The Association between the Body Mass Index, Chronic Obstructive Pulmonary Disease and SUV of the Non-Tumorous Lung in the Pretreatment [18F]FDG-PET/CT of Patients with Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. FDG-PET/CT Imaging
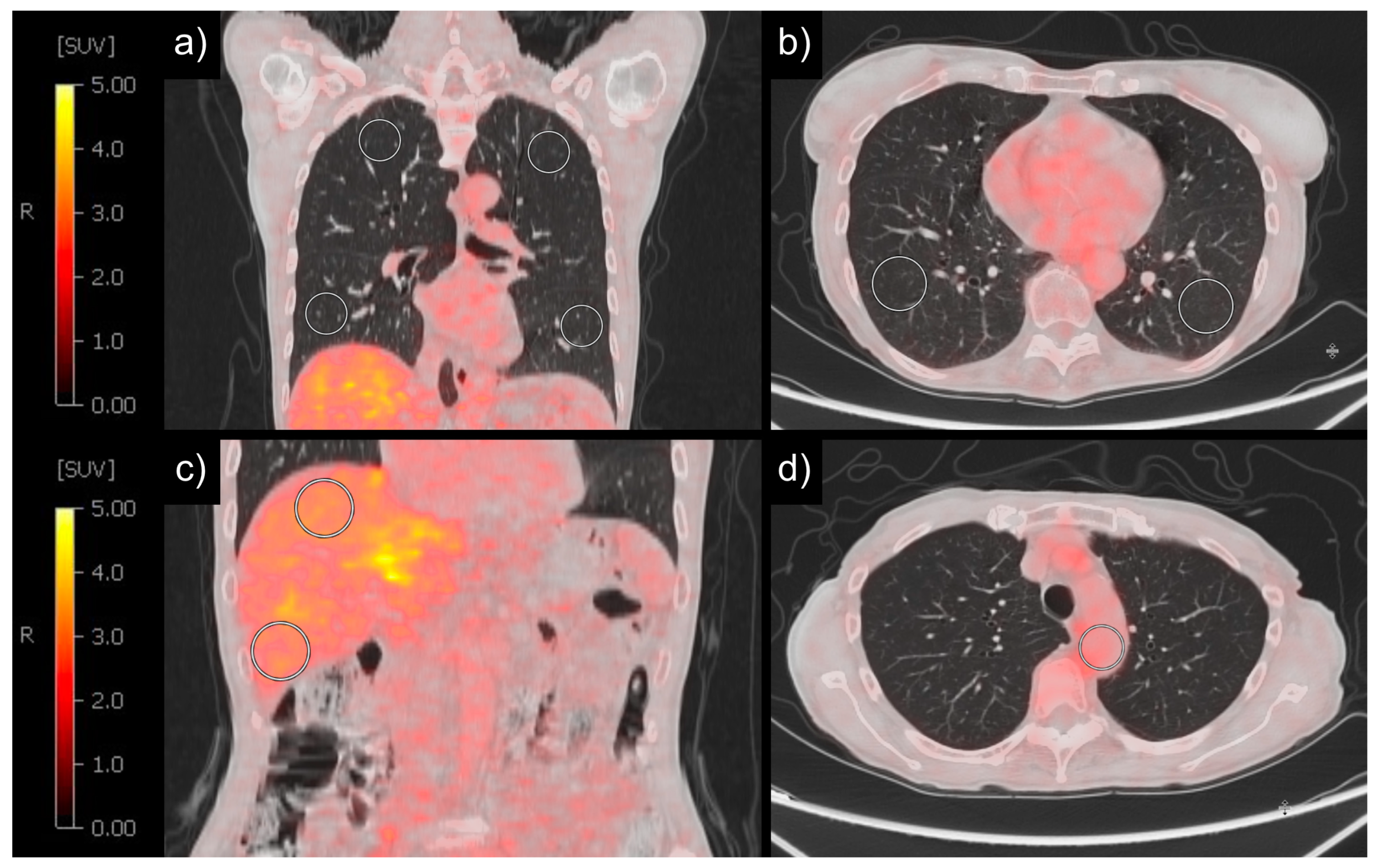
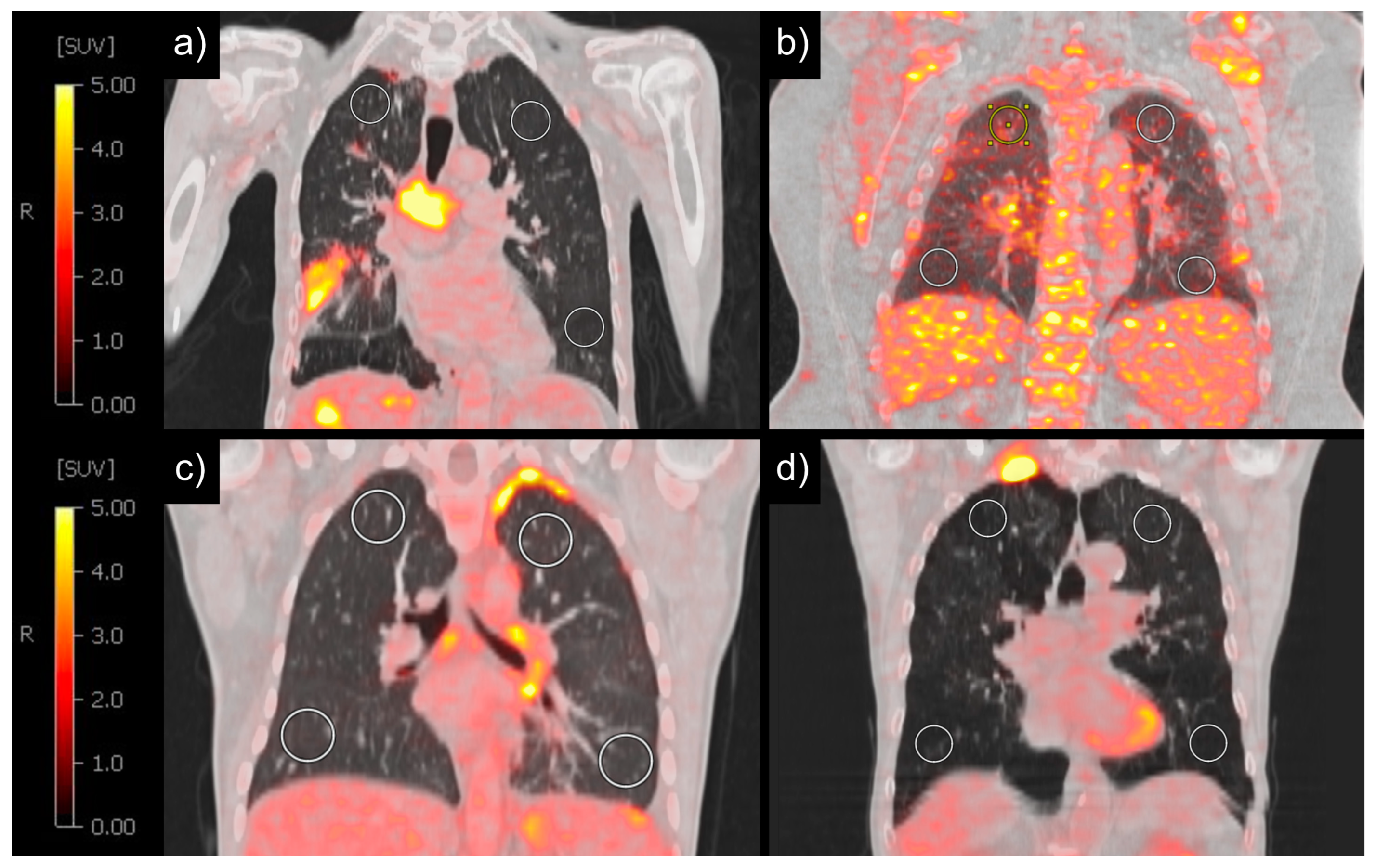
2.3. Image Analysis
2.4. Patient Characteristics
2.5. Statistical Analyses
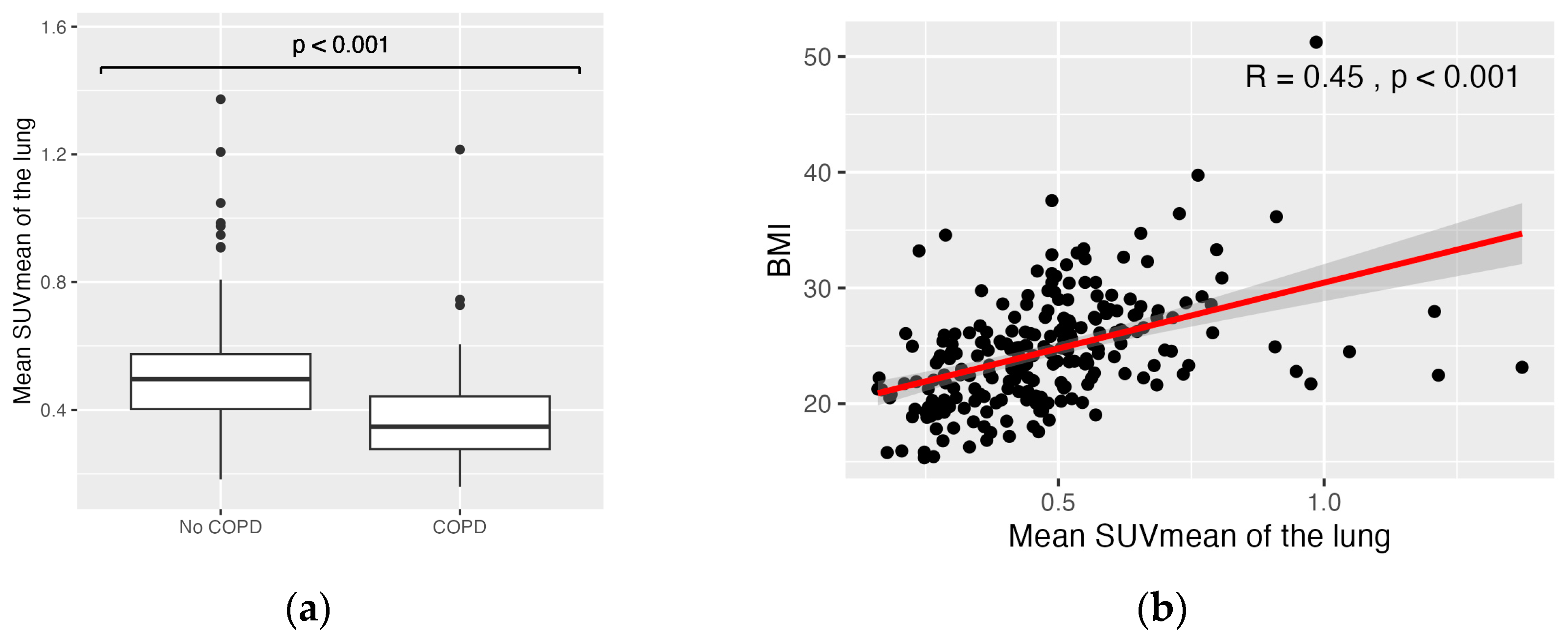
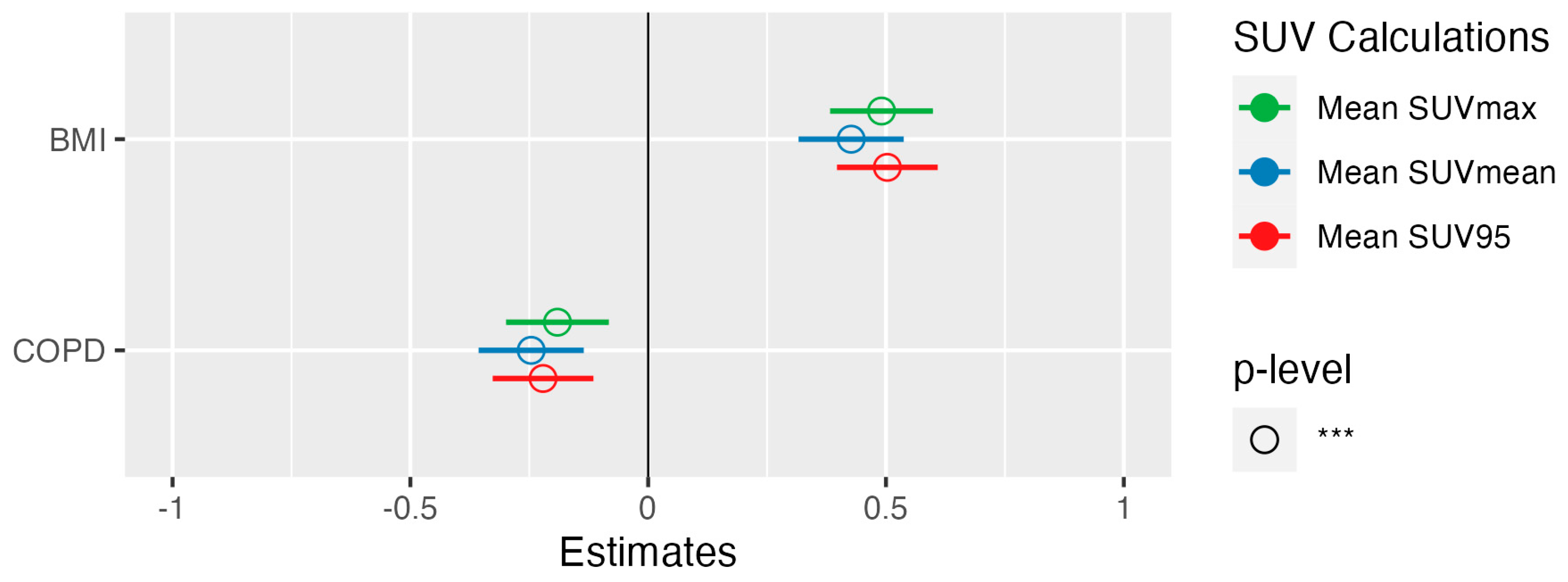
3. Results
3.1. Patient Characteristics
3.2. Association of Biological Variables and Semiquantitative PET Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| SUV Measurements | p |
|---|---|
| SUVMAX | |
| whole lung | 0.03 * |
| upper lung | 0.1603 |
| lower lung | 0.0114 * |
| TFL | 0.0069 ** |
| SUVMEAN | |
| whole lung | 0.3562 |
| upper lung | 0.2033 |
| lower lung | 0.2462 |
| TFL | 0.1932 |
| SUV95 | |
| whole lung | 0.3664 |
| upper lung | 0.5909 |
| lower lung | 0.104 |
| TFL | 0.0745 |
| SUVMEAN lung/liver | |
| whole lung | 0.2663 |
| upper lung | 0.0534 |
| lower lung | 0.1863 |
| TFL | 0.0724 |
| SUVMEAN lung/blood pool | |
| whole lung | 0.0139 * |
| upper lung | 0.0044 ** |
| lower lung | 0.0166 * |
| TFL | 0.0062 ** |
Appendix B
| Variable | Sex | Age | BMI | Smoking | PY | COPD | Stage | OP | Rad | PE | PCE | DM | CHD | Hb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUVMAX | ||||||||||||||
| whole lung | + | - | ||||||||||||
| upper lung | + | |||||||||||||
| lower lung | + | |||||||||||||
| TFL | + | |||||||||||||
| SUVMEAN | ||||||||||||||
| whole lung | + | - | ||||||||||||
| upper lung | + | - | - | |||||||||||
| lower lung | + | - | ||||||||||||
| TFL | + | - | ||||||||||||
| SUV95 | ||||||||||||||
| whole lung | + | - | ||||||||||||
| upper lung | + | - | - | |||||||||||
| lower lung | + | - | ||||||||||||
| TFL | + | |||||||||||||
| SUVMEAN lung/liver | ||||||||||||||
| whole lung | + | - | ||||||||||||
| upper lung | + | - | ||||||||||||
