The Role of Routine Electroencephalography in the Diagnosis of Seizures in Medical Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Ethics Statement
2.3. Reasons for Admission to the MICU
2.4. Indications for rEEG
2.5. Interpretation of EEG Patterns
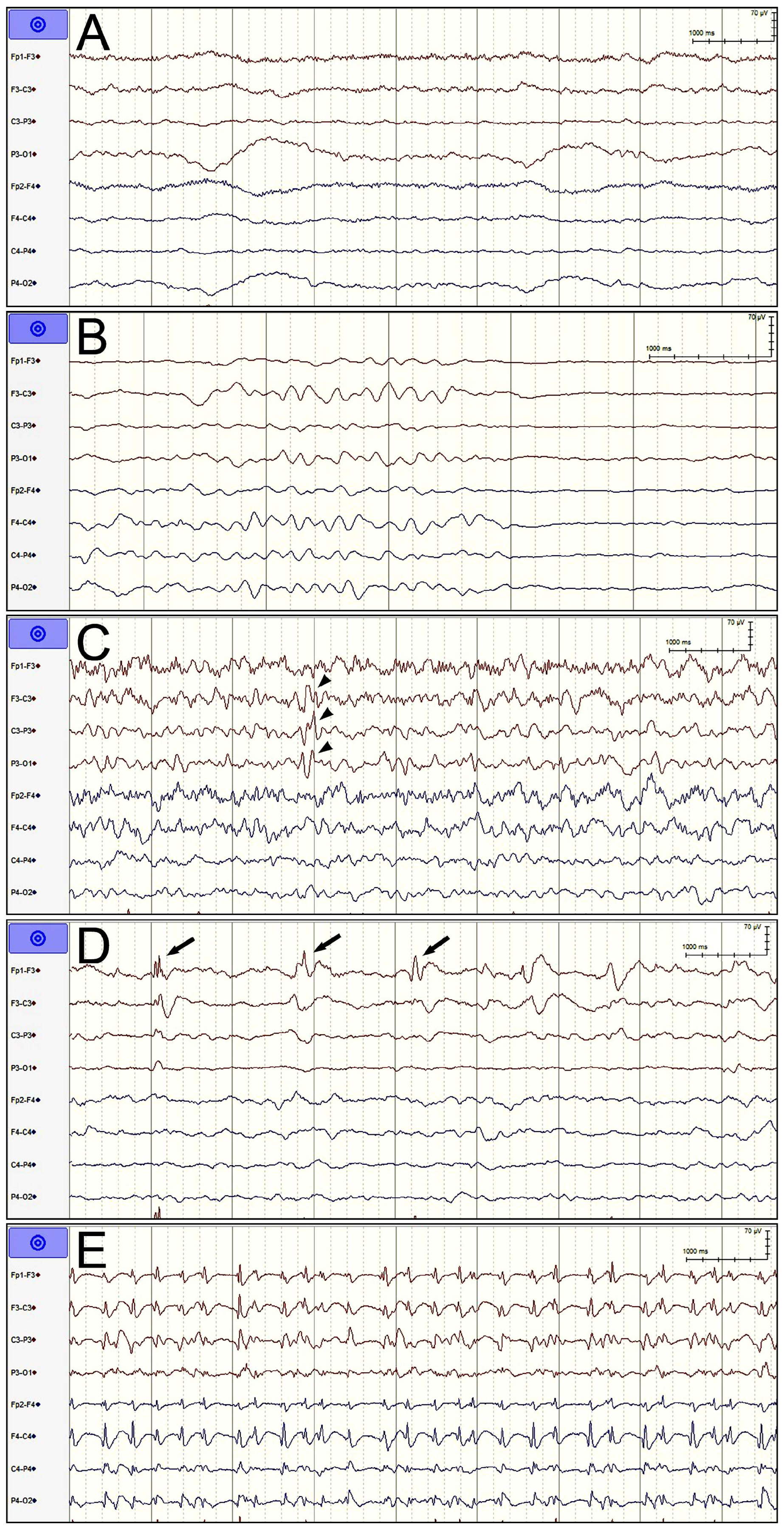
- Nonspecific slow wave: NSW indicated that no epileptic discharge met the ACNS requirements throughout the entire EEG recording except for slow waves and that the duration of suppression (amplitude < 10 μV) or attenuation (amplitude > 10 μV but less than half of the background amplitude) was less than 50% of the entire EEG recording duration.
