg. Obes. Relat. Dis. 2021, 17, 1890–1896. [Google Scholar] [CrossRef]
- Slater, B.J.; Dirks, R.C.; McKinley, S.K.; Ansari, M.T.; Kohn, G.P.; Thosani, N.; Qumseya, B.; Billmeier, S.; Daly, S.; Crawford, C.; et al. SAGES guidelines for the surgical treatment of gastroesophageal reflux (GERD). Surg. Endosc. 2021, 35, 4903–4917. [Google Scholar] [CrossRef]
- Tatum, R.P.; Soares, R.V.; Figueredo, E.; Oelschlager, B.K.; Pellegrini, C.A. High-resolution manometry in evaluation of factors responsible for fundoplication failure. J. Am. Coll. Surg. 2010, 210, 611–617. [Google Scholar] [CrossRef]
- Marjoux, S.; Roman, S.; Juget-Pietu, F.; Robert, M.; Poncet, G.; Boulez, J.; Mion, F. Impaired postoperative EGJ relaxation as a determinant of post laparoscopic fundoplication dysphagia: A study with high-resolution manometry before and after surgery. Surg. Endosc. 2012, 26, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.; Osler, T.; Lee, A.; Borrazzo, E. The role of preoperative high resolution manometry in predicting dysphagia after laparoscopic Nissen fundoplication. Surg. Endosc. 2018, 32, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, W.; Liu, S.; Wang, P.Q.; Qiu, M. The Role of Real-Time Continuous High-Resolution Manometry During Bougie-Free Laparoscopic Hill Repair for the Treatment of Gastroesophageal Reflux Disease. J. Gastrointest. Surg. 2021, 25, 1576–1578. [Google Scholar] [CrossRef]
- Ribolsi, M.; Savarino, E.; Rogers, B.; Rengarajan, A.; Coletta, M.D.; Ghisa, M.; Cicala, M.; Gyawali, C.P. High-resolution Manometry Determinants of Refractoriness of Reflux Symptoms to Proton Pump Inhibitor Therapy. J. Neurogastroenterol. Motil. 2020, 26, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tan, N.; Zhang, N.; Xiong, L.; Peng, S.; Lin, J.; Chen, M.; Xiao, Y. Predictors of proton pump inhibitor failure in non-erosive reflux disease: A study with impedance-pH monitoring and high-resolution manometry. Neurogastroenterol. Motil. 2016, 28, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, S.; Zhu, K.; Yu, W.; Wang, H.; Guo, J.; Gao, H. Relationship between esophageal motility and severity of gastroesophageal reflux disease according to the Los Angeles classification. Medicine 2019, 98, e15543. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.C.B.; Herbella, F.A.M.; Del Grande, L.M.; Patti, M.G. The Transdiaphragmatic Pressure Gradient and the Lower Esophageal Sphincter in the Pathophysiology of Gastroesophageal Reflux Disease: An Analysis of 500 Esophageal Function Tests. J. Gastrointest. Surg. 2023, 27, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.W.; Wang, Z.G.; Wu, J.M.; Tian, S.R.; Zhang, Y.; Zhan, X.L.; Du, X.; Wang, F.; Xin, R.H.; Xu, H. The relationship between the severity of reflux esophagitis and esophageal dynamics using high-resolution manometry. Chin. Med. J. 2017, 97, 3306–3311. [Google Scholar]
- Siboni, S.; Kristo, I.; Rogers, B.D.; De Bortoli, N.; Hobson, A.; Louie, B.; Lee, Y.Y.; Tee, V.; Tolone, S.; Marabotto, E.; et al. Improving the Diagnostic Yield of High-Resolution Esophageal Manometry for GERD: The “Straight Leg-Raise” International Study. Clin. Gastroenterol. Hepatol. 2023, 21, 1761–1770.e1761. [Google Scholar] [CrossRef]