| lower lung | + | - | ||||||||||||
| TFL | + | |||||||||||||
| SUVMEAN lung/blood pool | ||||||||||||||
| whole lung | + | - | ||||||||||||
| upper lung | + | - | ||||||||||||
| lower lung | + | |||||||||||||
| TFL | + | |||||||||||||
 | ||||||||||||||
References
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; Von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Fletcher, J.W. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. In Seminars in Ultrasound, CT and MRI; WB Saunders: Philadelphia, PA, USA, 2010; pp. 496–505. [Google Scholar]
- Nishino, M.; Chambers, E.S.; Chong, C.R.; Ramaiya, N.H.; Gray, S.W.; Marcoux, J.P.; Hatabu, H.; Janne, P.A.; Hodi, F.S.; Awad, M.M. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2016, 4, 289–293. [Google Scholar] [CrossRef]
- Owen, D.H.; Wei, L.; Bertino, E.M.; Edd, T.; Villalona-Calero, M.A.; He, K.; Shields, P.G.; Carbone, D.P.; Otterson, G.A. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non–small-cell lung cancer. Clin. Lung Cancer 2018, 19, e893–e900. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M. Overall survival and long-term safety of nivolumab (anti–programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J. Clin. Oncol. 2015, 33, 2004. [Google Scholar] [CrossRef]
- Castillo, R.; Pham, N.; Ansari, S.; Meshkov, D.; Castillo, S.; Li, M.; Olanrewaju, A.; Hobbs, B.; Castillo, E.; Guerrero, T. Pre-radiotherapy FDG PET predicts radiation pneumonitis in lung cancer. Radiat. Oncol. 2014, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.F.; van Elmpt, W.J.; Oberije, C.J.; Vegt, E.; Dingemans, A.-M.C.; Lambin, P.; Dekker, A.L.; De Ruysscher, D. [18F] fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Yu, J.; Fu, Z.; Liu, T.; Guo, S. (18)FDG PET-CT standardized uptake value for the prediction of radiation pneumonitis in patients with lung cancer receiving radiotherapy. Oncol. Lett. 2015, 10, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; Binkley, M.S.; Rigdon, J.; Carter, J.N.; Aggarwal, S.; Dudley, S.A.; Qian, Y.; Kumar, K.A.; Hara, W.Y.; Gensheimer, M. Pre-treatment non-target lung FDG-PET uptake predicts symptomatic radiation pneumonitis following Stereotactic Ablative Radiotherapy (SABR). Radiother. Oncol. 