- Suppression or burst suppression: We combined suppression and burst suppression/attenuation into a single category. The duration of suppression or attenuation was required to account for at least 50% of the total recording time. The burst duration was required to be longer than 0.5 s and for more than three phases [16]. This pattern usually indicates severe cortical dysfunction, which can result from being administered general anesthesia, having hypothermia or diffuse cortical damage, or being in a deep coma.
- Sporadic epileptiform discharges: Spikes, polyspikes, or sharp waves that met the definitions given by the ACNS but did not meet the definition of RPPs, ESz, or electroclinical seizures were interpreted as sporadic discharges regardless of EEG background [16]. These EEG findings often indicate cortical excitability, although the correlation between these EEG findings and epileptic seizures in unconscious patients is somewhat weak.
- Rhythmic and periodic patterns: Periodic discharges, rhythmic delta activity, and spike-and-wave or sharp-and-wave were all classified as RPPs and divided into generalized, lateralized, bilateral independent, unilateral independent, and multifocal patterns on the basis of where they occurred [16]. This type of EEG pattern has different correlations with epileptic seizures depending on where the pattern is detected, the morphology and frequency of the pattern, and whether the signal has plus modifiers (i.e., rhythmic, fast, or sharp waves superimposed on the RPP pattern). Lateralization, periodic discharges, higher frequency, and the combination of plus modifiers exhibit a higher correlation with epileptic seizures.
- Electrographic seizures: This type of epileptic seizure was diagnosed if epileptiform discharges averaging >2.5 Hz for ≥10 s or any pattern with the same definite evolution and lasting ≥10 s were detected. ESz lasting for ≥10 continuous min or for a total duration of ≥20% of any 60 min period of recording were defined as electrographic-status epilepticus and classified as ESz in our analysis [16].
2.6. Outcome Evaluation
2.7. Statistical Analysis
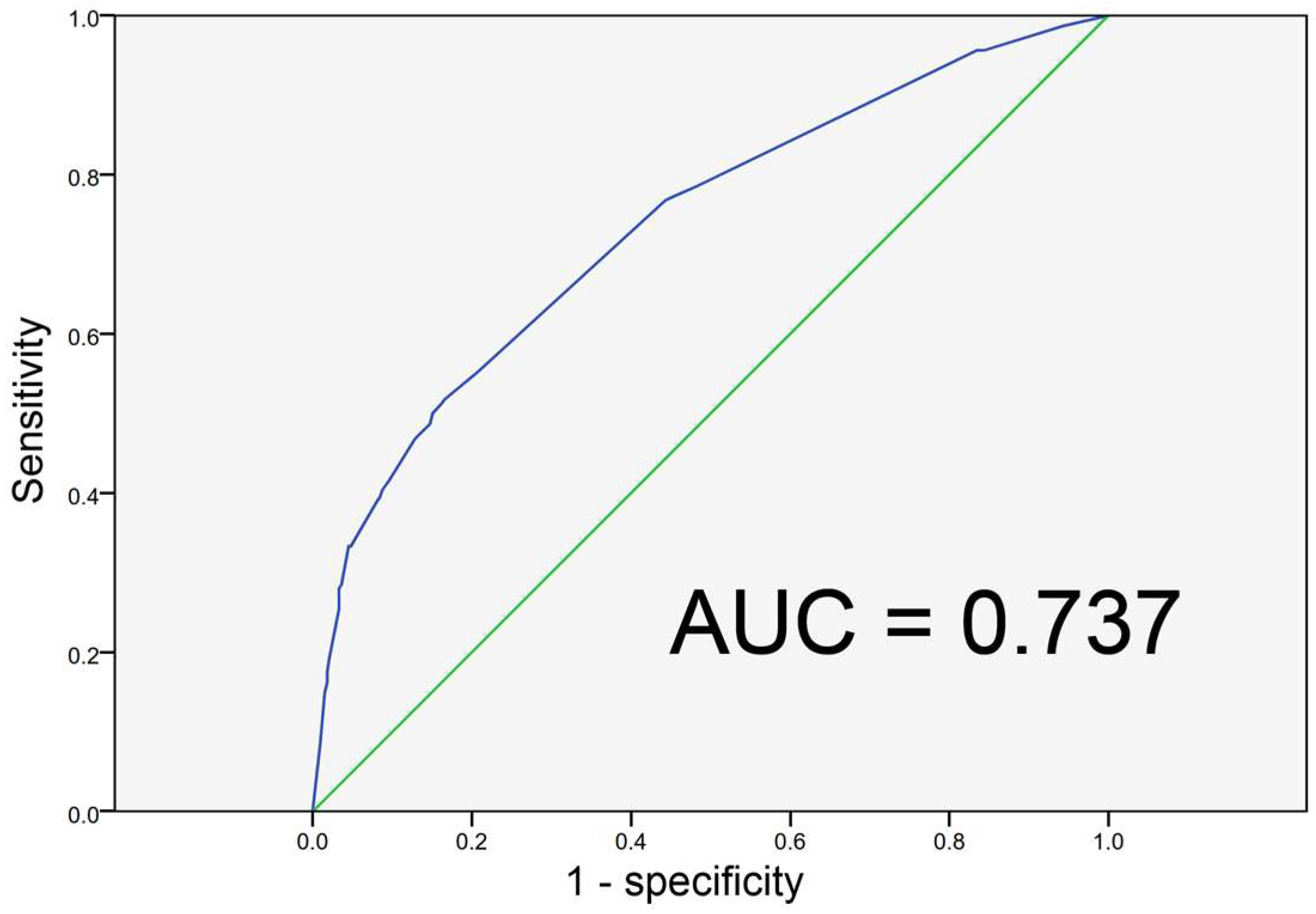
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panteliadis, C.P. Historical overview of electroencephalography: From antiquity to the beginning of the 21st century. J. Brain Neurol. Disord. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Benbadis, S.R. What type of EEG (or EEG-video) does your patient need? Expert Rev. Neurother. 