Revolutionizing Cardiology through Artificial Intelligence—Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment—A Comprehensive Review of the Past 5 Years
Abstract
1. Introduction
2. Literature Review
2.1. Methodology
- Studies examining the application of artificial intelligence in various branches of cardiology, such as arrhythmology, emergency cardiology, cardiomyopathies, cardiovascular imaging, congenital cardiovascular disease, electrocardiography, heart failure, heart transplantation, hypertension, pulmonary hypertension, infective endocarditis, ischemic heart disease, pericardial disease, peripheral heart disease, thromboembolic disease, and valvular diseases (this is a broad selection criterion focusing on the theme of studies relevant to the proposed review and represents the main topic of the article);
- Publications in English;
- Published within the last 5 years, between 2020 and 2024 (this temporal restriction ensures the timeliness and relevance of the information included in the review);
- Patient batches that included both adults and children (this criterion ensured a larger batch of studies covering cardiology);
- Studies in the form of an academic journal article.
- Articles in languages other than English;
- Retracted studies (eliminating retracted studies is essential to maintain the integrity and credibility of this review);
- Applications of artificial intelligence regarding technical functionality data of algorithms (excluding these studies may be justified to focus on the practical and clinical application of artificial intelligence in cardiology, rather than the technical aspects of algorithms);
- Studies in the form of posters, short papers, or only abstracts;
- Duplicate studies;
- Studies with a title and abstract that do not match the review topic.
2.2. Results
2.2.1. AI in Arrhythmias
2.2.2. AI in Cardiogenic Shock
2.2.3. AI in Cardiomyopathy
2.2.4. AI in Cardiovascular Imaging
2.2.5. AI in Congenital Heart Disease
2.2.6. AI in Electrocardiography
2.2.7. AI in Heart Failure
2.2.8. AI in Heart Transplant
2.2.9. AI in Hypertension
2.2.10. AI in Pulmonary Hypertension
2.2.11. AI in Infective Endocarditis
2.2.12. AI in Ischemic Heart Disease
2.2.13. AI in Pericardial Disease
2.2.14. AI in Peripheral Arterial Disease
2.2.15. AI in Thromboembolic Disease
2.2.16. AI in Valvular Disease
3. Discussion
3.1. Perspectives and Directions for the Application of Artificial Intelligence in Cardiology
3.1.1. Prevention
3.1.2. Screening
3.1.3. Diagnosis
3.1.4. Treatment
3.2. Ethical Considerations of AI in Cardiology
3.3. Bias Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACM | arrhythmogenic cardiomyopathy |
| ACR | acute cellular rejection |
| ACS | acute coronary syndrome |
| AF | atrial fibrillation |
| AI | artificial intelligence |
| AI-QCT | artificial intelligence-enabled quantitative coronary computed tomography |
| AMI | acute myocardial infarction |
| ARVD | arrhythmogenic heart disease |
| ATTR-CM | transthyretin amyloid cardiomyopathy |
| CCTA | coronary computed tomography angiography |
| CMR | cardiovascular magnetic resonance |
| CNN | convolutional neural network |
| DL | deep learning |
| DCM | dilated cardiomyopathy |
| DECT | dual-energy computed tomography |
| ECG | electrocardiogram |
| FFR | fractional flow reserve |
| HCA | hierarchical |
| KMCk | means clustering |
| IABP | intra-aortic balloon pump |
| ICA | invasive coronary angiography |
| IE | infective endocarditis |
| LA | left atrium |
| LAAT | left atrial appendage thrombus |
| LCA | latent class analysis |
| LV | left ventricle |
| LVH | left ventricular hypertrophy |
| MACE | major adverse cardiovascular events |
| MAPSE | mitral annular plane systolic excursion |
| MI | myocardial infarction |
| ML | machine learning |
| MLP | multiple layer perceptron |
| MRI | magnetic resonance imagining |
| PAP | pulmonary artery pressure |
| PCAT | per coronary adipose tissue |
| PPG | photoplethysmography |
| QCA | quantitative coronary angiography |
| RA | right atrium |
| RV | right ventricle |
| STEMI | ST-elevation myocardial infarction |
| TAVR | transcatheter aortic valve replacement |
| TTE | transthoracic echocardiography |
| TOE | transesophageal echocardiography |
| TCN | temporal convolutional network |
| XCB | machine learning model based on the xgboost |
References
- Beam, A.L.; Drazen, J.M.; Kohane, I.S.; Leong, T.-Y.; Manrai, A.K.; Rubin, E.J. Artificial Intelligence in Medicine. N. Engl. J. Med. 2023, 388, 1220–1221. [Google Scholar] [CrossRef]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Turco, J.V.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J Am Coll Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Gala, D.; Behl, H.; Shah, M.; Makaryus, A.N. The Role of Artificial Intelligence in Improving Patient Outcomes and Future of Healthcare Delivery in Cardiology: A Narrative Review of the Literature. Healthcare 2024, 12, 481. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Y.; Yang, Q.; Huo, T. Artificial Intelligence in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. J. Med. Res. 2023, 28, 242. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Shehab, M.; Abualigah, L.; Shambour, Q.; Abu-Hashem, M.A.; Shambour, M.K.Y.; Alsalibi, A.I.; Gandomi, A.H. Machine Learning in Medical Applications: A Review of State-of-the-Art Methods. Comput. Biol. Med. 2022, 145, 105458. [Google Scholar] [CrossRef]
- Yoon, C.H.; Torrance, R.; Scheinerman, N. Machine Learning in Medicine: Should the Pursuit of Enhanced Interpretability Be Abandoned? J. Med. Ethics 2022, 48, 581–585. [Google Scholar] [CrossRef]
- Kahr, M.; Kovács, G.; Loinig, M.; Brückl, H. Condition Monitoring of Ball Bearings Based on Machine Learning with Synthetically Generated Data. Sensors 2022, 22, 2490. [Google Scholar] [CrossRef]
- Mosqueira-Rey, E.; Hernández-Pereira, E.; Alonso-Ríos, D.; Bobes-Bascarán, J.; Fernández-Leal, Á. Human-in-the-Loop Machine Learning: A State of the Art. Artif. Intell. Rev. 2023, 56, 3005–3054. [Google Scholar] [CrossRef]
- Cho, H.; Keenan, G.; Madandola, O.O.; Dos Santos, F.C.; Macieira, T.G.R.; Bjarnadottir, R.I.; Priola, K.J.B.; Dunn Lopez, K. Assessing the Usability of a Clinical Decision Support System: Heuristic Evaluation. JMIR Hum. Factors 2022, 9, e31758. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Giallauria, F.; Carrizzo, A.; Visco, V.; Silverio, A.; Cesaro, A.; Calabrò, P.; De Luca, N.; Mancusi, C.; Masarone, D.; et al. Artificial Intelligence in Cardiovascular Prevention: New Ways Will Open New Doors. J. Cardiovasc. Med. 2023, 24 (Suppl. S2), e106–e115. [Google Scholar] [CrossRef]
- Busnatu, Ș.; Niculescu, A.-G.; Bolocan, A.; Petrescu, G.E.D.; Păduraru, D.N.; Năstasă, I.; Lupușoru, M.; Geantă, M.; Andronic, O.; Grumezescu, A.M.; et al. Clinical Applications of Artificial Intelligence—An Updated Overview. J. Clin. Med. 2022, 11, 2265. [Google Scholar] [CrossRef]
- Van den Eynde, J.; Lachmann, M.; Laugwitz, K.-L.; Manlhiot, C.; Kutty, S. Successfully Implemented Artificial Intelligence and Machine Learning Applications in Cardiology: State-of-the-Art Review. Trends Cardiovasc. Med. 2023, 33, 265–271. [Google Scholar] [CrossRef]
- Visco, V.; Ferruzzi, G.J.; Nicastro, F.; Virtuoso, N.; Carrizzo, A.; Galasso, G.; Vecchione, C.; Ciccarelli, M. Artificial Intelligence as a Business Partner in Cardiovascular Precision Medicine: An Emerging Approach for Disease Detection and Treatment Optimization. Curr. Med. Chem. 2021, 28, 6569–6590. [Google Scholar] [CrossRef]
- Soori, M.; Arezoo, B.; Dastres, R. Machine Learning and Artificial Intelligence in CNC Machine Tools, A Review. Sustain. Manuf. Serv. Econ. 2023, 2, 100009. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Pratap Singh, R.; Suman, R.; Rab, S. Significance of Machine Learning in Healthcare: Features, Pillars and Applications. Int. J. Intell. Netw. 2022, 3, 58–73. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Barratt, J.L.N.; Kennedy, P.J.; Kaufer, A.; Calarco, L.; Ellis, J.T. Machine Learning and Applications in Microbiology. FEMS Microbiol. Rev. 2021, 45, fuab015. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Polat, H. Prediction of Heart Disease Based on Machine Learning Using Jellyfish Optimization Algorithm. Diagnostics 2023, 13, 2392. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Bai, J.; Al-Sabaawi, A.; Santamaría, J.; Albahri, A.S.; Al-dabba, B.S.N.; Fadhel, M.A.; Manoufali, M.; Zhang, J.; Al-Timemy, A.H.; et al. A Survey on Deep Learning Tools Dealing with Data Scarcity: Definitions, Challenges, Solutions, Tips, and Applications. J. Big Data 2023, 10, 46. [Google Scholar] [CrossRef]
- Srivani, M.; Murugappan, A.; Mala, T. Cognitive Computing Technological Trends and Future Research Directions in Healthcare—A Systematic Literature Review. Artif. Intell. Med. 2023, 138, 102513. [Google Scholar] [CrossRef]
- Vinny, P.W.; Vishnu, V.Y.; Padma Srivastava, M.V. Artificial Intelligence Shaping the Future of Neurology Practice. Med. J. Armed Forces India 2021, 77, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Jiang, C.; Wang, X.; Wang, S.; Zheng, H.; Tang, H. Privacy-Preserving Construction of Generalized Linear Mixed Model for Biomedical Computation. Bioinformatics 2020, 36 (Suppl. S1), i128–i135. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.S. Data Analysis of COVID-2019 Epidemic Using Machine Learning Methods: A Case Study of India. Int. J. Inf. Technol. 2020, 12, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Hügle, M.; Omoumi, P.; van Laar, J.M.; Boedecker, J.; Hügle, T. Applied Machine Learning and Artificial Intelligence in Rheumatology. Rheumatol. Adv. Pract. 2020, 4, rkaa005. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pal, T.; Jaiswal, V. Heart Disease Prediction Using Convolutional Neural Network. In Cardiovascular and Coronary Artery Imaging; Elsevier: Amsterdam, The Netherlands, 2022; pp. 245–272. [Google Scholar]
- Teuwen, J.; Moriakov, N. Handbook of Medical Image Computing and Computer Assisted Intervention; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Xiong, Z.; Nash, M.P.; Cheng, E.; Fedorov, V.V.; Stiles, M.K.; Zhao, J. ECG Signal Classification for the Detection of Cardiac Arrhythmias Using a Convolutional Recurrent Neural Network. Physiol. Meas. 2018, 39, 094006. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Layard Horsfall, H.; Funnell, J.P.; Hanrahan, J.G.; Khan, D.Z.; Muirhead, W.; Stoyanov, D.; Marcus, H.J. Artificial Intelligence in Brain Tumour Surgery—An Emerging Paradigm. Cancers 2021, 13, 5010. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, B. Machine learning algorithms-a review. Int. J. Sci. Res. 2020, 9, 381–386. [Google Scholar]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, A.; Khayyat, M.M.; Zamzami, N. Predicting Heart Disease Using Collaborative Clustering and Ensemble Learning Techniques. Appl. Sci. 2023, 13, 13278. [Google Scholar] [CrossRef]
- Sahlab, N.; Sonji, I.; Weyrich, M. Graph-Based Association Rule Learning for Context-Based Health Monitoring to Enable User-Centered Assistance. Artif. Intell. Med. 2023, 135, 102455. [Google Scholar] [CrossRef]
- Kumar, K.A.; Gowri, S.; David, J.J.W.; Bevish Jinila, Y. An Efficient Association Rule Mining from Distributed Medical Database for Predicting Heart Disease. In Proceedings of the 2022 6th International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 29–31 March 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 791–795. [Google Scholar]
- Chaudhuri, A.K.; Das, A.; Addy, M. Identifying the Association Rule to Determine the Possibilities of Cardio Vascular Diseases (CVD). In Advances in Intelligent Systems and Computing; Springer: Singapore, 2021; pp. 219–229. [Google Scholar]
- Tran, K.-V.; Filippaios, A.; Noorishirazi, K.; Ding, E. False Atrial Fibrillation Alerts from Smartwatches Are Associated with Decreased Perceived Physical Well-Being and Confidence in Chronic Symptoms Management. Cardiol. Cardiovasc. Med. 2023, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Baj, G.; Gandin, I.; Scagnetto, A.; Bortolussi, L.; Cappelletto, C.; Di Lenarda, A.; Barbati, G. Comparison of Discrimination and Calibration Performance of ECG-Based Machine Learning Models for Prediction of New-Onset Atrial Fibrillation. BMC Med. Res. Methodol. 2023, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Nguyen, D.D.; Schram, M.; Albert, D.; Gollakota, S.; Shapiro, L.; Sridhar, A.R. Artificial Intelligence–Enabled Mobile Electrocardiograms for Event Prediction in Paroxysmal Atrial Fibrillation. Cardiovasc. Digit. Health J. 2023, 4, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Deng, H.; Liao, H.; Fang, X.; Zhan, X.; Wei, W.; Wu, S.; Xue, Y. An Artificial Intelligence-Enabled ECG Algorithm for Predicting the Risk of Recurrence in Patients with Paroxysmal Atrial Fibrillation after Catheter Ablation. J. Clin. Med. 2023, 12, 1933. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, Z.-Z.; Zhang, G.-G.; Guo, S.-D.; Rivera-Caravaca, J.M.; Wang, Y.-L.; Jin, Y.-Y.; Liu, Y. Validating Scores Predicting Atrial Fibrillation Recurrence Post Catheter Ablation in Patients with Concurrent Atrial Fibrillation and Pulmonary Diseases. Ann. Palliat. Med. 2021, 10, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Finkelstein, N.; Alyakin, A.; Gilotra, N.A.; Trost, J.; Schulman, S.P.; Saria, S. Using Machine Learning for Early Prediction of Cardiogenic Shock in Patients with Acute Heart Failure. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100308. [Google Scholar] [CrossRef]
- Bai, Z.; Hu, S.; Wang, Y.; Deng, W.; Gu, N.; Zhao, R.; Zhang, W.; Ma, Y.; Wang, Z.; Liu, Z.; et al. Development of a Machine Learning Model to Predict the Risk of Late Cardiogenic Shock in Patients with ST-Segment Elevation Myocardial Infarction. Ann. Transl. Med. 2021, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Antonescu, C.; Ravindranath, S.; Dong, J.; Lu, M.; Vicario, F.; Wondrely, L.; Thompson, P.; Swearingen, D.; Acharya, D. Early Prediction of Cardiogenic Shock Using Machine Learning. Front. Cardiovasc. Med. 2022, 9, 862424. [Google Scholar] [CrossRef] [PubMed]
- Jajcay, N.; Bezak, B.; Segev, A.; Matetzky, S.; Jankova, J.; Spartalis, M.; El Tahlawi, M.; Guerra, F.; Friebel, J.; Thevathasan, T.; et al. Data Processing Pipeline for Cardiogenic Shock Prediction Using Machine Learning. Front. Cardiovasc. Med. 2023, 10, 1132680. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Rayfield, C.; Soussi, S.; Berg, D.D.; Kennedy, J.N.; Sinha, S.S.; Baran, D.A.; Brant, E.; Mebazaa, A.; Billia, F.; et al. Machine Learning Approaches for Phenotyping in Cardiogenic Shock and Critical Illness. JACC Adv. 2022, 1, 100126. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Yao, R.; Chen, K.; Xu, Q.; Huang, R.; Mao, Z.; Yu, Y. Identification of Distinct Clinical Phenotypes of Cardiogenic Shock Using Machine Learning Consensus Clustering Approach. BMC Cardiovasc. Disord. 2023, 23, 426. [Google Scholar] [CrossRef] [PubMed]