2016, 119, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, N.; Garland, L.L.; Nia, E.; Betancourt, R.; Krupinski, E.; Kuo, P.H. 18F-FDG PET/CT can predict development of thyroiditis due to immunotherapy for lung cancer. J. Nucl. Med. Technol. 2018, 46, 260–264. [Google Scholar] [CrossRef]
- Ferrari, C.; Santo, G.; Merenda, N.; Branca, A.; Mammucci, P.; Pizzutilo, P.; Gadaleta, C.D.; Rubini, G. Immune checkpoint inhibitors in advanced NSCLC: [18F] FDG PET/CT as a troubleshooter in treatment response. Diagnostics 2021, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Rossi, S.; Toschi, L.; Lopci, E. Impact of antibiotic therapy and metabolic parameters in non-small cell lung cancer patients receiving checkpoint inhibitors. J. Clin. Med. 2021, 10, 1251. [Google Scholar] [CrossRef]
- Hribernik, N.; Huff, D.T.; Studen, A.; Zevnik, K.; Klanecek, Z.; Emamekhoo, H.; Skalic, K.; Jeraj, R.; Rebersek, M. Quantitative imaging biomarkers of immune-related adverse events in immune-checkpoint blockade-treated metastatic melanoma patients: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute number of new lesions on 18 F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef]
- Mu, W.; Tunali, I.; Qi, J.; Schabath, M.B.; Gillies, R.J. Radiomics of 18F fluorodeoxyglucose PET/CT images predicts severe immune-related adverse events in patients with NSCLC. Radiol. Artif. Intell. 2020, 2, e190063. [Google Scholar] [CrossRef]
- Forey, B.A.; Thornton, A.J.; Lee, P.N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm. Med. 2011, 11, 1–61. [Google Scholar] [CrossRef]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Clark, A.R.; Hoffman, E.A.; Tawhai, M.H. Metrics of lung tissue heterogeneity depend on BMI but not age. J. Appl. Physiol. 2018, 125, 328–339. [Google Scholar] [CrossRef]