- Lei, W.Y.; Liang, S.W.; Omari, T.; Chang, W.C.; Wong, M.W.; Hung, J.S.; Yi, C.H.; Liu, T.T.; Lin, L.; Gyawali, C.P.; et al. Transient Hiatal Separation During Straight Leg Raise Can Predict Reflux Burden in Gastroesophageal Reflux Disease Patients With Ineffective Esophageal Motility. J. Neurogastroenterol. Motil. 2022, 28, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.; Liu, T.T.; Yi, C.H.; Lei, W.Y.; Hung, J.S.; Omari, T.; Cock, C.; Liang, S.W.; Gyawali, C.P.; Chen, C.L. Analysis of contractile segment impedance during straight leg raise maneuver using high-resolution impedance manometry increases diagnostic yield in reflux disease. Neurogastroenterol. Motil. 2022, 34, e14135. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.Y.; Gyawali, C.P.; Chang, W.C.; Roman, S.; Wong, M.W.; Yi, C.H.; Liu, T.T.; Hung, J.S.; Liang, S.W.; Chen, C.L. Application of a novel straight leg raise test during high-resolution manometry can predict esophageal contractile reserve in patients with gastroesophageal reflux disease. Neurogastroenterol. Motil. 2021, 33, e13996. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.D.; Rengarajan, A.; Ali, I.A.; Hasak, S.L.; Hansalia, V.; Gyawali, C.P. Straight leg raise metrics on high-resolution manometry associate with esophageal reflux burden. Neurogastroenterol. Motil. 2020, 32, e13929. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.; Hasak, S.; Hansalia, V.; Gyawali, C.P. Trans-esophagogastric junction pressure gradients during straight leg raise maneuver on high-resolution manometry associate with large hiatus hernias. Neurogastroenterol. Motil. 2020, 32, e13836. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.; Geno, D.M.; Vela, M.F.; Crowell, M.D.; Katzka, D.A. Baseline impedance measured during high-resolution esophageal impedance manometry reliably discriminates GERD patients. Neurogastroenterol. Motil. 2017, 29, e12974. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.; Posner, S.; Sullivan, B.; Cornejo, J.; Davis, A.; Fields, M.; McIntosh, T.; Gellad, Z.; Shimpi, R.; Gyawali, C.P.; et al. Esophageal contractile segment impedance from high-resolution impedance manometry correlates with mean nocturnal baseline impedance and acid exposure time from 24-hour pH-impedance monitoring. Dis. Esophagus 2020, 33, doaa063. [Google Scholar] [CrossRef]
- Yadlapati, R.; Tye, M.; Roman, S.; Kahrilas, P.J.; Ritter, K.; Pandolfino, J.E. Postprandial High-Resolution Impedance Manometry Identifies Mechanisms of Nonresponse to Proton Pump Inhibitors. Clin. Gastroenterol. Hepatol. 2018, 16, 211–218.e211. [Google Scholar] [CrossRef]
- Nicodème, F.; Lin, Z.; Pandolfino, J.E.; Kahrilas, P.J. Esophagogastric Junction pressure morphology: Comparison between a station pull-through and real-time 3D-HRM representation. Neurogastroenterol. Motil. 2013, 25, e591–e598. [Google Scholar] [CrossRef] [PubMed]