- Bohm, A.; Jajcay, N.; Jankova, J.; Petrikova, K.; Bezak, B. Artificial Intelligence Model for Prediction of Cardiogenic Shock in Patients with Acute Coronary Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2022, 11 (Suppl. S1), zuac041-077. [Google Scholar] [CrossRef]
- Popat, A.; Yadav, S.; Patel, S.K.; Baddevolu, S.; Adusumilli, S.; Rao Dasari, N.; Sundarasetty, M.; Anand, S.; Sankar, J.; Jagtap, Y.G. Artificial Intelligence in the Early Prediction of Cardiogenic Shock in Acute Heart Failure or Myocardial Infarction Patients: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e50395. [Google Scholar] [CrossRef] [PubMed]
- Rong, F.; Xiang, H.; Qian, L.; Xue, Y.; Ji, K.; Yin, R. Machine Learning for Prediction of Outcomes in Cardiogenic Shock. Front. Cardiovasc. Med. 2022, 9, 849688. [Google Scholar] [CrossRef]
- Mo, Z.; Lu, Z.; Tang, X.; Lin, X.; Wang, S.; Zhang, Y.; Huang, Z. Construction and Evaluation of Prognostic Models of ECMO in Elderly Patients with Cardiogenic Shock Based on BP Neural Network, Random Forest, and Decision Tree. Am. J. Transl. Res. 2023, 15, 4639–4648. [Google Scholar] [PubMed]
- Cau, R.; Pisu, F.; Suri, J.S.; Montisci, R.; Gatti, M.; Mannelli, L.; Gong, X.; Saba, L. Artificial Intelligence in the Differential Diagnosis of Cardiomyopathy Phenotypes. Diagnostics 2024, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Haimovich, J.S.; Diamant, N.; Khurshid, S.; Di Achille, P.; Reeder, C.; Friedman, S.; Singh, P.; Spurlock, W.; Ellinor, P.T.; Philippakis, A.; et al. Artificial Intelligence-Enabled Classification of Hypertrophic Heart Diseases Using Electrocardiograms. Cardiovasc. Digit. Health J. 2023, 4, 48–59. [Google Scholar] [CrossRef]
- Beneyto, M.; Ghyaza, G.; Cariou, E.; Amar, J.; Lairez, O. Development and Validation of Machine Learning Algorithms to Predict Posthypertensive Origin in Left Ventricular Hypertrophy. Arch. Cardiovasc. Dis. 2023, 116, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, J.; Moghadasi, N.; Körperich, H.; Weise Valdés, E.; Sciacca, V.; Paluszkiewicz, L.; Burchert, W.; Piran, M. A Machine Learning Challenge: Detection of Cardiac Amyloidosis Based on Bi-Atrial and Right Ventricular Strain and Cardiac Function. Diagnostics 2022, 12, 2693. [Google Scholar] [CrossRef]
- Siontis, K.C.; Liu, K.; Bos, J.M.; Attia, Z.I.; Cohen-Shelly, M.; Arruda-Olson, A.M.; Zanjirani Farahani, N.; Friedman, P.A.; Noseworthy, P.A.; Ackerman, M.J. Detection of Hypertrophic Cardiomyopathy by an Artificial Intelligence Electrocardiogram in Children and Adolescents. Int. J. Cardiol. 2021, 340, 42–47. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-C.; Choi, D.; Choi, Y.-J.; Ju, L.; Kim, M.; Hong, J.-E.; Lee, H.-J.; Yoon, Y.E.; Park, J.-B.; Lee, S.-P.; et al. Differential Diagnosis of Common Etiologies of Left Ventricular Hypertrophy Using a Hybrid CNN-LSTM Model. Sci. Rep. 2022, 12, 20998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Deng, Y.; Liu, Y.; Su, X.; Zeng, X. Echocardiography-Based Machine Learning Algorithm for Distinguishing Ischemic Cardiomyopathy from Dilated Cardiomyopathy. BMC Cardiovasc. Disord. 2023, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Cau, R.; Pisu, F.; Porcu, M.; Cademartiri, F.; Montisci, R.; Bassareo, P.; Muscogiuri, G.; Amadu, A.; Sironi, S.; Esposito, A.; et al. Machine Learning Approach in Diagnosing Takotsubo Cardiomyopathy: The Role of the Combined Evaluation of Atrial and Ventricular Strain, and Parametric Mapping. Int. J. Cardiol. 2023, 373, 124–133. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, O.; Cammann, V.L.; Pancotti, C.; Di Vece, D.; Silverio, A.; Schweiger, V.; Niederseer, D.; Szawan, K.A.; Würdinger, M.; Koleva, I.; et al. Machine Learning-based Prediction of In-hospital Death for Patients with Takotsubo Syndrome: The InterTAK-ML Model. Eur. J. Heart Fail. 2023, 25, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.L.; Spencer, A.K.; Lau, H.A.; Nelson, M.W.; Giuliano, J.D.; Zabinski, J.W.; Boussios, C.; Curhan, G.; Gliklich, R.E.; Warnock, D.G. A New Approach to Identifying Patients with Elevated Risk for Fabry Disease Using a Machine Learning Algorithm. Orphanet J. Rare Dis. 2021, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.T.; Weston Hughes, J.; Sanchez, P.A.; Perez, M.; Ouyang, D.; Ashley, E.A. Multimodal Deep Learning Enhances Diagnostic Precision in Left Ventricular Hypertrophy. Eur. Heart J. Digit. Health 2022, 3, 380–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Wu, Y.; Zhang, B.; Zhou, C.; Gao, X.; Xie, X.; Li, X.; Yu, J.; Wang, X.; et al. Novel Algorithm for Diagnosis of Arrhythmogenic Cardiomyopathy and Dilated Cardiomyopathy: Key Gene Expression Profiling Using Machine Learning. J. Gene Med. 2023, 25, e3468. [Google Scholar] [CrossRef]
- Papageorgiou, V.E.; Zegkos, T.; Efthimiadis, G.; Tsaklidis, G. Analysis of Digitalized ECG Signals Based on Artificial Intelligence and Spectral Analysis Methods Specialized in ARVC. Int. J. Numer. Method. Biomed. Eng. 2022, 38, e3644. [Google Scholar] [CrossRef]
- Harmon, D.M.; Mangold, K.; Baez Suarez, A.; Scott, C.G.; Murphree, D.H., Jr.; Malik, A.; Attia, Z.I.; Lopez-Jimenez, F.; Friedman, P.A.; Dispenzieri, A.; et al. Postdevelopment Performance and Validation of the Artificial Intelligence-Enhanced Electrocardiogram for Detection of Cardiac Amyloidosis. JACC Adv. 2023, 2, 100612. [Google Scholar] [CrossRef]
- Cotella, J.I.; Slivnick, J.A.; Sanderson, E.; Singulane, C.; O’Driscoll, J.; Asch, F.M.; Addetia, K.; Woodward, G.; Lang, R.M. Artificial Intelligence Based Left Ventricular Ejection Fraction and Global Longitudinal Strain in Cardiac Amyloidosis. Echocardiography 2023, 40, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Su, C.; Qin, S.; Li, J.; Zeng, D.; Cai, Y.; Huang, T.; Wu, J. Deep Learn-Based Computer-Assisted Transthoracic Echocardiography: Approach to the Diagnosis of Cardiac Amyloidosis. Int. J. Cardiovasc. Imaging 2023, 39, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Jang, J.; Ruiz, J.; Desai, S.; Paghdar, S.; Malkani, S.; Yip, D.; Leoni, J.; Patel, P.; Lyle, M.; et al. (28) Artificial Intelligence to Predict Death or Transplant in ATTR Amyloidosis Cardiomyopathy. J. Heart Lung Transplant. 2023, 42, S22–S23. [Google Scholar] [CrossRef]
- Michalski, A.A.; Lis, K.; Stankiewicz, J.; Kloska, S.M.; Sycz, A.; Dudziński, M.; Muras-Szwedziak, K.; Nowicki, M.; Bazan-Socha, S.; Dabrowski, M.J.; et al. Supporting the Diagnosis of Fabry Disease Using a Natural Language Processing-Based Approach. J. Clin. Med. 2023, 12, 3599. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.; Aguiar, P.; Biondetti, G.; Warnock, D.; Kallish, S.; Nelson, M.; Giuliano, J.; Zabinksi, J.; Boussios, C.; Curhan, G.; et al. (751) Estimation of Arrhythmia Risk in Patients with Fabry Disease Using a Machine Learning Model. J. Heart Lung Transplant 2023, 42, S331. [Google Scholar] [CrossRef]
- Stolpe, G.; Didier, R.; Martel, H.; Claire, L.; Michel, N.; Sellami, S.; Essayagh, B.; Réant, P.; Donal, E.; Habib, G. Contribution of Artificial Intelligence and Left Atrial Strain in the Prediction of Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Results of a Multicentric Cohort. Arch. Cardiovasc. Dis. Suppl. 2023, 15, 237. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, C.; Zhao, S.; Xie, L.; Tian, Y. Cardiac Magnetic Resonance Radiomics for Disease Classification. Eur. Radiol. 2022, 33, 2312–2323. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Nakaura, T.; Yanagawa, M.; Fujita, S.; Kamagata, K.; Ito, R.; Kawamura, M.; Fushimi, Y.; Ueda, D.; Matsui, Y.; et al. Recent Advances in Artificial Intelligence for Cardiac CT: Enhancing Diagnosis and Prognosis Prediction. Diagn. Interv. Imaging 2023, 104, 521–528. [Google Scholar] [CrossRef]
- O’Brien, H.; Williams, M.C.; Rajani, R.; Niederer, S. Radiomics and Machine Learning for Detecting Scar Tissue on CT Delayed Enhancement Imaging. Front. Cardiovasc. Med. 2022, 9, 847825. [Google Scholar] [CrossRef]
- Wen, D.; Xu, Z.; An, R.; Ren, J.; Jia, Y.; Li, J.; Zheng, M. Predicting Haemodynamic Significance of Coronary Stenosis with Radiomics-Based Pericoronary Adipose Tissue Characteristics. Clin. Radiol. 2022, 77, e154–e161. [Google Scholar] [CrossRef]
- Lara-Hernández, A.; Rienmüller, T.; Juárez, I.; Pérez, M.; Reyna, F.; Baumgartner, D.; Makarenko, V.N.; Bockeria, O.L.; Maksudov, M.; Rienmüller, R.; et al. Deep Learning-Based Image Registration in Dynamic Myocardial Perfusion CT Imaging. IEEE Trans. Med. Imaging 2023, 42, 684–696. [Google Scholar] [CrossRef]
- Griffin, W.F.; Choi, A.D.; Riess, J.S.; Marques, H.; Chang, H.-J.; Choi, J.H.; Doh, J.-H.; Her, A.-Y.; Koo, B.-K.; Nam, C.-W.; et al. AI Evaluation of Stenosis on Coronary CTA, Comparison with Quantitative Coronary Angiography and Fractional Flow Reserve. JACC Cardiovasc. Imaging 2023, 16, 193–205. [Google Scholar] [CrossRef]
- Brandt, V.; Schoepf, U.J.; Aquino, G.J.; Bekeredjian, R.; Varga-Szemes, A.; Emrich, T.; Bayer, R.R., II; Schwarz, F.; Kroencke, T.J.; Tesche, C.; et al. Impact of Machine-Learning-Based Coronary Computed Tomography Angiography–Derived Fractional Flow Reserve on Decision-Making in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Eur. Radiol. 2022, 32, 6008–6016. [Google Scholar] [CrossRef]
- Li, X.-N.; Yin, W.-H.; Sun, Y.; Kang, H.; Luo, J.; Chen, K.; Hou, Z.-H.; Gao, Y.; Ren, X.-S.; Yu, Y.-T.; et al. Identification of Pathology-Confirmed Vulnerable Atherosclerotic Lesions by Coronary Computed Tomography Angiography Using Radiomics Analysis. Eur. Radiol. 2022, 32, 4003–4013. [Google Scholar] [CrossRef]
- Lyu, T.; Zhao, W.; Zhu, Y.; Wu, Z.; Zhang, Y.; Chen, Y.; Luo, L.; Li, S.; Xing, L. Estimating Dual-Energy CT Imaging from Single-Energy CT Data with Material Decomposition Convolutional Neural Network. Med. Image Anal. 2021, 70, 102001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, P.; Bian, Y.; Fan, Y.; Li, J.; Liu, X.; Shen, J.; Hu, Y.; Liao, X.; Wang, H.; et al. Establishment and Validation of an AI-Aid Method in the Diagnosis of Myocardial Perfusion Imaging. BMC Med. Imaging 2023, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Khunte, A.; Sangha, V.; Oikonomou, E.K.; Dhingra, L.S.; Aminorroaya, A.; Mortazavi, B.J.; Coppi, A.; Brandt, C.A.; Krumholz, H.M.; Khera, R. Detection of Left Ventricular Systolic Dysfunction from Single-Lead Electrocardiography Adapted for Portable and Wearable Devices. NPJ Digit. Med. 2023, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pieszko, K.; Hiczkiewicz, J.; Łojewska, K.; Uziębło-Życzkowska, B.; Krzesiński, P.; Gawałko, M.; Budnik, M.; Starzyk, K.; Wożakowska-Kapłon, B.; Daniłowicz-Szymanowicz, L.; et al. Artificial Intelligence in Detecting Left Atrial Appendage Thrombus by Transthoracic Echocardiography and Clinical Features: The Left Atrial Thrombus on Transoesophageal Echocardiography (LATTEE) Registry. Eur. Heart J. 2024, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Li, W.; Zhang, F.; Ouyang, W.; Wang, S.; Zhi, A.; Pan, X. Development of an Expert-Level Right Ventricular Abnormality Detection Algorithm Based on Deep Learning. Interdiscip. Sci. 2023, 15, 653–662. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Ghadimi, S.; Auger, D.C.; Croisille, P.; Viallon, M.; Mangion, K.; Berry, C.; Haggerty, C.M.; Jing, L.; et al. StrainNet: Improved Myocardial Strain Analysis of Cine MRI by Deep Learning from DENSE. Radiol. Cardiothorac. Imaging 2023, 5, e220196. [Google Scholar] [CrossRef]
- Yu, J.; Taskén, A.A.; Flade, H.M.; Skogvoll, E.; Berg, E.A.R.; Grenne, B.; Rimehaug, A.; Kirkeby-Garstad, I.; Kiss, G.; Aakhus, S. Automatic Assessment of Left Ventricular Function for Hemodynamic Monitoring Using Artificial Intelligence and Transesophageal Echocardiography. J. Clin. Monit. Comput. 2024, 38, 281–291. [Google Scholar] [CrossRef]
- Laumer, F.; Di Vece, D.; Cammann, V.L.; Würdinger, M.; Petkova, V.; Schönberger, M.; Schönberger, A.; Mercier, J.C.; Niederseer, D.; Seifert, B.; et al. Assessment of Artificial Intelligence in Echocardiography Diagnostics in Differentiating Takotsubo Syndrome from Myocardial Infarction. JAMA Cardiol. 2022, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Chang, C.-C.; Ko, C.-F.; Lee, Y.-H.; Tsai, Y.-L.; Chou, R.-H.; Chang, T.-Y.; Guo, S.-M.; Huang, P.-H. Artificial Intelligence Evaluation of Coronary Computed Tomography Angiography for Coronary Stenosis Classification and Diagnosis. Eur. J. Clin. Investig. 2024, 54, e14089. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, A.; Szabó, L.; Dohy, Z.; Kiss, M.; Merkely, B.; Gyires-Tóth, B.; Vágó, H. Automated T1 and T2 Mapping Segmentation on Cardiovascular Magnetic Resonance Imaging Using Deep Learning. Front. Cardiovasc. Med. 2023, 10, 1147581. [Google Scholar] [CrossRef] [PubMed]
- Ishikita, A.; McIntosh, C.; Hanneman, K.; Lee, M.M.; Liang, T.; Karur, G.R.; Roche, S.L.; Hickey, E.; Geva, T.; Barron, D.J.; et al. Machine Learning for Prediction of Adverse Cardiovascular Events in Adults with Repaired Tetralogy of Fallot Using Clinical and Cardiovascular Magnetic Resonance Imaging Variables. Circ. Cardiovasc. Imaging 2023, 16, e015205. [Google Scholar] [CrossRef] [PubMed]
- De Vries, I.R.; van Laar, J.O.E.H.; van der Hout-van der Jagt, M.B.; Clur, S.-A.B.; Vullings, R. Fetal Electrocardiography and Artificial Intelligence for Prenatal Detection of Congenital Heart Disease. Acta Obstet. Gynecol. Scand. 2023, 102, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Dong, B.; Lei, H.; Shi, G.; Wang, H.; Zhu, F.; Wen, C.; Zhang, Q.; Fu, L.; Gu, X.; et al. Artificial Intelligence-Assisted Auscultation in Detecting Congenital Heart Disease. Eur. Heart J. Digit. Health 2021, 2, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Rofeberg, V.; Bellinger, D.C.; Wypij, D.; Newburger, J.W. Machine Learning to Predict Executive Function in Adolescents with Repaired D-Transposition of the Great Arteries, Tetralogy of Fallot, and Fontan Palliation. J. Pediatr. 2022, 246, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Komatsu, M.; Komatsu, R.; Matsuoka, R.; Yasutomi, S.; Dozen, A.; Shozu, K.; Arakaki, T.; Machino, H.; Asada, K.; et al. Medical Professional Enhancement Using Explainable Artificial Intelligence in Fetal Cardiac Ultrasound Screening. Biomedicines 2022, 10, 551. [Google Scholar] [CrossRef]