- Heinzerling, L.; de Toni, E.N.; Schett, G.; Hundorfean, G.; Zimmer, L. Checkpoint Inhibitors. Dtsch. Arztebl. Int. 2019, 116, 119–126. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Demirev, A.K.; Kostadinova, I.D.; Gabrovski, I.R. 18F-FDG PET/CT in patients with parenchymal changes attributed to radiation pneumonitis. Mol. Imaging Radionucl. Ther. 2018, 27, 107. [Google Scholar] [CrossRef]
- Tatar, G.; Alçin, G.; Sengul Samanci, N.; Erol Fenercioglu, Ö.; Beyhan, E.; Cermik, T.F. Diagnostic impact of 18F-FDG PET/CT imaging on the detection of immune-related adverse events in patients treated with immunotherapy. Clin. Transl. Oncol. 2022, 24, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Thie, J.A. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. 2004, 45, 1431–1434. [Google Scholar] [PubMed]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef]
- Zhuang, M.; García, D.V.; Kramer, G.M.; Frings, V.; Smit, E.; Dierckx, R.; Hoekstra, O.S.; Boellaard, R. Variability and repeatability of quantitative uptake metrics in 18F-FDG PET/CT of non–small cell lung cancer: Impact of segmentation method, uptake interval, and reconstruction protocol. J. Nucl. Med. 2019, 60, 600–607. [Google Scholar] [CrossRef]
- Well, D.; Meier, J.; Mahne, A.; Houseni, M.; Hernandez-Pampaloni, M.; Mong, A.; Mishra, S.; Zhuge, Y.; Souza, A.; Udupa, J.; et al. Structural and functional changes in lung with increasing body mass index (BMI). J. Nucl. Med. 2007, 48, 292P. [Google Scholar]
- Sprinz, C.; Zanon, M.; Altmayer, S.; Watte, G.; Irion, K.; Marchiori, E.; Hochhegger, B. Effects of blood glucose level on 18F fluorodeoxyglucose (18F-FDG) uptake for PET/CT in normal organs: An analysis on 5623 patients. Sci. Rep. 2018, 8, 2126. [Google Scholar] [CrossRef] [PubMed]