- Chitose, S.I.; Shin, Y.; Sato, K.; Hamakawa, S.; Fukahori, M.; Ono, T.; Umeno, H. Three-dimensional imaging of upper esophageal sphincter resting pressure. Laryngoscope Investig. Otolaryngol. 2019, 4, 645–652. [Google Scholar] [CrossRef]
- Meyer, J.P.; Jones, C.A.; Walczak, C.C.; McCulloch, T.M. Three-dimensional manometry of the upper esophageal sphincter in swallowing and nonswallowing tasks. Laryngoscope 2016, 126, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Donnan, E.N.; Pandolfino, J.E. EndoFLIP in the Esophagus: Assessing Sphincter Function, Wall Stiffness, and Motility to Guide Treatment. Gastroenterol. Clin. N. Am. 2020, 49, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Hoppo, T.; McMahon, B.P.; Witteman, B.P.; Kraemer, S.J.; O’Rourke, R.W.; Gravesen, F.; Bouvy, N.D.; Jobe, B.A. Functional lumen imaging probe to assess geometric changes in the esophagogastric junction following endolumenal fundoplication. J. Gastrointest. Surg. 2011, 15, 1112–1120. [Google Scholar] [CrossRef]
- Lee, J.M.; Yoo, I.K.; Kim, E.; Hong, S.P.; Cho, J.Y. The Usefulness of the Measurement of Esophagogastric Junction Distensibility by EndoFLIP in the Diagnosis of Gastroesophageal Reflux Disease. Gut Liver 2021, 15, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Ponds, F.A.; Bredenoord, A.J.; Kessing, B.F.; Smout, A.J. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol. Motil. 2017, 29, e12908. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Lin, Z.; Kahrilas, P.J.; Sternbach, J.; Donnan, E.N.; Friesen, L.; Listernick, Z.; Mogni, B.; Pandolfino, J.E. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed with Manometry in Patients with Achalasia. Gastroenterology 2015, 149, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I. Chicago Classification ver. 4.0: Diagnosis of Peristaltic Disorder. Korean J. Gastroenterol. 2022, 79, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.P.; Meisenbach, L.M.; Chan, E.Y. Tailored Fundoplication with Endoluminal Functional Lumen Imaging Probe Allows for Successful Minimally Invasive Hiatal Hernia Repair. Surg. Laparosc. Endosc. Percutan Tech. 2018, 28, 178–182. [Google Scholar] [CrossRef]
- Su, B.; Callahan, Z.M.; Kuchta, K.; Linn, J.G.; Haggerty, S.P.; Denham, W.; Ujiki, M.B. Use of Impedance Planimetry (Endoflip) in Foregut Surgery Practice: Experience of More than 400 Cases. J. Am. Coll. Surg. 2020, 231, 160–171. [Google Scholar] [CrossRef]
- Dorsey, Y.C.; Posner, S.; Patel, A. Esophageal Functional Lumen Imaging Probe (FLIP): How Can FLIP Enhance Your Clinical Practice? Dig. Dis. Sci. 2020, 65, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Attaar, M.; Su, B.; Wong, H.J.; Kuchta, K.; Denham, W.; Haggerty, S.P.; Linn, J.; Ujiki, M.B. Intraoperative impedance planimetry (EndoFLIP™) results and development of esophagitis in patients undergoing peroral endoscopic myotomy (POEM). Surg. Endosc. 2021, 35, 4555–4562. [Google Scholar] [CrossRef]