- Gearhart, A.; Goto, S.; Deo, R.C.; Powell, A.J. An Automated View Classification Model for Pediatric Echocardiography Using Artificial Intelligence. J. Am. Soc. Echocardiogr. 2022, 35, 1238–1246. [Google Scholar] [CrossRef]
- Valente Silva, B.; Marques, J.; Nobre Menezes, M.; Oliveira, A.L.; Pinto, F.J. Artificial Intelligence-Based Diagnosis of Acute Pulmonary Embolism: Development of a Machine Learning Model Using 12-Lead Electrocardiogram. Rev. Port. Cardiol. 2023, 42, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Adedinsewo, D.; Hardway, H.D.; Morales-Lara, A.C.; Wieczorek, M.A.; Johnson, P.W.; Douglass, E.J.; Dangott, B.J.; Nakhleh, R.E.; Narula, T.; Patel, P.C.; et al. Non-Invasive Detection of Cardiac Allograft Rejection among Heart Transplant Recipients Using an Electrocardiogram Based Deep Learning Model. Eur. Heart J. Digit. Health 2023, 4, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Goto, S.; Niimi, N.; Katsumata, Y.; Goda, A.; Takei, M.; Saji, M.; Sano, M.; Fukuda, K.; Kohno, T.; et al. Improved Prediction of Sudden Cardiac Death in Patients with Heart Failure through Digital Processing of Electrocardiography. Europace 2023, 25, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Suzuki, S.; Motogi, J.; Nakai, H.; Matsuzawa, W.; Takayanagi, T.; Umemoto, T.; Hyodo, A.; Satoh, K.; Arita, T.; et al. Cardiovascular Events and Artificial Intelligence-Predicted Age Using 12-Lead Electrocardiograms. Int. J. Cardiol. Heart Vasc. 2023, 44, 101172. [Google Scholar] [CrossRef] [PubMed]
- Wouters, P.C.; van de Leur, R.R.; Vessies, M.B.; van Stipdonk, A.M.W.; Ghossein, M.A.; Hassink, R.J.; Doevendans, P.A.; van der Harst, P.; Maass, A.H.; Prinzen, F.W.; et al. Electrocardiogram-Based Deep Learning Improves Outcome Prediction Following Cardiac Resynchronization Therapy. Eur. Heart J. 2023, 44, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Wu, F.-H.; Hu, Y.-L.; Pan, R.-H.; Lin, C.-H.; Chen, Y.-F.; Tseng, G.-S.; Chan, Y.-K.; Wang, C.-L. Left Ventricular Hypertrophy Detection Using Electrocardiographic Signal. Sci. Rep. 2023, 13, 2556. [Google Scholar] [CrossRef] [PubMed]
- Zaver, H.B.; Mzaik, O.; Thomas, J.; Roopkumar, J.; Adedinsewo, D.; Keaveny, A.P.; Patel, T. Utility of an Artificial Intelligence Enabled Electrocardiogram for Risk Assessment in Liver Transplant Candidates. Dig. Dis. Sci. 2023, 68, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Naser, J.A.; Lopez-Jimenez, F.; Chang, A.Y.; Baez-Suarez, A.; Attia, Z.I.; Pislaru, S.V.; Pellikka, P.A.; Lin, G.; Kapa, S.; Friedman, P.A.; et al. Artificial Intelligence-Augmented Electrocardiogram in Determining Sex. Mayo Clin. Proc. 2023, 98, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Vaid, A.; Argulian, E.; Lerakis, S.; Beaulieu-Jones, B.K.; Krittanawong, C.; Klang, E.; Lampert, J.; Reddy, V.Y.; Narula, J.; Nadkarni, G.N.; et al. Multi-Center Retrospective Cohort Study Applying Deep Learning to Electrocardiograms to Identify Left Heart Valvular Dysfunction. Commun. Med. 2023, 3, 24. [Google Scholar] [CrossRef]
- Khan, M.S.; Arshad, M.S.; Greene, S.J.; Van Spall, H.G.C.; Pandey, A.; Vemulapalli, S.; Perakslis, E.; Butler, J. Artificial Intelligence and Heart Failure: A State-of-the-art Review. Eur. J. Heart Fail. 2023, 25, 1507–1525. [Google Scholar] [CrossRef]
- Almujally, N.A.; Aljrees, T.; Saidani, O.; Umer, M.; Faheem, Z.B.; Abuzinadah, N.; Alnowaiser, K.; Ashraf, I. Monitoring Acute Heart Failure Patients Using Internet-of-Things-Based Smart Monitoring System. Sensors 2023, 23, 4580. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Huttin, O.; Magnusson, M.; Ferreira, J.P.; Bozec, E.; Huby, A.-C.; Preud’homme, G.; Duarte, K.; Lamiral, Z.; Dalleau, K.; et al. Machine Learning-Derived Echocardiographic Phenotypes Predict Heart Failure Incidence in Asymptomatic Individuals. JACC Cardiovasc. Imaging 2022, 15, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.H.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of Patients with Heart Failure with Preserved Ejection Fraction Using Machine Learning-based Unsupervised Cluster Analysis. Eur. J. Heart Fail. 2020, 22, 148–158. [Google Scholar] [CrossRef]
- Bourazana, A.; Xanthopoulos, A.; Briasoulis, A.; Magouliotis, D.; Spiliopoulos, K.; Athanasiou, T.; Vassilopoulos, G.; Skoularigis, J.; Triposkiadis, F. Artificial Intelligence in Heart Failure: Friend or Foe? Life 2024, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Bachtiger, P.; Petri, C.F.; Scott, F.E.; Ri Park, S.; Kelshiker, M.A.; Sahemey, H.K.; Dumea, B.; Alquero, R.; Padam, P.S.; Hatrick, I.R.; et al. Point-of-Care Screening for Heart Failure with Reduced Ejection Fraction Using Artificial Intelligence during ECG-Enabled Stethoscope Examination in London, UK: A Prospective, Observational, Multicentre Study. Lancet Digit. Health 2022, 4, e117–e125. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.M.; Witt, D.R.; Friedman, P.A.; Attia, Z.I. Diagnosis and Treatment of New Heart Failure with Reduced Ejection Fraction by the Artificial Intelligence–Enhanced Electrocardiogram. Cardiovasc. Digit. Health J. 2021, 2, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-M.; Kim, K.-H.; Eisen, H.J.; Cho, Y.; Jeon, K.-H.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial Intelligence Assessment for Early Detection of Heart Failure with Preserved Ejection Fraction Based on Electrocardiographic Features. Eur. Heart J. Digit. Health 2021, 2, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Biswas, D.; Ryan, M.; Bernstein, B.S.; Rizvi, M.; Fairhurst, N.; Kaye, G.; Baral, R.; Searle, T.; Melikian, N.; et al. Artificial Intelligence Methods for Improved Detection of Undiagnosed Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2024, 11, 11728. [Google Scholar] [CrossRef] [PubMed]
- Akerman, A.P.; Porumb, M.; Scott, C.G.; Beqiri, A.; Chartsias, A.; Ryu, A.J.; Hawkes, W.; Huntley, G.D.; Arystan, A.Z.; Kane, G.C.; et al. Automated Echocardiographic Detection of Heart Failure with Preserved Ejection Fraction Using Artificial Intelligence. JACC Adv. 2023, 2, 100452. [Google Scholar] [CrossRef]
- Pană, M.-A.; Busnatu Ștefan, S.; Serbanoiu, L.-I.; Vasilescu, E.; Popescu, N.; Andrei, C.; Sinescu, C.-J. Reducing the Heart Failure Burden in Romania by Predicting Congestive Heart Failure Using Artificial Intelligence: Proof of Concept. Appl. Sci. 2021, 11, 11728. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Kashani, K.B. Artificial Intelligence in Heart Failure and Acute Kidney Injury: Emerging Concepts and Controversial Dimensions. Cardiorenal Med. 2024, 14, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Kamio, T.; Ikegami, M.; Machida, Y.; Uemura, T.; Chino, N.; Iwagami, M. Machine Learning-Based Prognostic Modeling of Patients with Acute Heart Failure Receiving Furosemide in Intensive Care Units. Digit. Health 2023, 9, 20552076231194933. [Google Scholar] [CrossRef] [PubMed]
- Naruka, V.; Arjomandi Rad, A.; Subbiah Ponniah, H.; Francis, J.; Vardanyan, R.; Tasoudis, P.; Magouliotis, D.E.; Lazopoulos, G.L.; Salmasi, M.Y.; Athanasiou, T. Machine Learning and Artificial Intelligence in Cardiac Transplantation: A Systematic Review. Artif. Organs 2022, 46, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, A.; Moustakidis, S.; Tzani, A.; Doulamis, I.; Kampaktsis, P. Prediction of Outcomes after Heart Transplantation by Machine Learning Models. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.0957. [Google Scholar] [CrossRef]
- Seraphin, T.P.; Luedde, M.; Roderburg, C.; van Treeck, M.; Scheider, P.; Buelow, R.D.; Boor, P.; Loosen, S.H.; Provaznik, Z.; Mendelsohn, D.; et al. Prediction of Heart Transplant Rejection from Routine Pathology Slides with Self-Supervised Deep Learning. Eur. Heart J. Digit. Health 2023, 4, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, I.; Toya, T.; Cohen-Shelly, M.; Park, H.W.; Ahmad, A.; Ozcan, A.; Noseworthy, P.A.; Kapa, S.; Lerman, L.O.; Attia, Z.I.; et al. Artificial Intelligence–Derived Cardiac Ageing Is Associated with Cardiac Events Post-Heart Transplantation. Eur. Heart J. Digit. Health 2022, 3, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Menon, N.; Ruiz, J.; Luce, C.; Brumble, L.; Bhattacharya, A.; Goswami, R. Developing a Risk Prediction Model for COVID-19 Infection in Heart Transplant Recipients Using Artificial Intelligence. Future Virol. 2023, 18, 1123–1136. [Google Scholar] [CrossRef]
- Glass, C.; Davis, R.; Xiong, B.; Dov, D.; Glass, M. The Use of Artificial Intelligence (AI) Machine Learning to Determine Myocyte Damage in Cardiac Transplant Acute Cellular Rejection. J. Heart Lung Transplant. 2020, 39, S59. [Google Scholar] [CrossRef]
- Al-Ani, M.A.; Bai, C.; Shickel, B.; Bledsoe, M.; Ahmed, M.M.; Vilaro, J.; Parker, A.; Aranda, J.; Jeng, E.; Bleiweis, M.; et al. (750) Determinants of Successful Bridging to Heart Transplantation on Temporary Percutaneous Left Ventricular Support—An Insight Using Artificial Intelligence. J. Heart Lung Transplant. 2023, 42, S331. [Google Scholar] [CrossRef]
- Peyster, E.G.; Arabyarmohammadi, S.; Janowczyk, A.; Azarianpour-Esfahani, S.; Sekulic, M.; Cassol, C.; Blower, L.; Parwani, A.; Lal, P.; Feldman, M.D.; et al. An Automated Computational Image Analysis Pipeline for Histological Grading of Cardiac Allograft Rejection. Eur. Heart J. 2021, 42, 2356–2369. [Google Scholar] [CrossRef]
- Lipkova, J.; Chen, T.Y.; Lu, M.Y.; Chen, R.J.; Shady, M.; Williams, M.; Wang, J.; Noor, Z.; Mitchell, R.N.; Turan, M.; et al. Deep Learning-Enabled Assessment of Cardiac Allograft Rejection from Endomyocardial Biopsies. Nat. Med. 2022, 28, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Giuste, F.O.; Sequeira, R.; Keerthipati, V.; Lais, P.; Mirzazadeh, A.; Mohseni, A.; Zhu, Y.; Shi, W.; Marteau, B.; Zhong, Y.; et al. Explainable Synthetic Image Generation to Improve Risk Assessment of Rare Pediatric Heart Transplant Rejection. J. Biomed. Inform. 2023, 139, 104303. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, P.J.G.; Jayabalan, M.; Ortega-Martorell, S.; Olier, I.; Medved, D.; Nilsson, J. Enhanced Survival Prediction Using Explainable Artificial Intelligence in Heart Transplantation. Sci. Rep. 2022, 12, 19525. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Morales, J.; Nativi-Nicolau, J.; Jang, J.; Patel, P.; Yip, D.; Leoni-Moreno, J.; Goswami, R. Artificial Intelligence 12 Lead ECG Based Heart Age Estimation and 1-Year Outcomes after Heart Transplantation. J. Heart Lung Transplant. 2022, 41, S213. [Google Scholar] [CrossRef]
- Agasthi, P.; Smith, S.D.; Murphy, K.M.; Buras, M.R.; Golafshar, M.; Herner, M.; Anand, S.; Pujari, S.; Allam, M.N.; Mookadam, F.; et al. Artificial Intelligence Helps Predict 5-Year Mortality and Graft Failure in Patients Undergoing Orthotopic Heart Transplantation. J. Heart Lung Transplant. 2020, 39, S142. [Google Scholar] [CrossRef]
- Ozcan, I.; Toya, T.; Cohen-Shelly, M.; Ahmad, A.; Corban, M.T.; Noseworthy, P.A.; Kapa, S.; Lerman, L.O.; Attia, Z.I.; Friedman, P.A.; et al. Artificial Intelligence Derived Age Algorithm after Heart Transplantation. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.2272. [Google Scholar] [CrossRef]
- Soh, D.C.K.; Ng, E.Y.K.; Jahmunah, V.; Oh, S.L.; San, T.R.; Acharya, U.R. A Computational Intelligence Tool for the Detection of Hypertension Using Empirical Mode Decomposition. Comput. Biol. Med. 2020, 118, 103630. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, F.; Núñez-Valdez, E.R.; Crespo, R.G.; García-Díaz, V. An Artificial Neural Network Approach for Predicting Hypertension Using NHANES Data. Sci. Rep. 2020, 10, 10620. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yuan, X.; Wang, W.; Liu, K.; Qin, Y.; Sun, X.; Ma, W.; Zou, Y.; Zhang, H.; Zhou, X.; et al. Value of a Machine Learning Approach for Predicting Clinical Outcomes in Young Patients with Hypertension. Hypertension 2020, 75, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; Malek, S.; Mhd Ali, A.; Wong, M.S.; Mosleh, M.; Milow, P. Determining Hypertensive Patients’ Beliefs towards Medication and Associations with Medication Adherence Using Machine Learning Methods. PeerJ 2020, 8, e8286. [Google Scholar] [CrossRef]
- Koshimizu, H.; Kojima, R.; Kario, K.; Okuno, Y. Prediction of Blood Pressure Variability Using Deep Neural Networks. Int. J. Med. Inform. 2020, 136, 104067. [Google Scholar] [CrossRef]
- Hamoud, B.; Kashevnik, A.; Othman, W.; Shilov, N. Neural Network Model Combination for Video-Based Blood Pressure Estimation: New Approach and Evaluation. Sensors 2023, 23, 1753. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, J.; Chen, Z.; Chen, J. Deep Learning-Based Non-Contact IPPG Signal Blood Pressure Measurement Research. Sensors 2023, 23, 5528. [Google Scholar] [CrossRef]
- Xing, W.; Shi, Y.; Wu, C.; Wang, Y.; Wang, X. Predicting Blood Pressure from Face Videos Using Face Diagnosis Theory and Deep Neural Networks Technique. Comput. Biol. Med. 2023, 164, 107112. [Google Scholar] [CrossRef] [PubMed]
- Visco, V.; Izzo, C.; Mancusi, C.; Rispoli, A.; Tedeschi, M.; Virtuoso, N.; Giano, A.; Gioia, R.; Melfi, A.; Serio, B.; et al. Artificial Intelligence in Hypertension Management: An Ace up Your Sleeve. J. Cardiovasc. Dev. Dis. 2023, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Xu, S.; Tran, S.; Garg, S.; Springer, M.; Karunanithi, M.; Mohawesh, R. A Survey: From Shallow to Deep Machine Learning Approaches for Blood Pressure Estimation Using Biosensors. Expert Syst. Appl. 2022, 197, 116788. [Google Scholar] [CrossRef]
- Herzog, L.; Ilan Ber, R.; Horowitz-Kugler, Z.; Rabi, Y.; Brufman, I.; Paz, Y.E.; Lopez-Jimenez, F. Causal Deep Neural Network-Based Model for First-Line Hypertension Management. Mayo Clin. Proc. Digit. Health 2023, 1, 632–640. [Google Scholar] [CrossRef]
- Khthir, R.; Santhanam, P. Artificial Intelligence (AI) Approach to Identifying Factors That Determine Systolic Blood Pressure in Type 2 Diabetes (Study from the LOOK AHEAD Cohort). Diabetes Metab. Syndr. 2021, 15, 102278. [Google Scholar] [CrossRef]
- Aryal, S.; Manandhar, I.; Mei, X.; Yeoh, B.S.; Tummala, R.; Saha, P.; Osman, I.; Zubcevic, J.; Durgan, D.J.; Vijay-Kumar, M.; et al. Combating Hypertension beyond GWAS: Microbiome and Artificial Intelligence as Opportunities for Precision Medicine. Camb. Prisms Precis. Med. 2023, 1, e26. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cheng, Y.T.; Cheung, B.M.Y. Machine Learning Algorithms Identify Hypokalaemia Risk in People with Hypertension in the United States National Health and Nutrition Examination Survey 1999–2018. Ann. Med. 2023, 55, 2209336. [Google Scholar] [CrossRef]