- Behazin, N.; Jones, S.B.; Cohen, R.I.; Loring, S.H. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J. Appl. Physiol. 2010, 108, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Parasuaraman, G.; Ayyasamy, L.; Aune, D.; Sen, A.; Nagarajan, R.; Rajkumar, P.; Velusamy, S.; Manickam, P.; Sivaprakasam, S. The association between body mass index, abdominal fatness, and weight change and the risk of adult asthma: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2023, 13, 7745. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.-D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Parini, S.; Azzolina, D.; Massera, F.; Mastromarino, M.G.; Papalia, E.; Baietto, G.; Curcio, C.; Crisci, R.; Rena, O.; Alloisio, M.; et al. The Overweight Paradox: Impact of Body Mass Index on Patients Undergoing VATS Lobectomy or Segmentectomy. Semin. Thorac. Cardiovasc. Surg. 2023, 35, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Kim, I.Y.; Sun, J.-M.; Lee, J.; Cha, H.-S.; Koh, E.-M.; Kim, H.; Lee, J. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep. 2019, 9, 14039. [Google Scholar] [CrossRef] [PubMed]
- Rogado, J.; Romero-Laorden, N.; Sanchez-Torres, J.M.; Ramos-Levi, A.M.; Pacheco-Barcia, V.; Ballesteros, A.I.; Arranz, R.; Lorenzo, A.; Gullon, P.; Garrido, A.; et al. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology 2020, 9, 1751548. [Google Scholar] [CrossRef]
- Albitar, H.A.H.; Duma, N.; Leventakos, K.; Gallo De Moraes, A. Pulmonary Complications Secondary to Immune Checkpoint Inhibitors. Int. J. Chronic. Dis. 2020, 2020, 4928648. [Google Scholar] [CrossRef][Green Version]
- Hickeson, M.; Yun, M.; Matthies, A.; Zhuang, H.; Adam, L.-E.; Lacorte, L.; Alavi, A. Use of a corrected standardized uptake value based on the lesion size on CT permits accurate characterization of lung nodules on FDG-PET. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1639–1647. [Google Scholar] [CrossRef]
- Khalaf, M.; Abdel-Nabi, H.; Baker, J.; Shao, Y.; Lamonica, D.; Gona, J. Relation between nodule size and 18 F-FDG-PET SUV for malignant and benign pulmonary nodules. J. Hematol. Oncol. 2008, 1, 13. [Google Scholar] [CrossRef]