2015, 15, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Hilkman, D.M.W.; van Mook, W.N.K.A.; Mess, W.H.; van Kranen-Mastenbroek, V.H.J.M. The use of continuous EEG monitoring in intensive care units in the Netherlands: A national durvey. Neurocrit. Care 2018, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Carrera, E.; Claassen, J.; Mayer, S.A.; Hirsch, L.J. Continuous electroencephalography in the medical intensive care unit. Crit. Care Med. 2009, 37, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Betjemann, J.P.; Navi, B.B.; Hegde, M.; Meisel, K.; Douglas, V.C.; Josephson, S.A. Diagnostic yield of electroencephalography in the medical and surgical intensive care unit. Neurocrit. Care 2013, 19, 336–341. [Google Scholar] [CrossRef]
- Limotai, C.; Ingsathit, A.; Thadanipon, K.; McEvoy, M.; Attia, J.; Thakkinstian, A. How and whom to monitor for seizures in an ICU: A systematic review and meta-analysis. Crit. Care Med. 2019, 47, e366–e373. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Coppler, P.J.; Solanki, P.; Westover, M.B.; Struck, A.F.; Baldwin, M.E.; Kurz, M.C.; Callaway, C.W. Sensitivity of continuous electroencephalography to detect ictal activity after cardiac arrest. JAMA Netw. Open 2020, 3, e203751. [Google Scholar] [CrossRef]
- Fenter, H.; Rossetti, A.O.; Beuchat, I. Continuous versus routine electroencephalography in the intensive care unit: A review of current evidence. Eur. Neurol. 2024, 87, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hilkman, D.M.; van Mook, W.N.; van Kranen-Mastenbroek, V.H. Continuous electroencephalographic-monitoring in the ICU: An overview of current strengths and future challenges. Curr. Opin Anaesthesiol. 2017, 30, 192–199. [Google Scholar] [CrossRef]
- Hill, C.E.; Blank, L.J.; Thibault, D.; Davis, K.A.; Dahodwala, N.; Litt, B.; Willis, A.W. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology 2019, 92, e9–e18. [Google Scholar] [CrossRef]
- Wagner, A.S.; Semmlack, S.; Frei, A.; Rüegg, S.; Marsch, S.; Sutter, R. Seizures and risks for recurrence in critically ill patients: An observational cohort study. J. Neurol. 2022, 269, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.; Taccone, F.S.; Horn, P.; Holtkamp, M.; Stocchetti, N.; Oddo, M. Neurointensive Care Section of the European Society of Intensive Care Medicine. Recommendations on the use of EEG monitoring in critically ill patients: Consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013, 39, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.T.; Abend, N.S.; Bleck, T.P.; Chapman, K.E.; Drislane, F.W.; Emerson, R.G.; Gerard, E.E.; Hahn, C.D.; Husain, A.M.; Kaplan, P.W.; et al. Consensus statement on continuous EEG in critically ill adults and children, part I: Indications. J. Clin. Neurophysiol. 2015, 32, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Claassen, J.; Hirsch, L.J. Continuous electroencephalogram monitoring in the intensive care unit. Anesth. Analg. 