- Kusunose, K.; Hirata, Y.; Tsuji, T.; Kotoku, J.; Sata, M. Deep Learning to Predict Elevated Pulmonary Artery Pressure in Patients with Suspected Pulmonary Hypertension Using Standard Chest X ray. Sci. Rep. 2020, 10, 19311. [Google Scholar] [CrossRef]
- Hardacre, C.J.; Robertshaw, J.A.; Barratt, S.L.; Adams, H.L.; MacKenzie Ross, R.V.; Robinson, G.R.E.; Suntharalingam, J.; Pauling, J.D.; Rodrigues, J.C.L. Diagnostic Test Accuracy of Artificial Intelligence Analysis of Cross-Sectional Imaging in Pulmonary Hypertension: A Systematic Literature Review. Br. J. Radiol. 2021, 94, 19311. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdottir, H.; Manduchi, L.; Michel, H.; Laumer, F.; Wellmann, S.; Ozkan, E.; Vogt, J.E. Interpretable Prediction of Pulmonary Hypertension in Newborns Using Echocardiograms. In Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2022; pp. 529–542. [Google Scholar]
- Chakravarty, K.; Antontsev, V.G.; Khotimchenko, M.; Gupta, N.; Jagarapu, A.; Bundey, Y.; Hou, H.; Maharao, N.; Varshney, J. Accelerated Repurposing and Drug Development of Pulmonary Hypertension Therapies for COVID-19 Treatment Using an AI-Integrated Biosimulation Platform. Molecules 2021, 26, 1912. [Google Scholar] [CrossRef]
- Rahaghi, F.N.; Nardelli, P.; Harder, E.; Singh, I.; Sánchez-Ferrero, G.V.; Ross, J.C.; San José Estépar, R.; Ash, S.Y.; Hunsaker, A.R.; Maron, B.A.; et al. Quantification of Arterial and Venous Morphologic Markers in Pulmonary Arterial Hypertension Using CT Imaging. Chest 2021, 160, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhou, T.; Lv, S.; Wang, M.; Chen, S.; Heidari, A.A.; Huang, X.; Chen, H.; Wang, L.; Wu, P. An Evolutionary Machine Learning for Pulmonary Hypertension Animal Model from Arterial Blood Gas Analysis. Comput. Biol. Med. 2022, 146, 105529. [Google Scholar] [CrossRef]
- Amodeo, I.; De Nunzio, G.; Raffaeli, G.; Borzani, I.; Griggio, A.; Conte, L.; Macchini, F.; Condò, V.; Persico, N.; Fabietti, I.; et al. A maChine and Deep Learning Approach to Predict pulmoNary hyperteNsIon in newbornS with Congenital Diaphragmatic Hernia (CLANNISH): Protocol for a Retrospective Study. PLoS ONE 2021, 16, e0259724. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; Bax, J.J. Using Deep Learning to Diagnose Pulmonary Hypertension. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1457–1458. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.J.; Lu, H.; Uthoff, J.; Garg, P.; Cogliano, M.; Taylor, J.; Metherall, P.; Zhou, S.; Johns, C.S.; Alabed, S.; et al. A Machine Learning Cardiac Magnetic Resonance Approach to Extract Disease Features and Automate Pulmonary Arterial Hypertension Diagnosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 236–245. [Google Scholar] [CrossRef]
- Charters, P.F.P.; Rossdale, J.; Brown, W.; Burnett, T.A.; Komber, H.M.E.I.; Thompson, C.; Robinson, G.; MacKenzie Ross, R.; Suntharalingam, J.; Rodrigues, J.C.L. Diagnostic Accuracy of an Automated Artificial Intelligence Derived Right Ventricular to Left Ventricular Diameter Ratio Tool on CT Pulmonary Angiography to Predict Pulmonary Hypertension at Right Heart Catheterisation. Clin. Radiol. 2022, 77, e500–e508. [Google Scholar] [CrossRef] [PubMed]
- Fortmeier, V.; Lachmann, M.; Körber, M.I.; Unterhuber, M.; von Scheidt, M.; Rippen, E.; Harmsen, G.; Gerçek, M.; Friedrichs, K.P.; Roder, F.; et al. Solving the Pulmonary Hypertension Paradox in Patients with Severe Tricuspid Regurgitation by Employing Artificial Intelligence. JACC Cardiovasc. Interv. 2022, 15, 381–394. [Google Scholar] [CrossRef]
- Liu, C.-M.; Shih, E.S.C.; Chen, J.-Y.; Huang, C.-H.; Wu, I.-C.; Chen, P.-F.; Higa, S.; Yagi, N.; Hu, Y.-F.; Hwang, M.-J.; et al. Artificial Intelligence-Enabled Electrocardiogram Improves the Diagnosis and Prediction of Mortality in Patients with Pulmonary Hypertension. JACC Asia 2022, 2, 258–270. [Google Scholar] [CrossRef]
- Lu, W.; Huang, J.; Shen, Q.; Sun, F.; Li, J. Identification of Diagnostic Biomarkers for Idiopathic Pulmonary Hypertension with Metabolic Syndrome by Bioinformatics and Machine Learning. Sci. Rep. 2023, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qin, W.; Lin, X.; Shan, Z.; Huang, L.; Shao, Q.; Wang, L.; Chen, M. Synergizing the Enhanced RIME with Fuzzy K-Nearest Neighbor for Diagnose of Pulmonary Hypertension. Comput. Biol. Med. 2023, 165, 107408. [Google Scholar] [CrossRef] [PubMed]
- Hyde, B.; Paoli, C.J.; Panjabi, S.; Bettencourt, K.C.; Bell Lynum, K.S.; Selej, M. A Claims-based, Machine-learning Algorithm to Identify Patients with Pulmonary Arterial Hypertension. Pulm. Circ. 2023, 13, e12237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, X.; Li, J.; Huang, L.; Li, H.; Feng, H.; Garcia, M.A.; Cao, Y.; Sun, Z.; Chai, S. Machine Learning Based on Computed Tomography Pulmonary Angiography in Evaluating Pulmonary Artery Pressure in Patients with Pulmonary Hypertension. J. Clin. Med. 2023, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Tsuji, T.; Kotoku, J.; Sata, M.; Kusunose, K. Echocardiographic Artificial Intelligence for Pulmonary Hypertension Classification. Heart 2024, 110, heartjnl-2023-323320. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Sakao, S.; Nagata, J.; Naito, A.; Sekine, A.; Sugiura, T.; Shigeta, A.; Nishiyama, A.; Yokota, H.; Shimizu, N.; et al. Artificial Intelligence-Based Model for Predicting Pulmonary Arterial Hypertension on Chest X-ray Images. BMC Pulm. Med. 2024, 24, 101. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdottir, H.; Ozkan, E.; Michel, H.; Chin-Cheong, K.; Manduchi, L.; Wellmann, S.; Vogt, J.E. Deep Learning Based Prediction of Pulmonary Hypertension in Newborns Using Echocardiograms. Int. J. Comput. Vis. 2024, 1–18. [Google Scholar] [CrossRef]
- Dwivedi, K.; Sharkey, M.; Delaney, L.; Alabed, S.; Rajaram, S.; Hill, C.; Johns, C.; Rothman, A.; Mamalakis, M.; Thompson, A.A.R.; et al. Improving Prognostication in Pulmonary Hypertension Using AI-Quantified Fibrosis and Radiologic Severity Scoring at Baseline CT. Radiology 2024, 310, e231718. [Google Scholar] [CrossRef]
- Griffiths, M.; Manlhiot, C.; Chinni, B.K.; Sleeper, L.A.; Abman, S.; Rosenzweig, E.; Romer, L.H.; Mullen, M.P.; Lin, S.; Benza, R.; et al. Abstract 15889: An Artificial Intelligence-Derived Pediatric Pulmonary Hypertension Risk Prediction Model from the Pediatric Pulmonary Hypertension Network (PPHNet) Registry. Circulation 2023, 148 (Suppl. S1), A15889. [Google Scholar] [CrossRef]
- Mamalakis, M.; Dwivedi, K.; Sharkey, M.; Alabed, S.; Kiely, D.; Swift, A.J. A Transparent Artificial Intelligence Framework to Assess Lung Disease in Pulmonary Hypertension. Sci. Rep. 2023, 13, 3812. [Google Scholar] [CrossRef]
- Tchuente Foguem, G.; Coulibaly, L.; Diamoutene, A. Combined Learning Models for Survival Analysis of Patients with Pulmonary Hypertension. Intell. Syst. Appl. 2024, 21, 200321. [Google Scholar] [CrossRef]
- Han, P.-L.; Jiang, L.; Cheng, J.-L.; Shi, K.; Huang, S.; Jiang, Y.; Jiang, L.; Xia, Q.; Li, Y.-Y.; Zhu, M.; et al. Artificial Intelligence-Assisted Diagnosis of Congenital Heart Disease and Associated Pulmonary Arterial Hypertension from Chest Radiographs: A Multi-Reader Multi-Case Study. Eur. J. Radiol. 2024, 171, 111277. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Weston, A.D.; Scott, C.G.; Kane, G.C.; Pellikka, P.A.; Carter, R.E. Machine Learning for Diagnosis of Pulmonary Hypertension by Echocardiography. Mayo Clin. Proc. 2024, 99, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.-C.; Leung, E.; He, Y.; Cheung, C.-C.; Oliver, M.O.Y.; Yu, Q.; Li, T.C.-M.; Lee, A.L.-H.; Yu, L.; Lui, G.C.-Y. A Machine Learning-Based Risk Score for Prediction of Infective Endocarditis among Patients with Staphylococcus Aureus Bacteraemia—The SABIER Score. J. Infect. Dis. 2024, jiae080. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Zhang, H.; Yang, J.; Chen, D.; Jiang, S. Elucidating Common Pathogenic Transcriptional Networks in Infective Endocarditis and Sepsis: Integrated Insights from Biomarker Discovery and Single-Cell RNA Sequencing. Front. Immunol. 2024, 14, 1298041. [Google Scholar] [CrossRef] [PubMed]
- Galizzi Fae, I.; Murta Pinto, P.H.O.; De Oliveira, G.B.; Taconeli, C.A.; De Andrade, A.B.; De Padua, L.B.; Diamante, L.C.; Ferrari, T.C.A.; Nunes, M.C.P. Cardiac Complications as a Major Predictor of In-Hospital Death in Infective Endocarditis Using Machine-Learning Algorithm Analysis. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655.1773. [Google Scholar] [CrossRef]
- Chen, Z.; Lalande, A.; Salomon, M.; Decourselle, T.; Pommier, T.; Qayyum, A.; Shi, J.; Perrot, G.; Couturier, R. Automatic Deep Learning-Based Myocardial Infarction Segmentation from Delayed Enhancement MRI. Comput. Med. Imaging Graph. 2022, 95, 102014. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, E.; Izquierdo Morcillo, C.; Raisi-Estabragh, Z.; Gkontra, P.; Aung, N.; Lekadir, K.; Petersen, S.E. New Imaging Signatures of Cardiac Alterations in Ischaemic Heart Disease and Cerebrovascular Disease Using CMR Radiomics. Front. Cardiovasc. Med. 2021, 8, 716577. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Lin, C.; Lin, C.-S.; Tsai, M.-C.; Chen, S.-J.; Tsai, S.-H.; Lin, W.-S.; Lee, C.-C.; Tsao, T.-P.; Cheng, C.-C. An Artificial Intelligence-Based Alarm Strategy Facilitates Management of Acute Myocardial Infarction. J. Pers. Med. 2021, 11, 1149. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, J.; Hou, Y.; Zhu, M.; Lu, Y.; Xu, Y.; Teliewubai, J.; Liu, W.; Xu, X.; Li, X.; et al. Early Detection of ST-Segment Elevated Myocardial Infarction by Artificial Intelligence with 12-Lead Electrocardiogram. Int. J. Cardiol. 2020, 317, 223–230. [Google Scholar] [CrossRef]
- Cho, Y.; Kwon, J.-M.; Kim, K.-H.; Medina-Inojosa, J.R.; Jeon, K.-H.; Cho, S.; Lee, S.Y.; Park, J.; Oh, B.-H. Artificial Intelligence Algorithm for Detecting Myocardial Infarction Using Six-Lead Electrocardiography. Sci. Rep. 2020, 10, 20495. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Lin, C.-S.; Tsai, C.-S.; Tsao, T.-P.; Cheng, C.-C.; Liou, J.-T.; Lin, W.-S.; Cheng, S.-M.; Lou, Y.-S.; Lee, C.-C.; et al. A Deep Learning Algorithm for Detecting Acute Myocardial Infarction. EuroIntervention 2021, 17, 765–773. [Google Scholar] [CrossRef]
- Velusamy, D.; Ramasamy, K. Ensemble of Heterogeneous Classifiers for Diagnosis and Prediction of Coronary Artery Disease with Reduced Feature Subset. Comput. Methods Programs Biomed. 2021, 198, 105770. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, L.J.; Al-Shourbaji, I.; Haruna, A.A.; Mohammed, I.A.; Ahmad, A.; Jibrin, M.B. Machine Learning Predictive Models for Coronary Artery Disease. SN Comput. Sci. 2021, 2, 350. [Google Scholar] [CrossRef]
- Li, D.; Xiong, G.; Zeng, H.; Zhou, Q.; Jiang, J.; Guo, X. Machine Learning-Aided Risk Stratification System for the Prediction of Coronary Artery Disease. Int. J. Cardiol. 2021, 326, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Brendel, J.M.; Walterspiel, J.; Hagen, F.; Kübler, J.; Paul, J.-F.; Nikolaou, K.; Gawaz, M.; Greulich, S.; Krumm, P.; Winkelmann, M. Coronary Artery Disease Evaluation during Transcatheter Aortic Valve Replacement Work-up Using Photon-Counting CT and Artificial Intelligence. Diagn. Interv. Imaging 2024. [Google Scholar] [CrossRef]
- Ihdayhid, A.R.; Sehly, A.; He, A.; Joyner, J.; Flack, J.; Konstantopoulos, J.; Newby, D.E.; Williams, M.C.; Ko, B.S.; Chow, B.J.W.; et al. Coronary Artery Stenosis and High-Risk Plaque Assessed with an Unsupervised Fully Automated Deep Learning Technique. JACC Adv. 2024, 2024, 100861. [Google Scholar] [CrossRef]
- Uzokov, J.; Alyavi, A.; Alyavi, B.; Abdullaev, A. How Artificial Intelligence Can Assist with Ischaemic Heart Disease. Eur. Heart J. 2024, ehae030. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, K.; Shiyovich, A.; Huck, D.; Berman, A.; Weber, B.; Gupta, S.; Cardoso, R.; Blankstein, R. Artificial Intelligence in Coronary Artery Calcium Scoring Detection and Quantification. Diagnostics 2024, 14, 125. [Google Scholar] [CrossRef]
- Park, M.J.; Choi, Y.J.; Shim, M.; Cho, Y.; Park, J.; Choi, J.; Kim, J.; Lee, E.; Kim, S.-Y. Performance of ECG-Derived Digital Biomarker for Screening Coronary Occlusion in Resuscitated out-of-Hospital Cardiac Arrest Patients: A Comparative Study between Artificial Intelligence and a Group of Experts. J. Clin. Med. 2024, 13, 1354. [Google Scholar] [CrossRef]
- Alkhamis, M.A.; Al Jarallah, M.; Attur, S.; Zubaid, M. Interpretable Machine Learning Models for Predicting In-Hospital and 30 Days Adverse Events in Acute Coronary Syndrome Patients in Kuwait. Sci. Rep. 2024, 14, 1243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xie, B.; Chen, Y.; Zeng, H.; Hu, J. Machine Learning in the Prediction of In-Hospital Mortality in Patients with First Acute Myocardial Infarction. Clin. Chim. Acta 2024, 554, 117776. [Google Scholar] [CrossRef]
- Kasim, S.; Amir Rudin, P.N.F.; Malek, S.; Aziz, F.; Wan Ahmad, W.A.; Ibrahim, K.S.; Muhmad Hamidi, M.H.; Raja Shariff, R.E.; Fong, A.Y.Y.; Song, C. Data Analytics Approach for Short- and Long-Term Mortality Prediction Following Acute Non-ST-Elevation Myocardial Infarction (NSTEMI) and Unstable Angina (UA) in Asians. PLoS ONE 2024, 19, e0298036. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Seringa, J.; Pinto, F.J.; Henriques, R.; Magalhães, T. Machine Learning Prediction of Mortality in Acute Myocardial Infarction. BMC Med. Inform. Decis. Mak. 2023, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Azdaki, N.; Salmani, F.; Kazemi, T.; Partovi, N.; Bizhaem, S.K.; Moghadam, M.N.; Moniri, Y.; Zarepur, E.; Mohammadifard, N.; Alikhasi, H.; et al. Which Risk Factor Best Predicts Coronary Artery Disease Using Artificial Neural Network Method? BMC Med. Inform. Decis. Mak. 2024, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Hu, H.; Hao, B.; Zhu, H.; Yan, T.; Zhang, J.; Wang, S.; Liu, S.; Zhang, T. Development of Machine Learning-Based Malignant Pericardial Effusion-Related Model in Breast Cancer: Implications for Clinical Significance, Tumor Immune and Drug-Therapy. Heliyon 2024, 10, e27507. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Lin, C.-S.; Cheng, C.-C.; Lin, C. A Deep Learning Algorithm for Detecting Acute Pericarditis by Electrocardiogram. J. Pers. Med. 2022, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Wu, C.-C.; Chen, H.-C.; Hung, C.-H.; Chen, T.-Y.; Lin, C.-H.R.; Chiu, I.-M. Development and Validation of a Deep Learning Pipeline to Measure Pericardial Effusion in Echocardiography. Front. Cardiovasc. Med. 2023, 10, 1195235. [Google Scholar] [CrossRef]