- Liu, J.; Dong, M.; Sun, X.; Li, W.; Xing, L.; Yu, J. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: A meta-analysis. PLoS ONE 2016, 11, e0146195. [Google Scholar] [CrossRef]
- Hyun, S.H.; Lee, K.H.; Choi, J.Y.; Kim, B.T.; Kim, J.; Zo, J.I.; Kim, H.; Kwon, O.J.; Ahn, H.K. Influence of Body Mass Index on the Prognostic Value of Tumor ¹⁸F-FDG Uptake in Stage I Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0145020. [Google Scholar] [CrossRef]
- Hyun, S.H.; Ahn, H.K.; Lee, J.H.; Choi, J.Y.; Kim, B.T.; Park, Y.H.; Im, Y.H.; Lee, J.E.; Nam, S.J.; Lee, K.H. Body Mass Index with Tumor 18F-FDG Uptake Improves Risk Stratification in Patients with Breast Cancer. PLoS ONE 2016, 11, e0165814. [Google Scholar] [CrossRef]
- Rennard, S.I. Inflammation and repair processes in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, S12–S16. [Google Scholar] [CrossRef]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Guenard, H.; Diallo, M.H.; Laurent, F.; Vergeret, J. Lung density and lung mass in emphysema. Chest 1992, 102, 198–203. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, X.; Wang, G. Risk Factors for Immune Checkpoint Inhibitor-Related Pneumonitis in Cancer Patients: A Systemic Review and Meta-Analysis. Respiration 2022, 101, 1035–1050. [Google Scholar] [CrossRef]
- Zeng, Z.; Qu, J.; Yao, Y.; Xu, F.; Lu, S.; Zhang, P.; Yao, Y.; Li, N.; Zhou, J.; Wang, Y. Clinical outcomes and risk factor of immune checkpoint inhibitors-related pneumonitis in non-small cell lung cancer patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 2022, 22, 458. [Google Scholar] [CrossRef] [PubMed]
- Yuji, H. Characteristics of Smoothing Filters to Achieve the Guideline Recommended Positron Emission Tomography Image without. Asia Ocean. J. Nucl. Med. Biol. 2018, 6, 15. [Google Scholar]
| Variable | Mean/Median | +/− SD/IQR |
|---|---|---|
| Physical characteristics | ||
| Age [y] | 68.0 | 14.25 |
| BMI [kg/m≤] | 24.49 | 4.67 |
| Smoking history | ||