2009, 109, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Waak, M.; Laing, J.; Nagarajan, L.; Lawn, N.; Harvey, A.S. Continuous electroencephalography in the intensive care unit: A critical review and position statement from an Australian and New Zealand perspective. Crit. Care Resusc. 2023, 25, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.J.; Fong, M.W.K.; Leitinger, M.; LaRoche, S.M.; Beniczky, S.; Abend, N.S.; Lee, J.W.; Wusthoff, C.J.; Hahn, C.D.; Westover, M.B.; et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J. Clin. Neurophysiol. 2021, 38, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Benbadis, S.R.; Beniczky, S.; Bertram, E.; MacIver, S.; Moshé, S.L. The role of EEG in patients with suspected epilepsy. Epilept. Disord. 2020, 22, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Hoedemaekers, C.; Hofmeijer, J.; Horn, J. Value of EEG in outcome prediction of hypoxic-ischemic brain injury in the ICU: A narrative review. Resuscitation 2023, 189, 109900. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.; Rüegg, S.; Kaplan, P.W. Epidemiology, diagnosis, and management of nonconvulsive status epilepticus: Opening Pandora’s box. Neurol. Clin. Pract. 2012, 2, 275–286. [Google Scholar] [CrossRef]
- Privitera, M.; Hoffman, M.; Moore, J.L.; Jester, D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994, 18, 155–166. [Google Scholar] [CrossRef]
- Towne, A.R.; Waterhouse, E.J.; Boggs, J.G.; Garnett, L.K.; Brown, A.J.; Smith, J.R.; DeLorenzo, R.J., Jr. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 2000, 54, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Rudin, D.; Grize, L.; Schindler, C.; Marsch, S.; Rüegg, S.; Sutter, R. High prevalence of nonconvulsive and subtle status epilepticus in an ICU of a tertiary care center: A three-year observational cohort study. Epilepsy Res. 2011, 96, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.O.; Schindler, K.; Sutter, R.; Rüegg, S.; Zubler, F.; Novy, J.; Oddo, M.; Warpelin-Decrausaz, L.; Alvarez, V. Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure: A multicenter randomized clinical trial. JAMA Neurol. 2020, 77, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.; Abel, J.H.; Schamberg, G.; Brown, E.N. Etiology of burst suppression EEG patterns. Front. Psychol. 2021, 12, 673529. [Google Scholar] [CrossRef]