- Su, B.; Novak, S.; Callahan, Z.M.; Kuchta, K.; Carbray, J.; Ujiki, M.B. Using impedance planimetry (EndoFLIP™) in the operating room to assess gastroesophageal junction distensibility and predict patient outcomes following fundoplication. Surg. Endosc. 2020, 34, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.K.; Choi, S.A.; Kim, W.H.; Hong, S.P.; Cakir, O.O.; Cho, J.Y. Assessment of Clinical Outcomes after Peroral Endoscopic Myotomy via Esophageal Distensibility Measurements with the Endoluminal Functional Lumen Imaging Probe. Gut Liver 2019, 13, 32–39. [Google Scholar] [CrossRef]
- VanDruff, V.N.; Amundson, J.R.; Joseph, S.; Che, S.; Kuchta, K.; Zimmermann, C.J.; Ishii, S.; Hedberg, H.M.; Ujiki, M.B. Impedance planimetry and panometry (EndoFLIP™) can replace manometry in preoperative anti-reflux surgery assessment. Surg. Endosc. 2023, 38, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.; Sweis, R.; Anggiansah, A.; Wong, T.; Telakis, E.; Knowles, K.; Wright, J.; Fox, M. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2013, 25, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Kathpalia, P.; Craft, J.; Tye, M.; Lin, Z.; Kahrilas, P.J.; Pandolfino, J.E. The relationship between esophageal acid exposure and the esophageal response to volumetric distention. Neurogastroenterol. Motil. 2018, 30, e13240. [Google Scholar] [CrossRef] [PubMed]
- Smeets, F.G.; Keszthelyi, D.; Bouvy, N.D.; Masclee, A.A.; Conchillo, J.M. Does Measurement of Esophagogastric Junction Distensibility by EndoFLIP Predict Therapy-responsiveness to Endoluminal Fundoplication in Patients With Gastroesophageal Reflux Disease? J. Neurogastroenterol. Motil. 2015, 21, 255–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwiatek, M.A.; Pandolfino, J.E.; Hirano, I.; Kahrilas, P.J. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP). Gastrointest. Endosc. 2010, 72, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Prescott, J.E.; Baumann, A.J.; Schauer, J.M.; Krause, A.; Donnan, E.N.; Kou, W.; Kahrilas, P.J.; Pandolfino, J.E. Validation of Clinically Relevant Thresholds of Esophagogastric Junction Obstruction Using FLIP Panometry. Clin. Gastroenterol. Hepatol. 2022, 20, e1250–e1262. [Google Scholar] [CrossRef] [PubMed]
- Amundson, J.R.; Kuchta, K.; Zimmermann, C.J.; VanDruff, V.N.; Joseph, S.; Che, S.; Ishii, S.; Hedberg, H.M.; Ujiki, M.B. Target distensibility index on impedance planimetry during fundoplication by choice of wrap and choice of bougie. Surg. Endosc. 2023, 37, 8670–8681. [Google Scholar] [CrossRef]
- Wu, H.; Attaar, M.; Wong, H.J.; Campbell, M.; Kuchta, K.; Denham, W.; Ujiki, M.B. Impedance Planimetry (Endoflip™) Shows That Length of Narrowing After Fundoplication Does Not Impact Dysphagia. J. Gastrointest. Surg. 2022, 26, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Attaar, M.; Wong, H.J.; Campbell, M.; Kuchta, K.; Denham, E.W., 3rd; Linn, J.; Ujiki, M.B. Impedance Planimetry (Endoflip) and Ideal Distensibility Ranges for Optimal Outcomes after Nissen and Toupet Fundoplication. J. Am. Coll. Surg. 2022, 235, 420–429. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, R.K.; Davila, D.; Frelich, M.J.; Gould, J.C. Esophagogastric junction distensibility is greater following Toupet compared to Nissen fundoplication. Surg. Endosc. 2017, 31, 193–198. [Google Scholar] [CrossRef]