| Pack years [y] | 34.39 | 26.95 |
| Blood count | ||
| Hb [g/dL] | 12.0 | 3.18 |
| Variable | No. | % |
| Physical characteristics | ||
| Biological sex | ||
| Female | 91 | 37.9 |
| Male | 149 | 62.1 |
| Tumor | ||
| Staging | ||
| I | 2 | 1.5 |
| II | 46 | 33.6 |
| III | 88 | 64.2 |
| IV | 1 | 0.7 |
| Histology | ||
| NSCLC adenocarcinoma | 137 | 57.1 |
| NSCLC squamous cell carcinoma | 65 | 27.1 |
| SCLC neuroendocrine carcinoma | 24 | 10 |
| Other lung cancer histology 1 | 14 | 5.8 |
| Clinical history | ||
| Thorax radiation before FDG-PET/CT | 63 | 26.3 |
| Lung operation | 23 | 9.6 |
| Smoking history | ||
| Nicotine consumption | 198 | 82.5 |
| in female patients | 69 | 75.8 |
| in male patients | 129 | 86.6 |
| Comorbidities | ||
| COPD | 57 | 23.8 |
| Pericardial effusion | 15 | 6.3 |
| Pleural effusion | 57 | 23.8 |
| Diabetes mellitus type II | 36 | 15.1 |
| Coronary heart disease | 34 | 14.2 |
| PET/CT Scanner | ||
| GE Discovery 690 | 137 | 57.1 |
| Siemens Biograph 20 | 42 | 17.5 |
| Siemens Biograph 40 | 19 | 7.9 |
| Siemens Biograph 64 | 14 | 5.8 |
| Philips Guardian Body | 12 | 5 |
| Other scanners | 16 | 6.7 |
| Variable | Sex | Age | BMI | Smoking | PY | COPD | Stage | OP | Rad | PE | PCE | DM | CHD | Hb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUVMAX | ||||||||||||||
| whole lung | + | - | - | |||||||||||
| upper lung | + | - | - | |||||||||||
| lower lung | + | - | - | |||||||||||
| TFL | + | - | ||||||||||||
| SUVMEAN | ||||||||||||||
| whole lung | + | - | - | |||||||||||
| upper lung | + | - | - | - | ||||||||||
| lower lung | + | - | - | |||||||||||
| TFL | + | - | ||||||||||||
| SUV95 | ||||||||||||||
| whole lung | + | - | - | |||||||||||
| upper lung | + | - | - | |||||||||||
| lower lung | + | - | - | |||||||||||
| TFL | + | - | ||||||||||||
| SUVMEAN lung/liver | ||||||||||||||
| whole lung | + | - | - | |||||||||||
| upper lung | + | - | - | - | ||||||||||
| lower lung | + | - | ||||||||||||
| TFL | + | - | ||||||||||||