- Wilder-Smith, A.J.; Yang, S.; Weikert, T.; Bremerich, J.; Haaf, P.; Segeroth, M.; Ebert, L.C.; Sauter, A.; Sexauer, R. Automated Detection, Segmentation, and Classification of Pericardial Effusions on Chest CT Using a Deep Convolutional Neural Network. Diagnostics 2022, 12, 1045. [Google Scholar] [CrossRef]
- Piccini, J.P.; Cunnane, R.; Steffel, J.; El-Chami, M.F.; Reynolds, D.; Roberts, P.R.; Soejima, K.; Steinwender, C.; Garweg, C.; Chinitz, L.; et al. Development and Validation of a Risk Score for Predicting Pericardial Effusion in Patients Undergoing Leadless Pacemaker Implantation: Experience with the Micra Transcatheter Pacemaker. Europace 2022, 24, 1119–1126. [Google Scholar] [CrossRef]
- McBane, R.D., II; Murphree, D.H.; Liedl, D.; Lopez-Jimenez, F.; Attia, I.Z.; Arruda-Olson, A.M.; Scott, C.G.; Prodduturi, N.; Nowakowski, S.E.; Rooke, T.W.; et al. Artificial Intelligence of Arterial Doppler Waveforms to Predict Major Adverse Outcomes among Patients Evaluated for Peripheral Artery Disease. J. Am. Heart Assoc. 2024, 13, e031880. [Google Scholar] [CrossRef]
- Rusinovich, Y.; Rusinovich, V.; Buhayenka, A.; Liashko, V.; Sabanov, A.; Holstein, D.J.F.; Aldmour, S.; Doss, M.; Branzan, D. Classification of Anatomic Patterns of Peripheral Artery Disease with Automated Machine Learning (AutoML). Vascular 2024, 17085381241236571. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, P.; Mohanarathinam, A. A Powerful Peripheral Arterial Disease Detection Using Machine Learning-Based Severity Level Classification Model and Hyper Parameter Optimization Methods. Biomed. Signal Process. Control 2024, 90, 105842. [Google Scholar] [CrossRef]
- Li, B.; Shaikh, F.; Zamzam, A.; Syed, M.H.; Abdin, R.; Qadura, M. A Machine Learning Algorithm for Peripheral Artery Disease Prognosis Using Biomarker Data. iScience 2024, 27, 109081. [Google Scholar] [CrossRef]
- Masoumi Shahrbabak, S.; Kim, S.; Youn, B.D.; Cheng, H.-M.; Chen, C.-H.; Mukkamala, R.; Hahn, J.-O. Peripheral Artery Disease Diagnosis Based on Deep Learning-Enabled Analysis of Non-Invasive Arterial Pulse Waveforms. Comput. Biol. Med. 2024, 168, 107813. [Google Scholar] [CrossRef]
- McBane, R.D., II; Murphree, D.H.; Liedl, D.; Lopez-Jimenez, F.; Arruda-Olson, A.; Scott, C.G.; Prodduturi, N.; Nowakowski, S.E.; Rooke, T.W.; Casanegra, A.I.; et al. Artificial Intelligence of Arterial Doppler Waveforms to Predict Major Adverse Outcomes among Patients with Diabetes Mellitus. J. Vasc. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Eisenberg, N.; Beaton, D.; Lee, D.S.; Aljabri, B.; Verma, R.; Wijeysundera, D.N.; Rotstein, O.D.; de Mestral, C.; Mamdani, M.; et al. Using Machine Learning (XGBoost) to Predict Outcomes after Infrainguinal Bypass for Peripheral Artery Disease. Ann. Surg. 2024, 279, 705–713. [Google Scholar] [CrossRef]
- Liu, L.; Bi, B.; Cao, L.; Gui, M.; Ju, F. Predictive Model, and Risk Analysis for Peripheral Vascular Disease in Type 2 Diabetes Mellitus Patients Using Machine Learning and Shapley Additive Explanation. Front. Endocrinol. 2024, 15, 1320335. [Google Scholar] [CrossRef]
- Nassour, N.; Akhbari, B.; Ranganathan, N.; Shin, D.; Ghaednia, H.; Ashkani-Esfahani, S.; DiGiovanni, C.W.; Guss, D. Using Machine Learning in the Prediction of Symptomatic Venous Thromboembolism Following Ankle Fracture. Foot Ankle Surg. 2024, 30, 110–116. [Google Scholar] [CrossRef]
- Chen, R.; Petrazzini, B.O.; Malick, W.A.; Rosenson, R.S.; Do, R. Prediction of Venous Thromboembolism in Diverse Populations Using Machine Learning and Structured Electronic Health Records. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 491–504. [Google Scholar] [CrossRef]
- Pan, S.; Bian, L.; Luo, H.; Conway, A.; Qiao, W.; Win, T.; Wang, W. Risk Factor Analysis and Prediction Model Construction for Surgical Patients with Venous Thromboembolism: A Prospective Study. Interdiscip. Nurs. Res. 2024. [Google Scholar] [CrossRef]
- Grdinic, A.G.; Radovanovic, S.; Gleditsch, J.; Jørgensen, C.T.; Asady, E.; Pettersen, H.H.; Delibasic, B.; Ghanima, W. Developing a Machine Learning Model for Bleeding Prediction in Patients with Cancer-Associated Thrombosis Receiving Anticoagulation Therapy. J. Thromb. Haemost. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chiasakul, T.; Lam, B.D.; McNichol, M.; Robertson, W.; Rosovsky, R.P.; Lake, L.; Vlachos, I.S.; Adamski, A.; Reyes, N.; Abe, K.; et al. Artificial Intelligence in the Prediction of Venous Thromboembolism: A Systematic Review and Pooled Analysis. Eur. J. Haematol. 2023, 111, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, H.; Geng, X.; Li, Y.; Zhao, M.; Li, F.; Li, Z.; Ji, H.; Tian, H. Artificial Intelligence-Based Prediction of Lower Extremity Deep Vein Thrombosis Risk after Knee/Hip Arthroplasty. Clin. Appl. Thromb. Hemost. 2023, 29, 107602962211392. [Google Scholar] [CrossRef]
- Wang, K.Y.; Ikwuezunma, I.; Puvanesarajah, V.; Babu, J.; Margalit, A.; Raad, M.; Jain, A. Using Predictive Modeling and Supervised Machine Learning to Identify Patients at Risk for Venous Thromboembolism Following Posterior Lumbar Fusion. Glob. Spine J. 2023, 13, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.J.; Souto, J.C.; Lecumberri, R.; Obispo, B.; Sanchez, A.; Aparicio, J.; Aguayo, C.; Gutierrez, D.; Palomo, A.G.; Fanjul, V.; et al. Development of a Predictive Model of Venous Thromboembolism Recurrence in Anticoagulated Cancer Patients Using Machine Learning. Thromb. Res. 2023, 228, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, M.; Goumidi, L.; Iglesias, M.-J.; Munsch, G.; Bruzelius, M.; Ibrahim-Kosta, M.; Butler, L.; Odeberg, J.; Morange, P.-E.; Tregouet, D.A. Explainable Artificial Neural Network for Recurrent Venous Thromboembolism Based on Plasma Proteomics. In Computational Methods in Systems Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 108–121. [Google Scholar]
- Contreras-Luján, E.E.; García-Guerrero, E.E.; López-Bonilla, O.R.; Tlelo-Cuautle, E.; López-Mancilla, D.; Inzunza-González, E. Evaluation of Machine Learning Algorithms for Early Diagnosis of Deep Venous Thrombosis. Math. Comput. Appl. 2022, 27, 24. [Google Scholar] [CrossRef]
- Seo, J.W.; Park, S.; Kim, Y.J.; Hwang, J.H.; Yu, S.H.; Kim, J.H.; Kim, K.G. Artificial Intelligence-Based Iliofemoral Deep Venous Thrombosis Detection Using a Clinical Approach. Sci. Rep. 2023, 13, 967. [Google Scholar] [CrossRef]
- Alhwiti, T.; Aldrugh, S.; Megahed, F.M. Predicting In-Hospital Mortality after Transcatheter Aortic Valve Replacement Using Administrative Data and Machine Learning. Sci. Rep. 2023, 13, 10252. [Google Scholar] [CrossRef]
- Strange, G.; Stewart, S.; Watts, A.; Playford, D. Enhanced Detection of Severe Aortic Stenosis via Artificial Intelligence: A Clinical Cohort Study. Open Heart 2023, 10, e002265. [Google Scholar] [CrossRef]
- Ueda, D.; Matsumoto, T.; Ehara, S.; Yamamoto, A.; Walston, S.L.; Ito, A.; Shimono, T.; Shiba, M.; Takeshita, T.; Fukuda, D.; et al. Artificial Intelligence-Based Model to Classify Cardiac Functions from Chest Radiographs: A Multi-Institutional, Retrospective Model Development and Validation Study. Lancet Digit. Health 2023, 5, e525–e533. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chaudhary, R.; Bliden, K.P.; Tantry, U.S.; Gurbel, P.A.; Visweswaran, S.; Harinstein, M.E. Meta-Analysis of the Performance of AI-Driven ECG Interpretation in the Diagnosis of Valvular Heart Diseases. Am. J. Cardiol. 2024, 213, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Roshanitabrizi, P.; Rwebembera, J.; Okello, E.; Beaton, A.; Linguraru, M.G.; Sable, C.A. Using Artificial Intelligence for Rheumatic Heart Disease Detection by Echocardiography: Focus on Mitral Regurgitation. J. Am. Heart Assoc. 2024, 13, e031257. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Wyler von Ballmoos, M.C.; Moccetti, F.; Douverny, A.; Wolfrum, M.; Imamoglu, Z.; Mohler, A.; Gülan, U.; Kim, W.-K. A Fully Automated Artificial Intelligence-Driven Software for Planning of Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.D.; Tabada, G.; Allen, A.; Sung, S.H.; Go, A.S. Large-Scale Identification of Aortic Stenosis and Its Severity Using Natural Language Processing on Electronic Health Records. Cardiovasc. Digit. Health J. 2021, 2, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, G.; Zhao, L.; Zhao, S.; Xue, X.; Zhong, Y.; Yamauchi, H.; Tsukihara, H.; Maeda, E.; Ino, K.; Tomii, N.; et al. Automatic Aortic Valve Cusps Segmentation from CT Images Based on the Cascading Multiple Deep Neural Networks. J. Imaging 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Dasi, A.; Lee, B.; Polsani, V.; Yadav, P.; Dasi, L.P.; Thourani, V.H. Predicting Pressure Gradient Using Artificial Intelligence for Transcatheter Aortic Valve Replacement. JTCVS Technol. 2024, 23, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Desai, K.; Slostad, B.; Bhayani, S.; Arnold, J.H.; Ouwerkerk, W.; Hummel, Y.; Lam, C.S.P.; Ezekowitz, J.; Frost, M.; et al. Fully Automated Artificial Intelligence Assessment of Aortic Stenosis by Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-F.; Xie, Y.-L.; Wu, Q.-S.; He, J.; Lin, X.-F.; Qiu, Z.-H.; Chen, L.-W. A Predictive Model for Postoperative Adverse Outcomes Following Surgical Treatment of Acute Type A Aortic Dissection Based on Machine Learning. J. Clin. Hypertens. 2024, 26, 251–261. [Google Scholar] [CrossRef]
- Zhou, M.; Lei, L.; Chen, W.; Luo, Q.; Li, J.; Zhou, F.; Yang, X.; Pan, Y. Deep Learning-Based Diagnosis of Aortic Dissection Using an Electrocardiogram: Development, Validation, and Clinical Implications of the AADE Score. Kardiol. Pol. 2024, 82, 63–71. [Google Scholar] [CrossRef]
- Irtyuga, O.; Babakekhyan, M.; Kostareva, A.; Uspensky, V.; Gordeev, M.; Faggian, G.; Malashicheva, A.; Metsker, O.; Shlyakhto, E.; Kopanitsa, G. Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods. J. Pers. Med. 2023, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Bates, K.; Therrien, J.; Grossman, Y.; Kodaira, M.; Pressacco, J.; Rosati, A.; Dagenais, F.; Leask, R.L.; Lachapelle, K. Thoracic Aortic Aneurysm Risk Assessment. JACC Adv. 2023, 2, 100637. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Frohnert, P.P.; Gluliani, E.R.; Friedberg, M.; Johnson, W.J.; Tauxe, W.N. Statistical Investigation of Correlations between Serum Potassium Levels and Electrocardiographic Findings in Patients on Intermittent Hemodialysis Therapy. Circulation 1970, 41, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Marina-Breysse, M. Current and Future Use of Artificial Intelligence in Electrocardiography. J. Cardiovasc. Dev. Dis. 2023, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Xing, W.; Chen, Q.; Liu, X.; Pu, Y.; Xin, F.; Jiang, H.; Yin, Z.; Tao, D.; et al. Robust Artificial Intelligence Tool for Atrial Fibrillation Diagnosis: Novel Development Approach Incorporating Both Atrial Electrograms and Surface ECG and Evaluation by Head-to-head Comparison with Hospital-based Physician ECG Readers. J. Am. Heart Assoc. 2024, 13, e032100. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Vafaei Sadr, A.; Abedi, V.; Zand, R. Many Models, Little Adoption—What Accounts for Low Uptake of Machine Learning Models for Atrial Fibrillation Prediction and Detection? J. Clin. Med. 2024, 13, 1313. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, Z.; Yang, C.; Liu, J.; Liang, H. Machine Learning for Detecting Atrial Fibrillation from ECGs: Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2024, 25, 8. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Raheem, A.; Waheed, S.; Karim, M.; Khan, N.U.; Jawed, R. Prediction of Major Adverse Cardiac Events in the Emergency Department Using an Artificial Neural Network with a Systematic Grid Search. Int. J. Emerg. Med. 2024, 17, 4. [Google Scholar] [CrossRef]
- Abusnina, W.; Elhouderi, E.; Walters, R.W.; Al-Abdouh, A.; Mostafa, M.R.; Liu, J.L.; Mazozy, R.; Mhanna, M.; Ben-Dor, I.; Dufani, J.; et al. Sex Differences in the Clinical Outcomes of Patients with Takotsubo Stress Cardiomyopathy: A Meta-Analysis of Observational Studies. Am. J. Cardiol. 2024, 211, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.; Delmas, C.; Campelo-Parada, F.; Lhermusier, T.; Bouisset, F.; Elbaz, M.; Nader, V.; Blanco, S.; Roncalli, J.; Carrié, D. Takotsubo Cardiomyopathy. Rev. Cardiovasc. Med. 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, D.; Monaco, S.; Ting, T.W.; Narasimhalu, K.; Hsieh, J.; Kam, S.; Lim, J.Y.; Lim, W.K.; Davila, S.; Bylstra, Y.; et al. Cluster Analysis and Visualisation of Electronic Health Records Data to Identify Undiagnosed Patients with Rare Genetic Diseases. Sci. Rep. 2024, 14, 5056. [Google Scholar] [CrossRef] [PubMed]
- van Assen, M.; Razavi, A.C.; Whelton, S.P.; De Cecco, C.N. Artificial Intelligence in Cardiac Imaging: Where We Are and What We Want. Eur. Heart J. 2023, 44, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.M.; Katsaggelos, A.K.; Hammond, K.J.; Hong, H.; Ahmad, F.S.; Ouyang, D.; Shah, S.J.; McCarthy, P.M.; Thomas, J.D. Deep Learning for Cardiovascular Imaging: A Review. JAMA Cardiol. 2023, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Jone, P.-N.; Gearhart, A.; Lei, H.; Xing, F.; Nahar, J.; Lopez-Jimenez, F.; Diller, G.-P.; Marelli, A.; Wilson, L.; Saidi, A.; et al. Artificial Intelligence in Congenital Heart Disease. JACC Adv. 2022, 1, 100153. [Google Scholar] [CrossRef]
- Dahiya, E.S.; Kalra, A.M.; Lowe, A.; Anand, G. Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review. Sensors 2024, 24, 1318. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Park, J.J.; Hur, T.; Hua, C.-H.; Hussain, M.; Lee, S.; Choi, D.-J. Application and Potential of Artificial Intelligence in Heart Failure: Past, Present, and Future. Int. J. Heart Fail. 2024, 6, 11. [Google Scholar] [CrossRef]
- Dogan, S.; Barua, P.D.; Tuncer, T.; Acharya, U.R. An Accurate Hypertension Detection Model Based on a New Odd-Even Pattern Using Ballistocardiograph Signals. Eng. Appl. Artif. Intell. 2024, 133, 108306. [Google Scholar] [CrossRef]
- Becerra-Muñoz, V.M.; Gómez Sáenz, J.T.; Escribano Subías, P. La importancia de los datos en la hipertensión arterial pulmonar: De los registros internacionales al machine learning. Med. Clin. 2024. [Google Scholar] [CrossRef]
- Perek, S.; Nussinovitch, U.; Sagi, N.; Gidron, Y.; Raz-Pasteur, A. Prognostic Implications of Ultra-Short Heart Rate Variability Indices in Hospitalized Patients with Infective Endocarditis. PLoS ONE 2023, 18, e0287607. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Uzun Ozsahin, D.; Ozgocmen, C.; Balcioglu, O.; Ozsahin, I.; Uzun, B. Diagnostic AI and Cardiac Diseases. Diagnostics 2022, 12, 2901. [Google Scholar] [CrossRef] [PubMed]