- Westhall, E.; Rossetti, A.O.; van Rootselaar, A.F.; Wesenberg Kjaer, T.; Horn, J.; Ullén, S.; Friberg, H.; Nielsen, N.; Rosén, I.; Åneman, A.; et al. Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 2016, 86, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, B.J.; Tjepkema-Cloostermans, M.C.; Tromp, S.C.; van den Bergh, W.M.; Foudraine, N.A.; Kornips, F.H.M.; Drost, G.; Scholten, E.; Bosch, F.H.; Beishuizen, A.; et al. Early electroencephalography for outcome prediction of postanoxic coma: A prospective cohort study. Ann. Neurol. 2019, 86, 203–214. [Google Scholar] [CrossRef]
- Lybeck, A.; Friberg, H.; Aneman, A.; Hassager, C.; Horn, J.; Kjærgaard, J.; Kuiper, M.; Nielsen, N.; Ullén, S.; Wise, M.P.; et al. Prognostic significance of clinical seizures after cardiac arrest and target temperature management. Resuscitation 2017, 114, 146–151. [Google Scholar] [CrossRef]
- Barbella, G.; Lee, J.W.; Alvarez, V.; Novy, J.; Oddo, M.; Beers, L.; Rossetti, A.O. Prediction of regaining consciousness despite an early epileptiform EEG after cardiac arrest. Neurology 2020, 94, e1675–e1683. [Google Scholar] [CrossRef]
- Koren, J.; Herta, J.; Draschtak, S.; Pötzl, G.; Pirker, S.; Fürbass, F.; Hartmann, M.; Kluge, T.; Baumgartner, C. Prediction of rhythmic and periodic EEG patterns and seizures on continuous EEG with early epileptiform discharges. Epilepsy Behav. 2015, 49, 286–289. [Google Scholar] [CrossRef]
- Koren, J.; Herta, J.; Draschtak, S.; Pötzl, G.; Fürbass, F.; Hartmann, M.; Kluge, T.; Gruber, A.; Baumgartner, C. Early Epileptiform Discharges and Clinical Signs Predict Nonconvulsive Status Epilepticus on Continuous EEG. Neurocrit. Care 2018, 29, 388–395. [Google Scholar] [CrossRef]
- Witsch, J.; Frey, H.P.; Schmidt, J.M.; Velazquez, A.; Falo, C.M.; Reznik, M.; Roh, D.; Agarwal, S.; Park, S.; Connolly, E.S.; et al. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol. 2017, 74, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Ruiz, A.; Vlachy, J.; Lee, J.W.; Gilmore, E.J.; Ayer, T.; Haider, H.A.; Gaspard, N.; Ehrenberg, J.A.; Tolchin, B.; Fantaneanu, T.A.; et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017, 74, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Struck, A.F.; Ustun, B.; Ruiz, A.R.; Lee, J.W.; LaRoche, S.M.; Hirsch, L.J.; Gilmore, E.J.; Vlachy, J.; Haider, H.A.; Rudin, C.; et al. Association of an Electroencephalography-Based Risk Score With Seizure Probability in Hospitalized Patients. JAMA Neurol. 2017, 74, 1419–1424. [Google Scholar] [CrossRef]
- Zawar, I.; Briskin, I.; Hantus, S. Risk factors that predict delayed seizure detection on continuous electroencephalogram (cEEG) in a large sample size of critically ill patients. Epilepsia Open 2022, 7, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.H. EEG in ICU: A monitoring tool for critically ill patient. Bangladesh Crit. Care J. 2014, 2, 28–34. [Google Scholar] [CrossRef]