- Kwiatek, M.A.; Kahrilas, K.; Soper, N.J.; Bulsiewicz, W.J.; McMahon, B.P.; Gregersen, H.; Pandolfino, J.E. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J. Gastrointest. Surg. 2010, 14, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.; Helm, M.; Hetzel, E.; Gould, J.C. Is that ‘floppy’ fundoplication tight enough? Surg. Endosc. 2020, 34, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
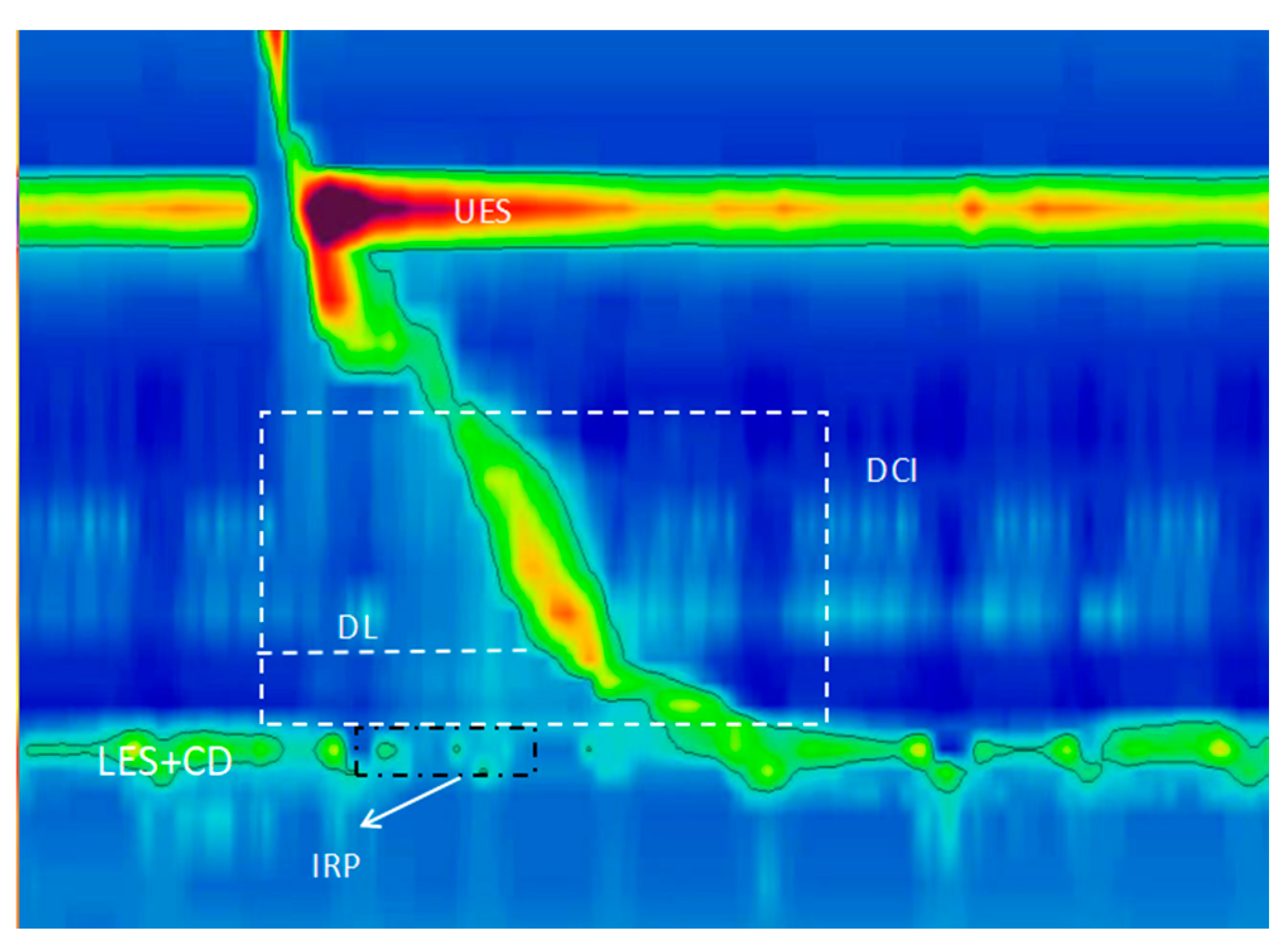


| Application | Representation | Main Parameters Involved |
|---|---|---|
| Understanding pathophysiologies | Impairment of the anti-reflux barrier | LES, CD, EGJ-CI |
| TLESR | LES, CD | |
| Motor disorders of the Esophageal body | DCI | |
| Auxiliary diagnosis | the decrease of EGJ pressure, | LESP |
| HH | LES, CD | |
| IEM/absent contractility | DCI | |
| Differential diagnosis of esophageal motility disorders | EGJOO | IRP, DCI |
| AC | IRP | |
| esophageal hypermotility | DCI | |
| DES | DCI, DL | |
| Guide anti-reflux surgical | - | DCI, MRS, IRP, LES, CD, LESP |
| Predicting the efficacy of PPI | - | DCI, LES, CD |
| Predicting acid exposure and severity | - | EGJ-CI, DCI, LES |
| Application of new equipment | HRIM | BI, CSI |
| 3D-HREM | - |
| Study | Subject Groups | Threshold | Algorithm | Conclusion |
|---|---|---|---|---|
| Humayra Dervin et al. [31], 2023 | BE (n = 25) NERD (n = 25) AET4–6% (n = 25) FH (n = 25) | 21.2 mmHg·cm | Recommend threshold 20 mmHg above gastric pressure | The distinction between Barrett’s esophagus/NERD and FH had a sensitivity of 72% and a specificity of 72%. |
| Jasper D et al. [24], 2017 | HC (n = 65) GERD (n = 452) | 47 mmHg·cm | threshold 2 mmHg above gastric pressure | The sensitivity of GERD diagnosis was 54%, and the positive predictive value was 46%. |
| Rengarajan A et al. [13], 2020 | GERD (n = 482) | 39.3 mmHg·cm | threshold 0 mmHg above gastric pressure | The EGJ-CIs were independent predictors of AET abnormalities. |
| S.Tolone et al. [10], 2015 | GERD (n = 91) FH (n = 39) | 13 mmHg·cm | threshold 2 mmHg above gastric pressure | The EGJ-CI is associated with AET, reflux episodes, and mucosal injuries. And, a cut-off value of 5 has the highest sensitivity (89%) and specificity (63%) in distinguishing GERD from FH. |
| Gor, P et al. [28], 2016 | normal controls (n = 21), GERD (n = 188) | 39.3 mmHg·cm | gastric baseline (rather than correction to a value above the gastric baseline) during a period of quiet rest | EGJ-CI is a novel HREM metric that has potential to complement or replace currently used basal LES and EGJ parameters. |
| Nicodème F et al. [32], 2014 | normal controls (n = 75) PPI-NRs (n = 88) | 39 mmHg·cm | threshold 2 mmHg above gastric pressure | The EGJ-CI may help distinguish between PPI-NRs patients with functional heartburn and patients with refractory GERD. |
| Benjamin D Rogers et al. [30], 2021 | Health volunteers (n = 484) | - | threshold 20 mmHg above gastric pressure | The 5th percentile EGJ-CI value was 6.9 to 12.1 mmHg·cm. |