- El Sherbini, A.; Rosenson, R.S.; Al Rifai, M.; Virk, H.U.H.; Wang, Z.; Virani, S.; Glicksberg, B.S.; Lavie, C.J.; Krittanawong, C. Artificial Intelligence in Preventive Cardiology. Prog. Cardiovasc. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bușilă, C.; Stuparu-Crețu, M.; Nechita, A.; Grigore, C.A.; Balan, G. Good Glycemic Control for a Low Cardiovascular Risk in Children Suffering from Diabets. Rev. De Chim. 2017, 68, 358–361. [Google Scholar] [CrossRef]
- Kanegae, H.; Suzuki, K.; Fukatani, K.; Ito, T.; Harada, N.; Kario, K. Highly Precise Risk Prediction Model for New-onset Hypertension Using Artificial Intelligence Techniques. J. Clin. Hypertens. 2020, 22, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.S.; Talukder, A.; Awal, M.A.; Siddiqui, M.M.U.; Ahamad, M.M.; Ahammed, B.; Rawal, L.B.; Alizadehsani, R.; Abawajy, J.; Laranjo, L.; et al. Machine Learning Approaches for Predicting Hypertension and Its Associated Factors Using Population-Level Data from Three South Asian Countries. Front. Cardiovasc. Med. 2022, 9, 839379. [Google Scholar] [CrossRef]
- Oh, G.C.; Ko, T.; Kim, J.-H.; Lee, M.H.; Choi, S.W.; Bae, Y.S.; Kim, K.H.; Lee, H.-Y. Estimation of Low-Density Lipoprotein Cholesterol Levels Using Machine Learning. Int. J. Cardiol. 2022, 352, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qin, S.; Wang, J.; Li, J.; Wang, H.; Li, H.; Chen, Z.; Li, C.; Wang, J.; Yuan, J. Develop and Evaluate a New and Effective Approach for Predicting Dyslipidemia in Steel Workers. Front. Bioeng. Biotechnol. 2020, 8, 839. [Google Scholar] [CrossRef]
- Correia, M.; Kagenaar, E.; van Schalkwijk, D.B.; Bourbon, M.; Gama-Carvalho, M. Machine Learning Modelling of Blood Lipid Biomarkers in Familial Hypercholesterolaemia versus Polygenic/EnvironmentalDyslipidaemia. Sci. Rep. 2021, 11, 3801. [Google Scholar] [CrossRef]
- Bușilă, C.; Stuparu-Crețu, M.; Barna, O.; Balan, G. Dyslipidemia in Children as a Risk Factor for Cardiovascular Diseases. Biotechnol. Biotechnol. Equip. 2017, 31, 1192–1197. [Google Scholar] [CrossRef]
- Adedinsewo, D.A.; Pollak, A.W.; Phillips, S.D.; Smith, T.L.; Svatikova, A.; Hayes, S.N.; Mulvagh, S.L.; Norris, C.; Roger, V.L.; Noseworthy, P.A.; et al. Cardiovascular Disease Screening in Women: Leveraging Artificial Intelligence and Digital Tools. Circ. Res. 2022, 130, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Thao, V.; Borah, B.J.; Attia, I.Z.; Medina Inojosa, J.; Kapa, S.; Carter, R.E.; Friedman, P.A.; Lopez-Jimenez, F.; Yao, X.; et al. Cost Effectiveness of an Electrocardiographic Deep Learning Algorithm to Detect Asymptomatic Left Ventricular Dysfunction. Mayo Clin. Proc. 2021, 96, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.; Farina, J.M.; Chao, C.-J.; Ayoub, C.; Jeong, J.; Patel, B.N.; Banerjee, I.; Arsanjani, R. The Role of Artificial Intelligence in Echocardiography. J. Imaging 2023, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Nedadur, R.; Wang, B.; Tsang, W. Artificial Intelligence for the Echocardiographic Assessment of Valvular Heart Disease. Heart 2022, 108, 1592–1599. [Google Scholar] [CrossRef]
- Almansouri, N.E.; Awe, M.; Rajavelu, S.; Jahnavi, K.; Shastry, R.; Hasan, A.; Hasan, H.; Lakkimsetti, M.; AlAbbasi, R.K.; Gutiérrez, B.C.; et al. Early Diagnosis of Cardiovascular Diseases in the Era of Artificial Intelligence: An in-Depth Review. Cureus 2024, 16, e55869. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Shrestha, S.; Kagiyama, N.; Hamirani, Y.; Kulkarni, H.; Yanamala, N.; Bing, R.; Chin, C.W.L.; Pawade, T.A.; Messika-Zeitoun, D.; et al. A Machine-Learning Framework to Identify Distinct Phenotypes of Aortic Stenosis Severity. JACC Cardiovasc. Imaging 2021, 14, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, X.; Lin, X.; Chen, X.; Wang, W.; Liu, B.; Li, Y.; Pu, H.; Zhang, L.; Huang, D.; et al. Automated Analysis of Doppler Echocardiographic Videos as a Screening Tool for Valvular Heart Diseases. JACC Cardiovasc. Imaging 2022, 15, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Jin, J.; Jiang, X.; Yang, L.; Fan, S.; Zhang, Q.; Chi, M. Artificial Intelligence Applied in Cardiovascular Disease: A Bibliometric and Visual Analysis. Front. Cardiovasc. Med. 2024, 11, 1323918. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, P.; Hong, Z.; Li, L.; Liu, N.; Bian, Z.; Chen, X.; Wu, H.; Zhao, S. Machine Learning in Risk Prediction of Continuous Renal Replacement Therapy after Coronary Artery Bypass Grafting Surgery in Patients. Clin. Exp. Nephrol. 2024, 1–11. [Google Scholar] [CrossRef]
- Bivolaru, S.; Constantin, A.; Vlase, C.M.; Gutu, C. COPD Patients’ Behaviour When Involved in the Choice of Inhaler Device. Healthcare 2023, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- De Ramón Fernández, A.; Ruiz Fernández, D.; Gilart Iglesias, V.; Marcos Jorquera, D. Analyzing the Use of Artificial Intelligence for the Management of Chronic Obstructive Pulmonary Disease (COPD). Int. J. Med. Inform. 2022, 158, 104640. [Google Scholar] [CrossRef] [PubMed]
- Al Namat, R.; Duceac, L.D.; Chelaru, L.; Dabija, M.G.; Guțu, C.; Marcu, C.; Popa, M.V.; Popa, F.; Bogdan Goroftei, E.R.; Țarcă, E. Post-Coronary Artery Bypass Grafting Outcomes of Patients with/without Type-2 Diabetes Mellitus and Chronic Kidney Disease Treated with SGLT2 Inhibitor Dapagliflozin: A Single-Center Experience Analysis. Diagnostics 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Moinul, M.; Amin, S.A.; Kumar, P.; Patil, U.K.; Gajbhiye, A.; Jha, T.; Gayen, S. Exploring Sodium Glucose Cotransporter (SGLT2) Inhibitors with Machine Learning Approach: A Novel Hope in Anti-Diabetes Drug Discovery. J. Mol. Graph. Model. 2022, 111, 108106. [Google Scholar] [CrossRef]
- Vidal-Perez, R.; Grapsa, J.; Bouzas-Mosquera, A.; Fontes-Carvalho, R.; Vazquez-Rodriguez, J.M. Current Role and Future Perspectives of Artificial Intelligence in Echocardiography. World J. Cardiol. 2023, 15, 284–292. [Google Scholar] [CrossRef]
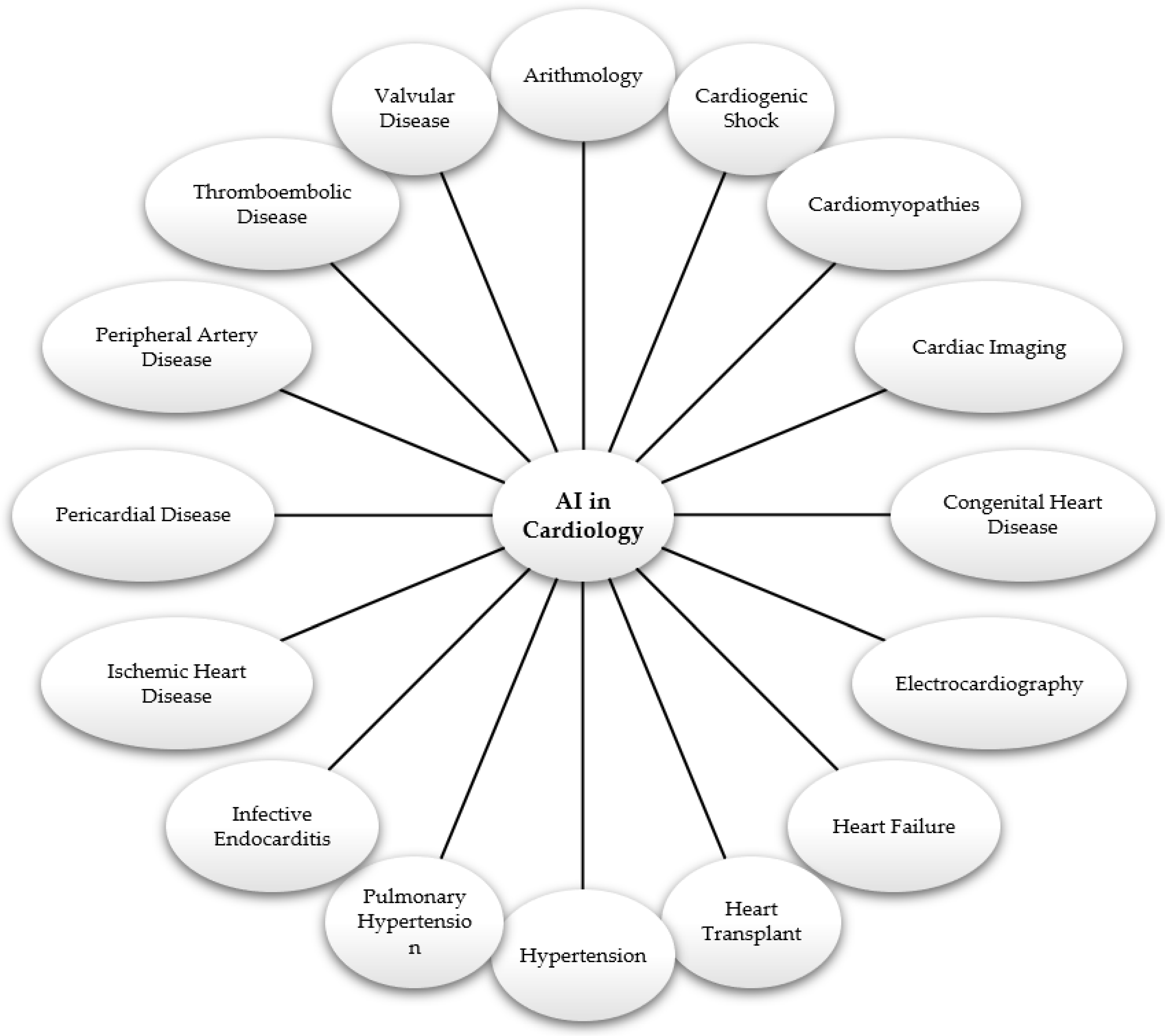
| Term | Definitions | References |
|---|---|---|
| Artificial intelligence (AI) | Artificial intelligence (AI) is a subtype of information technology that through algorithms can analyze (receive, process, and interpret) medical information and perform complex mathematical calculations, simulating artificially what happens in the human mind during learning. | [14] |
| Machine learning (ML) | Machine learning (ML) is the ability of computer systems to automatically learn from existing data and past experiences to find patterns and make future predictions. ML is a well-known subtype of AI and can be grouped into three categories: supervised learning, unsupervised learning, and reinforcement learning. In medicine, ML can incorporate and manage various data resources (from clinical and biological observations to wearable devices and environmental information) to create models that can predict and diagnose certain diseases. Additionally, ML can personalize disease treatment to improve the healthcare system. In conclusion, ML is one of the fastest, most convenient, and cost-effective ways of detecting disease through artificial intelligence technology. | [15,16,17,18] |
| Deep learning (DL) | Deep learning (DL) is a subtype of machine learning that can analyze massive amounts of data to provide greater accuracy in creating concepts and accurately predicting pathologies. DL is currently one of the most applied algorithms for medical purposes, alongside support vector machine (SVM) and artificial neural network (ANN). | [4,19] |
| Cognitive computing | Cognitive computing systems are artificial intelligence systems that are part of machine learning and understand, reason, and enhance human brain capabilities by combining virtual technology and natural language processing. | [20] |
| Supervised learning | Training the ML algorithm using labeled examples consisting of inputs and outputs provided by an expert is a phenomenon known as supervised learning. Supervised learning encompasses artificial neural networks (ANNs), support vector machine (SVM), decision tree, random forest, fuzzy logic, naive Bayes (NB), K-nearest neighbor (KNN), and regression. | [17,21] |
| Unsupervised learning | This involves training the ML algorithm to process data and perform classification of samples without category information, thus without human intervention. Unsupervised learning includes clustering algorithms and association rule-learning algorithms. | [21,22] |
| Reinforcement learning | Reinforcement learning is a subtype of machine learning that can be considered a combination of supervised and unsupervised learning and can facilitate efforts to increase algorithm accuracy. It is a learning strategy for optimal learning regarding a specific criterion in a given situation. This algorithm receives feedback on its performance by comparing rewards obtained during training with the chosen criterion. | [23,24] |
| Convolutional neural networks (CNNs) | Deep learning (DL), a method primarily used in image processing and understanding or classifying images, involves models similar to those used in the visual cortex for processing images. Convolutional neural networks (CNNs) are neural networks similar to regular neural networks, as they are composed of neurons with weights that can be learned. However, CNNs explicitly assume that inputs have specific structures, such as images. | [21,25,26] |
| Recurrent neural networks (RNNs) | RNNs are different from CNNs in that the input data are of variable size, which can be processed by the RNN; moreover, the outputs of intermediate-layer neurons are cyclically captured in the original input. When many recurrent neurons exist in a recurrent layer, the sequential data are processed in parallel through different weights, allowing RNNs to generate multiple representations and create effective feature space separation. | [27] |
| Deep neural networks (DNNs) | A DL architecture with multiple layers between the input and output layers. | [21] |
| Artificial neural network (ANN) | An ML technique that processes information in an architecture comprising many layers (“neurons”), with each interneuronal connection extracting the desired parameters incrementally from the training data. | [21,28] |
| Support vector machine (SVM) | A supervised learning model that can efficiently perform linear and nonlinear classifications, implicitly mapping their inputs into high-dimensional feature spaces. | [29] |
| Decision tree (DT) | This nonparametric supervised learning method is visualized as a graph representing the choices and their outcomes in the form of a tree; each tree consists of branches (values that a node can take) and nodes (attributes in the group to be classified). | [30] |
| Random tree (RT) | This is an ensemble classification technique that uses “parallel ensembling”, fitting several decision tree classifiers in parallel on dataset subsamples. | [30] |
| Naïve Bayes (NB) | A classification technique assuming independence among predictors, Naive Bayes is a tool that works with the most basic knowledge of probability. Bayes’ rule is a formula that determines the probability that Y will happen with a given X. The Bayes technique makes the naive assumption of independence of all characteristics. It attempts to find probabilities based on known prior probabilities that have been learned from training data. | [29] |
| Fuzzy logic | Fuzzy logic is part of supervised learning which allows multiple possible truth values to be processed through the same variable. | [30] |
| K-nearest neighbor (KNN) | This non-generalizing learning algorithm or an “instance-based learning” does not focus on constructing a general internal model but rather stores all instances corresponding to the training data in an n-dimensional space and classifies new data points based on similarity measures. | [30] |
| Regression | This is an algorithm using a logistic function to estimate probabilities that can overfit high-dimensional datasets, being suitable for datasets that can be linearly separated. | [30] |
| Clustering algorithms | Data clustering is an essential part of extracting information from databases and is part of unsupervised learning. There are several ways to split the data, the most important of which are horizontal and vertical collaborative clustering. | [31] |
| Association rule-learning algorithms | Association rule learning and correlation learning methods are used to find and weigh contextual relations between modeled context entities. In the presence of a training dataset, a unique classification strategy is introduced, which can effectively increase classification performance. | [32,33,34] |
| Year of Study | Author | Application | Data Source | Machine Learning Method | Future Direction | |
|---|---|---|---|---|---|---|
| Arrhythmias | 2023 | Tran, K.-V. [35] | AF detection | mECG | CNN | Studies to improve the AI algorithm of commercial wearable devices for AF detection |
| 2023 | Baj, G. [36] | Prediction of new-onset AF | ECG | CNN XGB LR | Integration of demographic information (gender, age) or clinical information as predictors in addition to ECG; clinical interpretability is a fundamental step to building predictive tools for clinical usage | |
| 2023 | Raghunath, A. [37] | Prediction of AF | mECG | CNN DNN | Differentiating atrial fibrillation from atrial flutter using the availability of information such as clinical indicators, socio-economic status, or racial background | |
| 2023 | Jiang, J. [38] | Prediction AF recurrence 12 months after catheter ablation | ECG | CNN | Larger batches of patients are analyzed to improve applicability and accuracy. In addition, a prospective study is needed; other studies assess recurrence after more than 12 months | |
| 2021 | Bai, Y. [39] | Prediction of AF recurrence after catheter ablation | ECG | CNN | To generalize the premise, larger batches of patients were analyzed, with more pathologies | |
| Cardiogenic Shock | 2022 | Rahman, F. [40] | Predicting patients at high risk of developing cardiogenic shock (CS) | Demographic, vital signs, laborator, medication | DT RF SVM KNN LR | Future studies will assess how early identification of cardiogenic shock and potential effects on prompt treatment may alter patient outcomes |