- Azabou, E.; Magalhaes, E.; Braconnier, A.; Yahiaoui, L.; Moneger, G.; Heming, N.; Annane, D.; Mantz, J.; Chrétien, F.; Durand, M.C.; et al. Early standard electroencephalogram abnormalities predict mortality in septic intensive care unit patients. PLoS ONE 2015, 10, e0139969. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 560) | Clinical Seizure | p Value | |
|---|---|---|---|---|
| Yes (n = 219) | No (n = 341) | |||
| Age (years) | 74 (63–84) | 71 (60–81) | 77 (64–85) | 0.0003 |
| Gender (Male) | 281 (50%) | 110 (50%) | 171 (50%) | >0.9999 |
| GCS score | 8 (5–10) | 8 (4–10) | 8 (5–10) | 0.4933 |
| Etiology of illness | 0.2676 | |||
| Respiratory failure | 185 (33%) | 63 (29%) | 122 (36%) | |
| Cardiogenic diseases | 139 (25%) | 56 (26%) | 83 (24%) | |
| Sepsis/septic shock | 126 (23%) | 50 (23%) | 76 (22%) | |
| Other disorders | 110 (20%) | 50 (237%) | 60 (1927%) | |
| Seizure-related rEEG | 265 (47%) | 205 (94%) | 60 (18%) | <0.0001 |
| rEEG patterns | <0.0001 | |||
| NSW | 325 (58%) | 90 (41%) | 235 (69%) | |
| SBS | 69 (12%) | 20 (9%) | 49 (14%) | |
| SED | 54 (10%) | 27 (12%) | 27 (8%) | |
| RPPs | 90 (16%) | 63 (29%) | 27 (8%) | |
| ESz | 22 (4%) | 19 (9%) | 3 (1%) | |
| Seizure within 24 h after rEEG | 82 (15%) | 82 (37%) | 0 (0%) | <0.0001 |
| Seizure after rEEG | 105 (19%) | 105 (48%) | 0 (0%) | <0.0001 |
| Post-rEEG ASM | 290 (52%) | 194 (89%) | 96 (28%) | <0.0001 |
| Repeated rEEG | 91 (16%) | 64 (29%) | 27 (8%) | <0.0001 |
| GOS score | 3 (1–3) | 3 (1–3) | 3 (1–4) | 0.3870 |
| Unfavorable outcome | 430 (77%) | 175 (80%) | 255 (75%) | 0.1826 |
| Death | 228 (41%) | 92 (42%) | 136 (40%) | 0.6596 |
| EEG Patterns | Respiratory Failure (n = 185) | Cardiovascular Diseases (n = 139) | Sepsis/Septic Shock (n = 126) | Other Disorders (n = 110) | p Value |
|---|---|---|---|---|---|
| Age (years) | 78 (67–86) | 71 (60–83) | 74 (66–83) | 68 (57–82) | 0.0012 |
| Male gender | 86 (56%) | 73 (53%) | 64 (51%) | 58 (53%) | 0.6553 |
| GCS score | 8 (6–10) | 4 (3–7) | 8 (7–10) | 10 (8–14) | <0.0001 |
| rEEG patterns | <0.0001 | ||||
| NSW | 120 (65%) | 48 (35%) | 81 (64%) | 76 (69%) | |
| SBS | 10 (5%) | 49 (35%) | 10 (8%) | 0 (0%) | |
| SED | 18 (10%) | 10 (7%) | 15 (12%) | 11 (10%) | |
| RPPs | 32 (17%) | 24 (17%) | 15 (12%) | 19 (17%) | |
| ESz | 5 (3%) | 8 (6%) | 5 (4%) | 4 (4%) | |
| Seizure-related rEEG | 91 (49%) | 62 (45%) | 58 (46%) | 54 (49%) | 0.8272 |
| Clinical seizure | 63 (34%) | 56 (40%) | 50 (40%) | 50 (45%) | 0.2671 |
| Seizure within 24 h after rEEG | 23 (12%) | 27 (19%) | 21 (17%) | 11 (10%) | 0.1342 |
| Seizure after rEEG | 32 (17%) | 32 (23%) | 27 (21%) | 14 (13%) | 0.1623 |
| Post-rEEG ASM | 88 (48%) | 78 (56%) | 67 (53%) | 57 (52%) | 0.4827 |
| GOS score | 3 (1–3) | 1 (1–3) | 3 (1–3) | 3 (2–4) | <0.0001 |