| D. WANG et al. [33], 2016 | Twenty-one achalasia patients, 68 GERD patients, and 21 healthy controls | - | gastric baseline (rather than correction to a value above the gastric baseline) during a period of quiet rest | The EGJ-CI has clinical utility in assessing EGJ barrier function at baseline and after surgical intervention to the EGJ. |
| References | Population | Volumes Distension | Process | Findings |
|---|---|---|---|---|
| Diagnosis | ||||
| Tucker et al. [95], 2013 | Twenty-one HV and 18 patients with typical GERD symptoms | 20–30 mL | enteral anesthesia | EndoFLIP technology cannot be used to diagnose GERD. |
| Lee et al. [84], 2021 | ERD (n = 204), NERD (n = 310), and 277 normal subjects | 40 mL | not very clear | The measurement of EGJ distensibility was helpful in the diagnosis of GERD. The EGJ distensibility of GERD patients was higher than that of normal subjects, regardless of the presence of reflux esophagitis. |
| Carlson et al. [96], 2018 | 25 patients | 10–70 mL | conscious sedation | No correlation was found between EGJ-DI and reflux parameters including AET, number of reflux episodes, and longest reflux episodes. |
| Smeets et al. [97], 2015 | GERD (n = 42) and 25 patients receiving TIF treatment were followed up for 6 months | 20–30 mL | - Induction general anesthesia and conscious sedation | EndoFLIP technique has no additional value in preoperative diagnosis. |
| Kwiatek et al. [98], 2010 | GERD (n = 20) and controls (n = 20) | 10–40 mL | - Esophagogastroduodenoscopy, conscious sedation | GERD patients exhibited two- to threefold increased EGJ distensibility compared with controls, particularly at 20 to 30 mL distention volumes. |
| Differential Diagnosis | ||||
| Carlson et al. [99], 2022 | asymptomatic volunteers (n = 35), primary esophageal motility evaluation patients (n = 687) | 40–70 mL | Esophagogastroduodenoscopy, conscious sedation | Normal EGJ opening: EGJ-DI > 2–3 mm2/mm Hg and EGJ diameter > 12–16 mm, EGJ-DI < 2 mm2/mm Hg and EGJ diameter < 12 mm suggest EGJ outflow obstruction) |
| Ponds et al. [85], 2017 | 13 patients of achalasia | 20–50 mL | Conscious without sedation | EGJ distensibility measured can diagnose achalasia despite normal IRP (<15 mmHg). |
| Anti-reflux surgical | ||||
| Julia R. Amundson et al. [100], 2023 | TFHB (n = 147), TFFB (n = 69), TFNB (n = 78), NFHB (n = 20), NFFB (n = 19) | 30–40 mL (8 cm) 60 mL (16 cm) | general anesthesia | The CCDI > 3.5 mm2/mmHg (40 mL fill) should be sought in abnormal motility patients, regardless of wrap or bougie, to avoid postoperative dysphagia. TFFB abnormal motility patients with FDI > 3.6 mm2/mmHg (40 mL fill) also developed zero postoperative dysphagia. FDI > 6.2 mm2/mmHg (40 mL fill) was seen in all postoperative hernia recurrences. |
| Wu, H et al. [101], 2022 | GERD (n = 111) (26% Nissen, 74% Toupet) | 30–40 mL | Intra-operative, General anesthesia | A LON of 2.5–4.5 cm and DI of 2.5–3.6 mm2/mmHg after fundoplication led to better postoperative quality of life. |