| 2021 | Bai, Z. [41] | Predictive model of evolution towards CS in patients with STEMI | Demographic, pre-existing diagnoses, ECG, laboratory | LASSO LR SVM DT | Larger groups of patients; blood glucose analysis used as a predictive factor of CS | |
| 2022 | Chang, Y. [42] | CS prediction 2 h before the need for the first intervention | Demographic, vital signs, laboratory | XGB MLP TCN | Future studies to include the integration of HF or AMI specific elements to increase accuracy | |
| 2023 | Jajcay, N. [43] | Predicting CS in acute coronary syndrome (ACS) patients | Demographic, vital signs, laboratory, ECG | KNN | Superior computational power would include more models for analysis and allow the imputation analysis of analyzing more datasets | |
| 2022 | Jentzer, J. C. [44] | Phenotype CS | Laboratory | HC LCA KMCk | Integration of multi-biomarker/imaging (ECG, echocardiography, angiography) to understand the differences in underlying pathophysiology that separate these clinical subphenotypes could improve phenotyping | |
| 2023 | Wang, L. [45] | Clinical phenotypes of CS | Demographic and medical history, vital signs, laboratory, treatment | CA | Association between endpoints within individual SCAI stages and ML-derived phenotypes whose aim is to characterize disease severity as it evolves over the course of a hospital stay | |
| 2022 | Bohm, A. [46] | Clinical predictive model of progression to CS in patients with ACS | Demographic, vital signs, laboratory | LR | Validation on an external cohort | |
| 2023 | Popat, A. [47] | Early prediction of CS in acute heart failure or MI | Demographic, Laboratory | ML | Conducting studies also outside the USA | |
| 2022 | Rong, F. [48] | Predicting the 30-day mortality of elderly patients with CS | Demographic, vital sign, laboratory, comorbidities, Echocardiograpy | Cox model LASSO SAPSII | Larger groups of patients | |
| 2023 | Mo, Z. [49] | Assessing the prognosis of ECMO treatment in elderly patients with CS | Demographic, vital sign, laboratory, comorbidities, medications | RF DT | A larger batch of patients followed for more than 6 months | |
| Cardiomyopathy | 2024 | Cau, R. [50] | Differential diagnosis of cardiomyopathy phenotypes | Demographic, vital sign, laboratory, ECG | CNN | Randomized trials are crucial |
| 2023 | Haimovich, J. S. [51] | Differential diagnosis between LV hypertrophy: cardiac amyloidosis and hypertrophic cardiomyopathy | Demographic, vital signs, laboratory, medication, ECG | CNN | Studies on cardiomyopathies to include the athletic heart | |
| 2023 | Beneyto, M. [52] | Predicting hypertensive origin in left ventricular hypertrophy (LVH) | Clinical, Laboratory, ECG, echocardiograpy | DT RF SVM | Additional studies from non-tertiary centers | |
| 2022 | Eckstein, J. [53] | Diagnosis of cardiac amyloidosis (CA) | Demographic, clinical, echocardiogray, CMR | KNN SVM DT | Multicenter evaluation of patients with early stage cardiac amyloidosis | |
| 2021 | Siontis, K. C. [54] | Diagnosis of hypertrophic cardiomyopathy (HCM) in children and adolescents | Demographi, ECG, | CNN | Multicenter studies in children under 5 years | |
| 2020 | Ko, W.-Y. [55] | Diagnosis of HCM particularly in younger patients | Demographic, ECG | CNN | Further refinement and external validation | |
| 2022 | Hwang, I.-C. [56] | Differential diagnosis of LVH | Echocardiography | CNN | Future studies that include rare LVH etiologies: Fabry disease, Danon syndrome, transthyretin amyloidosis | |
| 2023 | Zhou, M. [57] | Differentiating ischemic cardiomyopathy from dilated cardiomyopathy | Echocardiography | RF LR CNN XGB | Multicenter external validation on larger patient batches | |
| 2023 | Cau, R. [58] | Diagnosis of Takotsubo cardiomyopathy | Demographic, Echocardiography | RT | Longitudinal and prospective studies to assess predictive performance in different cohorts and validate these findings | |
| 2023 | De Filippo, O. [59] | The prediction of prognosis in hospital patients with Takotsubo syndrome | Demographic, ECG, Echocardiograpy, laboratory, medications | LR CA | Multicenter studies beyond European and Asian ethnicities | |
| 2021 | Jefferies, J. L. [60] | Predictive screening model for potential patients with Fabry disease | Demographic, clinical, echocardiography, medications, laboratory | ML | Future clinical implementation studies | |
| 2022 | Sotto, J. [61] | The prediction of etiology of LVH | ECG Echocardiograpy | CNN | Multicenter studies to identify other causes of ventricular hypertrophy, such as Fabry disease or cardiac amyloidosis | |
| 2023 | Zhang, Y. [62] | Diagnosis of arrhythmogenic cardiomyopathy (ACM) and dilated cardiomyopathy (DCM) | Echocardiograpy, Genes | CA RF | Checking for other genes involved in pathogenesis of ACM and DCM | |
| 2022 | Papageorgiou, V. E. [63] | Detection of an arrhythmogenic heart disease (ARVD) | ECG | CNN | Checking integration into clinical practice | |
| 2023 | Harmon, D.M. [64] | Validation study of cardiac amyloidosis diagnosis | Demographic, ECG | KNN SVM DT CNN | Future multicentric studies to validate the diagnosis in different ethnicities in the presence of left bundle branch block or LVH | |
| 2023 | Cotella, J. I. [65] | Measuring ejection fraction and longitudinal strain in cardiac amyloidosis | Echocardiograpy | LR | Studies on larger batches of patients | |
| 2023 | Zhang, X. [66] | Non-invasive diagnostic method for myocardial amyloidosis | Echocardiograpy | RF SVM LR | Studies on larger, multicentre patient groups | |
| 2023 | Goswami, R. [67] | Predicting death or transplantation of transthyretin amyloid cardiomyopathy (ATTR-CM) | Hemodynamic, clinical, Echocardiograpy | CNN | Larger groups of patients in multicenter studies | |
| 2023 | Michalski, A. A. [68] | Diagnosis of Fabry disease | Demographics, clinical, Echocardiograpy, laboratory | NLP | Prospective studies on larger groups of patients with improved NLP | |
| 2023 | Jefferies, J. [69] | Predicting the risk of arrhythmias in Fabry disease | Demographics, Clinical data, ECG, Echocardiograpy | ML | Multicentric studies | |
| 2023 | Stolpe, G. [70] | The prediction of sudden cardiac death in HCM | Demographic, Clinical data, Echocardiograpy | RF | Definition of sensitivity and specificity | |
| Cardiovascular Imaging | 2022 | Zhang, X. [71] | A predictive model for classifying HCM, DCM, and healthy patients | CMR | RF | Multicentric studies |
| 2023 | Tatsugami, F. [72] | Prediction of myocardial infarction or cardiac death | CT cardiac | CNN | External validation for cardiac CT on larger datasets | |
| 2022 | O’Brien, H. [73] | Diagnosis of myocardial scars | MRI-LGE CT-DE | SVM | Larger studies with larger CT-DE data to establish optimal imaging parameters and characteristics | |
| 2022 | Wen, D. [74] | Identification of hemodynamically significant coronary artery stenosis | CCTA FFR | DT | Validation studies on larger groups of patients | |
| 2023 | Lara-Hernández, A. [75] | Quantitative myocardial perfusion | CCTA | DL | Extending the method to other dynamic imaging modalities | |
| 2023 | Griffin, W.F. [76] | Detection and grading of coronary stenoses | CCTA QCA FFR | ML | Application of the method after the results of the INVICTUS trial | |
| 2022 | Bandt, V. [77] | Identify significant CAD pre-TAVR | CCTA | ML | Additional studies on larger batches | |
| 2022 | Li, X.-N. [78] | Highlighting the vulnerability of the coronary artery plate | CCTA | ML | Validation studies | |
| 2021 | Lyu, T. [79] | Deep learning approach in reducing the radiation dose of CT | CT cardiac | CNN | Additional studies on larger batches | |
| 2023 | Zhang, R. [80] | Diagnosis of myocardial perfusion imaging | SPECT-CT | CNN | The larger cohort in the next stage of our study | |
| 2023 | Khunte, A. [81] | Detection of left ventricular systolic dysfunction | ECG, Echocardiography | CNN | Use of this algorithm as a screening method for LV systolic dysfunction among individuals with no clinical disease | |
| 2024 | Pieszko, K. [82] | Prediction of left atrial appendage thrombus (LAT) | TTE TOE | LR, DT | Studies in different populations to assess the performance and to evaluate performance in specific subgroups based on sex or race | |
| 2023 | Liu, Z. [83] | Diagnosis of right ventricular abnormalities | CMR | DNN | Another validation study by comparing with human experts | |
| 2023 | Wang, Y. [84] | Improved myocardial strain analysis | CMR | CNN | Larger batches | |
| 2024 | Yu, J. [85] | Assessment of LV function | TOE | ML | Larger batches | |
| 2023 | Laumer, F. [86] | Differential diagnosis of Takotsubo syndrome (TTS) and acute myocardial infarction (AMI) | TTE | CNN | Future studies on a larger population are to differentiate the two conditions in the acute phase | |
| 2024 | Lee, D. [87] | Detecting obstructive CAD | CCTA | DL | Larger batches | |
| 2023 | Kapalos, A. [88] | Segmentation of cardiac T1 and T2 mapping images | CMR | DNN | Future work will focus on ensuring the accurate measurement of tissue properties | |
| Congenital Heart Disease | 2023 | Ishikita, A. [89] | Prediction of adverse cardiovascular events in adults with repaired tetralogy of Fallot | CMR Clinical data | RF | Follow-up studies including multiple centers with longer follow-up |
| 2023 | De Vries, I.R. [90] | Screening of congenital heart disease (CHD) | ECG | ANN | Future work should aim to improve the signal processing chain to omit or reduce the need for combining multiple heartbeats, potentially further allowing for the additional analysis of arrhythmias and better performance | |
| 2021 | Lv, J. [91] | Screening and detecting CHD in children | Heart sounds | CNN | Larger patient cohorts | |
| 2022 | Majeed, A. [92] | A greater risk of developing executive function deficits in children with complex CHD | Demographic, medical and surgical history, family social class | RF DT | Larger patient cohorts | |
| 2022 | Sakai, A. [93] | Detection of small ventricular septal defects | Fetal cardiac ultrasound | DNN | Multicenter joint research. | |
| 2022 | Gearhart, A. [94] | Analysis of pediatric echocardiograms | ETT | CNN | Future work could incorporate transfer learning to reduce the manual workload | |
| Electrocardiography | 2023 | Valente Silva, B. [95] | Diagnosis of pulmonary embolism | ECG | DNN | External samples from other centers |
| 2023 | Adedinsewo, D. [96] | Detecting moderate-to-severe acute cellular rejection (ACR) among heart transplant (HT) recipients | Demographic, clinical characteristic, ECG, TTE | CNN | Future directions would include evaluating the potential benefit of combining AI-ECG screening with blood testing methods, evaluation of the AI-ECG for detecting ACR and antibody-mediated rejection (AMR) combined | |
| 2023 | Shiraishi, Y. [97] | Risk of sudden cardiac death | ECG | CNN RNN | Studies on larger batches of patients; future studies will include batches of patients treated with angiotensin receptor-neprilysin inhibitors and sodium-glucose cotransporter 2 inhibitors | |
| 2023 | Hirota, N. [98] | The biological age of the patient is associated with cardiovascular events | ECG | CNN | The clinical significance of AgeDiff in patients older than 60 years should be re-evaluated in different cohorts, such as multicenter cohorts or the general population | |
| 2023 | Wouters, P. C. [99] | Effects of cardiac resynchronization therapy | ECG | DNN | Prospective studies; the results need to be validated in a patient group that received a CRT-P device | |
| 2023 | Raghunath, A. [37] | Onset of atrial fibrillation | ECG | CNN RNN | Future studies with characteristic information for the population (e.g., racial background) | |
| 2023 | Liu, C.-W. [100] | Prediction of LVH | ECG | CNN DNN | Analyzing other surrogate end points in cardiovascular diseases (such as left ventricular diastolic dysfunction, new-onset myocardial infarction, or heart failure) | |
| 2023 | Zaver, H. [101] | Predicting cardiac events and incident AF in patients who have received a liver transplant | ECG | CNN DNN | Larger patient cohorts | |
| 2023 | Naser, J. [102] | Concentration of sex hormones | ECG; Laboratory | CNN | Larger patient cohorts | |
| 2023 | Vaid, A. [103] | Valvular diseases | ECG | CNN | Further external validation from another health system | |
| 2023 | Jiang, J. [38] | Predicting the risk of recurrence in patients with paroxysmal atrial fibrillation after catheter ablation | ECG | CNN | Further calibration and validation using high-quality prospective studies | |
| Heart Failure | 2023 | Khan, M. S. [104] | Early diagnosis of HF, stratifying HF disease severity | Demographic, clinical parameters, ECG, TTE | SVM ANN CNN | More datasets are needed for validation and increased transparency through an understanding of AI models |
| 2023 | Almujally, N. A. [105] | Remote monitoring of patients with acute heart failure | Demographic, clinical, laboratory | CNN RNN | The IoT-based system can be further expanded with different types of wearable medical healthcare devices that can be operated on tablets or smartphones | |
| 2022 | Kobayashi, M. [106] | Predicting heart failure incidence in asymptomatic individuals | Demographic, TTE, laboratory, medication, cardiovascular risk factors | CA DT | A further prospective multicenter study is needed to assess the applicability | |
| 2020 | Segar, M.W. [107] | Phenomapping of patients with HF with preserved ejection fraction (HFpEF) | TTE, Laboratory, | CA | The use of more comprehensive data, as well as a larger number of patient variables, may have yielded different results | |
| 2024 | Bourazana, A. [108] | HF diagnosis, monitoring, and management | Clinical examinations, TTE, laboratory | SVM ANN CNN | Issues that remain unresolved concern diagnosis, classification, and treatment. Future studies will assess heart failure with preserved ejection fraction (HFpEF) | |
| 2022 | Bachtiger, P. [109] | Screening for HF with reduced ejection fraction | ECG ETT | CNN | Screening for further priority cardiovascular diseases, such as valvular heart pathology, using AI-enabled phonocardiography | |
| 2021 | Harmon, D. M. [110] | Diagnosis of HF with reduced ejection fraction (HFrEF) | ECG | CNN | Prospective evaluation of the AI-ECG for ventricular dysfunction for treatment of HF in the acute setting | |
| 2021 | Kwon, J.-M. [111] | Early detection of HFpEF | ECG | DLM | The algorithm used must be further validated in patients with HFrEF in other countries | |
| 2024 | Wu, J. [112] | Predicting mortality in patients with undiagnosed HFpEF | Demographic, ETT | NLP | Other observational studies | |