| Unfavorable outcome | 152 (82%) | 123 (88%) | 97 (77%) | 58 (53%) | <0.0001 |
| Death | 65 (35%) | 89 (64%) | 48 (38%) | 26 (24%) | <0.0001 |
| Characteristics | NSW (n = 325) | SBS (n = 69) | SED (n = 54) | RPPs (n = 90) | ESz (n = 22) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 74 (63–84) | 70 (61–83) | 73 (58–85) | 77 (66–86) | 71 (63–83) | 0.2507 |
| Male gender | 175 (54%) | 36 (52%) | 25 (46%) | 34 (38%) | 11 (50%) | 0.1001 |
| GCS score | 9 (7–11) | 3 (3–4) | 9 (7–10) | 7 (4–9) | 5 (4–9) | <0.0001 |
| Etiology of illness | <0.0001 | |||||
| Respiratory failure | 120 (37%) | 10 (14%) | 18 (33%) | 32 (36%) | 5 (23%) | |
| Cardiovascular diseases | 48 (15%) | 49 (71%) | 10 (19%) | 24 (27%) | 8 (36%) | |
| Sepsis/septic shock | 81(25%) | 10 (14%) | 15 (28%) | 15(17%) | 5 (23%) | |
| Other disorders | 76 (23%) | 0 (0%) | 11 (20%) | 19 (21%) | 4 (18%) | |
| Seizure-related rEEG | 135 (42%) | 24 (35%) | 30 (56%) | 62 (69%) | 14 (64%) | <0.0001 |
| Clinical seizure | 90 (28%) | 20 (29%) | 27 (50%) | 63 (70%) | 19 (86%) | <0.0001 |
| Seizure within 24 h after rEEG | 10 (3%) | 7 (10%) | 13 (24%) | 36 (40%) | 16 (73%) | <0.0001 |
| Seizure after rEEG | 21 (6%) | 9 (13%) | 15 (28%) | 42 (47%) | 18 (82%) | <0.0001 |
| Post-rEEG ASM | 122 (36%) | 34 (49%) | 41 (76%) | 76 (84%) | 17 (77%) | <0.0001 |
| Repeated rEEG | 15 (5%) | 9 (13%) | 14 (26%) | 39 (43%) | 14 (64%) | <0.0001 |
| GOS score | 3 (1–4) | 1 (1–1) | 3 (1–3) | 1 (1–3) | 2 (1–3) | <0.0001 |
| Unfavorable outcome | 225 (69%) | 66 (96%) | 38 (70%) | 82 (91%) | 19 (86%) | <0.0001 |
| Death | 103 (32%) | 56 (81%) | 15 (28%) | 46 (51%) | 8 (36%) | <0.0001 |
| Characteristics | Seizure within 24 h after rEEG | Seizure after rEEG | ||||
|---|---|---|---|---|---|---|
| Yes (n = 82) | No (n = 478) | p Value | Yes (n = 105) | No (n = 455) | p Value | |
| Etiology of illness | 0.1342 | 0.1623 | ||||
| Respiratory failure | 23 (28%) | 162 (34%) | 32 (30%) | 153 (34%) | ||
| Cardiogenic diseases | 27 (33%) | 112 (23%) | 32 (30%) | 107 (24%) | ||
| Sepsis/septic shock | 21 (26%) | 105 (22%) | 27 (26%) | 99 (22%) | ||
| Other disorders | 11 (13%) | 99 (21%) | 14 (13%) | 96 (21%) | ||
| GCS score | 6 (3–8) | 8 (6–10) | <0.0001 | 7 (4–9) | 8 (5–10) | 0.0002 |
| Seizure-related rEEG | 75 (91%) | 190 (40%) | <0.0001 | 91 (87%) | 174 (38%) | <0.0001 |
| Post-rEEG ASM | 80 (98%) | 210 (44%) | 0.0274 | 100 (95%) | 190 (42%) | <0.0001 |
| rEEG patterns | <0.0001 | <0.0001 | ||||
| NSW | 10 (12%) | 315 (66%) | 21 (20%) | 304 (67%) | ||
| SBS | 7 (9%) | 62 (13%) | 9 (9%) | 60 (13%) | ||
| SED | 13 (16%) | 41 (9%) | 15 (14%) | 39 (9%) | ||
| RPPs | 36 (44%) | 54 (11%) | 42 (40%) | 48 (11%) | ||