| Min P. Kim et al. [88], 2018 | Forty patients underwent minimally invasive hiatal hernia repair with fundoplication | 30 mL | Intra-operative, General anesthesia | EndoFLIP can be used to help tailor how tight to close the crus and how tight to create the fundoplication. (decided to aim for a DI > 0.5 mm2/mm Hg, as measured with 30 mL in the balloon.) |
| Wu, H et al. [102], 2022 | Two hundred fifty patients (171 Toupet, 79 Nissen) | 30–40 mL | Intra-operative, General anesthesia | The ideal distensibility index range of Toupet patients with the 30 and 40 mL balloon fills was 2.6 to 3.7 mm2/mmHg. For Nissen patients, the 30 and 40 mL ideal threshold was a distensibility index of ≥2.2 mm2/mmHg. |
| Su, B et al. [92], 2019 | 175 patients underwent laparoscopic fundoplication | 20–40 mL | Intra-operative, General anesthesia | EndoFLIP measurements correlate well with patient outcomes, with a final DI between 2 and 3.5 mm2/mmHg potentially being ideal. And EndoFLIP measurements correlate well with patient outcomes. |
| DeHaan et al. [103], 2017 | 75 patients underwent fundoplications | 30–40 mL | conscious sedation | EGJ distensibility can be determined in real-time intraoperatively and that fundoplication results in a decreased distensibility of the EGJ in patients with GERD. |
| Monika et al. [104], 2010 | Ten controls and ten Nissen FP patients were studied | 30–60 mL | conscious sedation | After FP, the stretchability of EGJ decreased and the shrinkage length increased. |
| Smeets et al. [97], 2015 | 42 GERD patients and 25 patients receiving TIF treatment were followed up for 6 months | 20–30 mL | induction of general anesthesia and conscious sedation | EndoFLIP technology has no added value in the postoperative evaluation of endovascular anti-reflux therapy. |
| Turner et al. [105], 2020 entry 4 | Forty-three GERD patients | 30 mL | general anesthesia | The EndoFLIP probe is a useful tool that can provide feedback during gastric fundus folding surgery and allows surgeons to customize the geometry of the package to optimize symptom outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Huang, Y.; He, L.; Chen, D.; Wu, S.; Tian, Y.; Zheng, J.; Yang, J.; Song, G. Utilizing Esophageal Motility Tests in Diagnosing and Evaluating Gastroesophageal Reflux Disease. Diagnostics 2024, 14, 1467. https://doi.org/10.3390/diagnostics14141467
Yang W, Huang Y, He L, Chen D, Wu S, Tian Y, Zheng J, Yang J, Song G. Utilizing Esophageal Motility Tests in Diagnosing and Evaluating Gastroesophageal Reflux Disease. Diagnostics. 2024; 14(14):1467. https://doi.org/10.3390/diagnostics14141467
Chicago/Turabian StyleYang, Wangliu, Yurong Huang, Lei He, Dongmei Chen, Sheng Wu, Yan Tian, Juan Zheng, Jie Yang, and Gengqing Song. 2024. "Utilizing Esophageal Motility Tests in Diagnosing and Evaluating Gastroesophageal Reflux Disease" Diagnostics 14, no. 14: 1467. https://doi.org/10.3390/diagnostics14141467
APA StyleYang, W., Huang, Y., He, L., Chen, D., Wu, S., Tian, Y., Zheng, J., Yang, J., & Song, G. (2024). Utilizing Esophageal Motility Tests in Diagnosing and Evaluating Gastroesophageal Reflux Disease. Diagnostics, 14(14), 1467. https://doi.org/10.3390/diagnostics14141467