| SUVMEAN lung/blood pool | ||||||||||||||
| whole lung | + | - | - | |||||||||||
| upper lung | + | - | - | - | ||||||||||
| lower lung | + | - | ||||||||||||
| TFL | + | - | ||||||||||||
 | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehlte, L.; Walter, J.; Daisenberger, L.; Kuhnle, F.; Ingenerf, M.; Schmid-Tannwald, C.; Brendel, M.; Kauffmann-Guerrero, D.; Heinzerling, L.; Tufman, A.; et al. The Association between the Body Mass Index, Chronic Obstructive Pulmonary Disease and SUV of the Non-Tumorous Lung in the Pretreatment [18F]FDG-PET/CT of Patients with Lung Cancer. Diagnostics 2024, 14, 1139. https://doi.org/10.3390/diagnostics14111139
Wehlte L, Walter J, Daisenberger L, Kuhnle F, Ingenerf M, Schmid-Tannwald C, Brendel M, Kauffmann-Guerrero D, Heinzerling L, Tufman A, et al. The Association between the Body Mass Index, Chronic Obstructive Pulmonary Disease and SUV of the Non-Tumorous Lung in the Pretreatment [18F]FDG-PET/CT of Patients with Lung Cancer. Diagnostics. 2024; 14(11):1139. https://doi.org/10.3390/diagnostics14111139
Chicago/Turabian StyleWehlte, Lukas, Julia Walter, Lea Daisenberger, Felix Kuhnle, Maria Ingenerf, Christine Schmid-Tannwald, Matthias Brendel, Diego Kauffmann-Guerrero, Lucie Heinzerling, Amanda Tufman, and et al. 2024. "The Association between the Body Mass Index, Chronic Obstructive Pulmonary Disease and SUV of the Non-Tumorous Lung in the Pretreatment [18F]FDG-PET/CT of Patients with Lung Cancer" Diagnostics 14, no. 11: 1139. https://doi.org/10.3390/diagnostics14111139
APA StyleWehlte, L., Walter, J., Daisenberger, L., Kuhnle, F., Ingenerf, M., Schmid-Tannwald, C., Brendel, M., Kauffmann-Guerrero, D., Heinzerling, L., Tufman, A., Pfluger, T., & Völter, F. (2024). The Association between the Body Mass Index, Chronic Obstructive Pulmonary Disease and SUV of the Non-Tumorous Lung in the Pretreatment [18F]FDG-PET/CT of Patients with Lung Cancer. Diagnostics, 14(11), 1139. https://doi.org/10.3390/diagnostics14111139