| ESz | 16 (20%) | 6 (1%) | 18 (17%) | 4 (1%) | ||
| Repeated rEEG | 42 (51%) | 49 (10%) | <0.0001 | 52 (50%) | 39 (9%) | <0.0001 |
| GOS score | 1 (1–3) | 3 (1–4) | 0.0010 | 2 (1–3) | 3 (1–4) | 0.0008 |
| Unfavorable outcome | 77 (94%) | 353 (74%) | <0.0001 | 98 (93%) | 332 (73%) | <0.0001 |
| Death | 42 (51%) | 186 (39%) | 0.0361 | 50 (48%) | 178 (39%) | 0.1101 |
| Unfavorable Outcome (n = 430) | Death (n = 228) | ||||
|---|---|---|---|---|---|
| Characteristics | Odds Ratio (95% CI) | p Value | Characteristics | Odds Ratio (95% CI) | p Value |
| Age > 68 years | 3.542 (2.172–5.776) | <0.0001 | Age > 59 years | 2.150 (1.291–3.581) | 0.0032 |
| Male gender | 0.915 (0.565–1.482) | 0.7180 | Male gender | 1.581 (1.072–2.331) | 0.0207 |
| Etiology of illness a | Etiology of illness a | ||||
| Sepsis/septic shock | 1.986 (1.037–3.804) | 0.0384 | Sepsis/septic shock | 1.572 (0.862–2.866) | 0.1401 |
| Respiratory failure | 2.805 (1.529–5.146) | 0.0009 | Respiratory failure | 1.335 (0.762–2.341) | 0.3126 |
| Cardiovascular diseases | 3.005 (1.396–6.470) | 0.0049 | Cardiovascular diseases | 2.447 (1.289–4.645) | 0.0062 |
| GCS ≤ 9 | 4.536 (2.795–7.359) | <0.0001 | GCS ≤ 5 | 3.021 (1.827–4.993) | <0.0001 |
| rEEG Pattern b | rEEG Pattern b | ||||
| SBS | 4.000 (1.115–14.353) | 0.0335 | SBS | 3.324 (1.555–7.107) | 0.0019 |
| SED | 0.860 (0.413–1.789) | 0.6859 | SED | 0.856 (0.433–1.693) | 0.6551 |
| RPPs | 3.383 (1.360–8.416) | 0.0087 | RPPs | 1.654 (0.940–2.911) | 0.0810 |
| ESz | 1.183 (0.254–5.516) | 0.8307 | ESz | 0.522 (0.172–1.583) | 0.2505 |
| Seizure within 24 h after rEEG | 4.260 (1.335–13.593) | 0.0143 | Seizure within 24 h after rEEG | 1.316 (0.701–2.470) | 0.3928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-L.; Chen, P.-Y.; Chen, I.-A.; Lin, S.-K. The Role of Routine Electroencephalography in the Diagnosis of Seizures in Medical Intensive Care Units. Diagnostics 2024, 14, 1111. https://doi.org/10.3390/diagnostics14111111
Hsiao C-L, Chen P-Y, Chen I-A, Lin S-K. The Role of Routine Electroencephalography in the Diagnosis of Seizures in Medical Intensive Care Units. Diagnostics. 2024; 14(11):1111. https://doi.org/10.3390/diagnostics14111111
Chicago/Turabian StyleHsiao, Cheng-Lun, Pei-Ya Chen, I-An Chen, and Shinn-Kuang Lin. 2024. "The Role of Routine Electroencephalography in the Diagnosis of Seizures in Medical Intensive Care Units" Diagnostics 14, no. 11: 1111. https://doi.org/10.3390/diagnostics14111111
APA StyleHsiao, C.-L., Chen, P.-Y., Chen, I.-A., & Lin, S.-K. (2024). The Role of Routine Electroencephalography in the Diagnosis of Seizures in Medical Intensive Care Units. Diagnostics, 14(11), 1111. https://doi.org/10.3390/diagnostics14111111