| 2023 | Akerman, A. P. [113] | Detection of HF | ETT ECG | CNN | Future work is required to guide more transparent and patient-level interpretation | |
| 2021 | Pană, M.-A. [114] | Predicting HF exacerbation | Patient’s voice | SVM ANN KNN | Larger batches of patients | |
| 2024 | Cheungpasitporn, W. [115] | Treatment for HF patients with acute kidney disease | Demographic, vital signs, laboratory, medication | ML | External validation | |
| 2023 | Kamio, T. [116] | Predicting clinical outcomes in acute heart failure | Demographic, vital signs, laboratory, comorbidities, medication | CNN SVM LSVCe XGB | Further research is required to determine the generalizability of these conclusions to other populations and settings | |
| Heart Transplant | 2022 | Naruka, V. [117] | Predicting graft failure and mortality | Demographic, biomarker, CMR | ANN CNN RF SVM DT | Prospective multicenter studies collecting data on the immunosuppression regime or the causative factors for length of hospital stay were not studied |
| 2021 | Briasoulis, A. [118] | Predicting survival and acute rejection after HT | Clinical and Laboratory | LR DT SVM KNN | Future studies with analysis of other predictors after heart transplantation | |
| 2023 | Seraphin, T. P. [119] | Predicting the degree of cellular rejection from pathology | Pathological archive | CNN | Validation in larger cohorts for clinical-grade AI biomarkers | |
| 2022 | Ozcan, I. [120] | Predicting major adverse cardiovascular events (MACE) risk post-HT | Clinical and laboratory, ECG | CNN | Further research is to guide screening and treatment strategies for HT patients using this algorithm | |
| 2023 | Sharma, S. [121] | Predicting the susceptibility of HT patients to COVID-19 | Demographic, Clinical, Laboratory | CNN MLP | Further research and implementation of these technologies in diagnosis, monitoring, and detection | |
| 2020 | Glass, C. [122] | Identify myocyte damage in HT acute cellular rejection (ACR) | Comorbidities | CNN | Validation in prospective studies with larger cohorts | |
| 2023 | Al-Ani, M. A. [123] | Predicting survival rates in intra-aortic balloon pump (IABP) used as a bridge to HT compared to the Impella | Clinical, Laboratory, Related Disease | XGB | External validation | |
| 2021 | Peyster, E. G. [124] | Histological grading of cardiac allograft rejection | Endomyocardial biopsy slides | SVM | External validation | |
| 2022 | Lipkova, J. [125] | Assessment of cardiac allograft rejection from | Endomyocardial biopsy slides | CNN | Larger studies | |
| 2023 | GIuste, F.O. [126] | Enhancing risk assessment of rare pediatric heart transplant rejection | Heart Biopsy | CNN DNN | Future work will focus on generating additional expert-annotated examples of cellular rejection signs to further improve and validate our model’s performance | |
| 2022 | Lisboa, P. J. G. [127] | Survival prediction in heart transplantation | Clinical, Demographic | GANN PRN | Prospective studies | |
| 2022 | Ruiz Morales, J. [128] | Predicting outcomes after HT | ECG | CNN | Larger studies | |
| 2020 | Agasthi, P. [129] | Predicting 5-year mortality and graft failure in patients with HT | Demographic, clinical | GBM | Larger studies | |
| 2021 | Ozcan, I. [130] | Predicting MACE after HT | ECG | CNN | Larger studies | |
| Hypertension | 2020 | Soh, D. C. K. [131] | Diagnosis of hypertension | ECG | KNN | Future studies with larger cohorts and validation studies |
| 2020 | López-Martínez, F. [132] | Predicting hypertension | Demographic, laboratory, vital signs | ANN | Future studies with new risk factors | |
| 2020 | Wu, X. [133] | Clinical prediction of hypertension in young patients | Clinical, comorbidities | XGB | Larger studies | |
| 2020 | Aziz, F. [134] | Effectiveness of hypertension treatment | Demographic, clinical, treatment | ANN SVR | Studies on larger batches of patients | |
| 2020 | Koshimizu, H [135] | Blood pressure variability | Demographic, vital signs | DNN | Longer-term studies | |
| 2023 | Hamoud, B. [136] | Estimating blood pressure | Videos of subjects | CNN | Extending the training set with subjects who are older or estimating other vital signs such as heart rate, oxygen saturation, and body temperature | |
| 2023 | Cheng, H. [137] | Blood pressure prediction | IPPG | CNN | Larger studies | |
| 2023 | Xing, W. [138] | Hypertension screening and disease prevention | Facial videos | DNN | Larger studies | |
| 2023 | Visco, V. [139] | Hypertension prediction and management | Clinical, Laboratory, TTE | ANN SVM KNN | Implementation studies | |
| 2022 | Maqsood, S. [140] | Blood pressure estimation and measurement | PPG ECG | DNN | Future studies will combat the “Black Box” because they lack the declarative knowledge necessary to explain the outcomes further | |
| 2023 | Herzog, L. [141] | Hypertension management | Demograpic, clinical, Comorbidities | DNN | Validation of results with a randomized controlled clinical trial | |
| 2021 | Khthir, R. [142] | Predictive factors for hypertension in patients with type 2 diabetes | Demographic, Clinical data, Laboratory | RF | Larger studies | |
| 2023 | Aryal, S. [143] | Evidence that blood pressure is closely correlated with the microbiota | Demographic, clinical, laboratory, genetic tests | XGB RF NB | Larger studies and prospective studies | |
| 2023 | Lin, Z. [144] | Prediction of hypokalemia in patients with hypertension | Laboratory, comorbidities, antihypertensive medications | RF | Prospective studies | |
| Pulmonary Hypertension | 2020 | Kusunose, K. [145] | Confirmation of the need for RHC in patients with suspected pulmonary hypertension (PH) | Clinical Findings CXR ETT PAP | CNN | Further validation is necessary to determine the feasibility of CXR and larger numbers for the differentiation of pre- and post-capillary PH |
| 2021 | Hardacre, C. J. [146] | MRI may allow for reduction of right heart catheterization (RHC) | CMR, laboratory | CNN DT RF | Future studies with AI to diagnose PAH that can be applied to CT | |
| 2022 | Ragnarsdottir, H. [147] | Prediction of PH in newborns | ECG | CNN | Larger batches of patients | |
| 2021 | Chakravarty, K. [148] | PH therapies for COVID-19 treatment | Clinical, laboratory | RF | Further validation is necessary | |
| 2021 | Rahaghi, F. N. [149] | Detection of pulmonary arterial disease | Demographic, PAP, CT imaging, ECG, ETT, Laboratory | CNN | Future studies on the multicenter registry; further work is to determine the specificity and sensitivity of such metrics as well as their value for prognostication and monitoring response to therapeutic intervention | |
| 2022 | Shi, B. [150] | Prediction of PH | Biomarkers, Hemodynamics, PAP | DT RF RT LR SVM | Prospective studies on human subjects | |
| 2021 | Amodeo, I. [151] | Predict PH in newborns with congenital diaphragmatic hernia | CMR images | CNN SVM | Future prospective multicenter cohort study for validation | |
| 2022 | Van der Bijl, P. [152] | Diagnosis of PH | ETT CXR | ANN | studies including patients with tricuspid regurgitation | |
| 2021 | Swift, A. J. [153] | Diagnosis of PH | CMR PAP, ETT | SVM LR | Larger cohorts | |
| 2022 | Charters, P. F. P. [154] | Predicting survival in patients with suspected PH | ETT, CTPA | ML | Larger studies with larger cohorts | |
| 2022 | Fortmeier, V. [155] | Predicting PH in patients with severe TR | ETT PAP demographic, laboratory, comorbidities | XGB | Randomized controlled trials to investigate whether specifically patients with elevations of predicted mPAP still benefit from transcatheter interventions | |
| 2022 | Liu, C.-M. [156] | Predicting future risk for cardiovascular mortality in patients with PH | ECG ETT | CNN | Further studies on larger cohorts are necessary for validation | |
| 2023 | Lu, W. [157] | Diagnostic biomarkers for idiopathic pulmonary hypertension (IPAH) | Gene | RF | Larger studies | |
| 2023 | Yu, X. [158] | PH diagnosis | ETT, PAP CT images | KNN | Larger studies | |
| 2023 | Hyde, B. [159] | Distinguishing between patients with PAH and those without PAH at 6 months before diagnosis | Demographics, diagnoses, laboratory, medications | RF | External validation | |
| 2023 | Zhang, N. [160] | Diagnosis of PH | ETT, CTPA | SVM XGB | Future studies will explore the volumetric information of heart and pulmonary artery morphology as well as the spatial relationship between different intra- and extra-cardiac structures to improve the accuracy of PAP parameter evaluation | |
| 2024 | Hirata, Y. [161] | Predicting PH | RHC | LR | Validation and further research to assess clinical utility in PH diagnosis and treatment decision making | |
| 2024 | Imai, S. [162] | PAH detection | CXR PAP | CNN | Future studies should include diseases with CXR images similar to PAH, such as cardiac hypertrophy, and collect data from diverse clinical settings | |
| 2024 | Ragnarsdottir, H. [163] | Assessing PH in newborns | ETT | CNN | Future studies could be applied to ECHOs from the adult population, with retraining required | |
| 2024 | Dwivedi, K. [164] | Improving PH prognosis by detecting pulmonary fibrosis | Demographic, CT pulmonary angiograms, ETT | CNN | Validation study | |
| 2023 | Griffiths, M. [165] | Risk prediction model for pediatric PH | Demographics, imaging, hemodynamics, laboratory, comorbidities | RF | Future studies could be applied to the adult population | |
| 2023 | Mamalakis, M. [166] | Diagnosis of PH | CT images | CNN | Larger studies | |
| 2024 | Tchuente Foguem, G. [167] | Prognosis of survival of PH | Demographics, ETT | SVM | Larger studies | |
| 2024 | Han, P.-L. [168] | Diagnosis of congenital heart disease and associated PAH | CXR, CMR ETT | RF | Larger studies | |
| 2024 | Anand, V. [169] | Diagnosis PH | ETT PAP ECG | ML | Larger studies | |
| Infective Endocarditis | 2024 | Lai, C. K.-C. [170] | Predicting infective endocarditis (IE) | Demographic, clinical, ETT, comorbidities | RF | Multicentric study and prospective studies |
| 2024 | Yi, C. [171] | Common biomarkers in IE and sepsis | Laboratory tests, genes | RF LASSO | Future research will require more extensive datasets and experimental validation. Future studies also demonstrate differences between subtypes of sepsis, including significant variations in the abundance and expression levels of immune cell populations | |
| 2023 | Galizzi Fae, I. [172] | Risk stratification of patients hospitalized with IE | Laboratory, imaging, treatment, comorbidities | LR DT | Larger studies | |
| Ischemic Heart Disease | 2022 | Chen, Z. [173] | Myocardial infarction segmentation | CMR | CNN | Future studies that can differentiate old myocardial scar from acute myocardial infarction |
| 2021 | Rauseo, E. [174] | Diagnosis of chronic ischemic disease | CMR | SVM RF | Further studies to determine whether there is a correlation between the radiomic features and the extent of myocardial scarring | |
| 2021 | Liu, W.-C. [175] | Diagnosis of acute myocardial infarction (AMI) at the emergency department (ED) | Clinical, ECG, Laboratory | DL | Future studies on large-scale, multi-institute, prospective, or randomized control studies are necessary to further confirm real-world performance | |
| 2020 | Zhao, Y. [176] | Early detection of ST-segment elevated myocardial infarction (STEMI) | ECG | CNN | Future studies should be validated in various ethnics | |
| 2020 | Cho, Y. [177] | Diagnosis of AMI | Demographic, ECG, laboratory | CNN | A prospective study specifically designed to confirm the accuracy of data from various wearable or portable ECG devices is warranted to apply the DLA to these devices | |
| 2021 | Liu, W.-C. [178] | Detecting AMI | Demographic, ECG, laboratory, angiography | DL LR | Further prospective validation with prehospital and in-hospital ECG tests is needed to confirm the performance of our DLM | |
| 2023 | Ciccarelli, M. [11] | Prevention of cardiovascular disease (CAD) | Genetic and epigenetic variables, clinical risk factor | RF | Future studies for the prevention of other pathologies | |
| 2021 | Velusamy, D. [179] | Diagnosis and prediction of CAD | Demographic, clinical, laboratory, risk factors | RF SVM KNN | Larger studies | |
| 2021 | Muhammad, L. J. [180] | Prediction of CAD | Demographic, clinical, diagnosis | Naive Bayes; SVM RF DT | Future studies on other ethnic groups | |
| 2021 | Li, D. [181] | Prediction of CAD | Demographic, clinical, laboratory | ML | Future studies for prediction of other pathologies | |
| 2024 | Brendel, J. M. [182] | Assessment of CAD in patients undergoing workup for transcatheter aortic valve replacement (TAVR) | CT angiography, laboratory, ETT, comorbidities | CNN | Multicentric study | |
| 2024 | Ihdayhid, A. R. [183] | CAD diagnosis and identification of high-risk plaque | Demographic, CCTA ECG | CNN | Future research is needed to investigate the prognostic impact and value of incorporating deep learning techniques into clinical practice compared to the standard of care | |
| 2024 | Uzokov, J. [184] | Diagnosis of ischemic heart disease (IHD) | ECG ETT CCTA | DL | Further management using cloud-based innovative digital technologies and artificial intelligence (AI) | |
| 2024 | Abdelrahman, K. [185] | Coronary artery calcium scoring detection and quantification | ECG CT imaging | KNN SVM CNN ANN | Future studies to distinguish between non-coronary calcifications such as valvular calcification and other high-density objects (e.g., metal implants) from coronary calcifications | |
| 2024 | Park, M. J. [186] | Predicting coronary occlusion in resuscitated out-of-hospital cardiac arrest (OHCA) patients | ECG, Clinical, Biomarkers | DL | Further validation in larger, prospective studies to establish efficacy across diverse clinical settings | |
| 2024 | Alkhamis, M. A. [187] | Predicting in-hospital and 30 days adverse events in ACS | Demographic, clinical, ECG, ETT, CCTA | RF XGB SVM LR | Future studies for prediction of 1-year adverse events in ACS | |
| 2024 | Zhu, X.; Xie, B. [188] | Prediction of death in patients with first AMI | Demographic Clinical Laboratory ECG | LR, RF XGB SVM MLP | Validating studies on other ethnic groups | |
| 2024 | Kasim, S. [189] | Predicting outcomes in non-ST-segment elevation myocardial infarction (NSTEMI) or unstable angina (UA) | Demographic medication | XGB SVM NB RF GLM | Continuous development, testing, and validation of these ML algorithms hold the promise of enhanced risk stratification, thereby revolutionizing future management strategies and patient outcomes | |
| 2023 | Oliveira, M. [190] | AMI mortality prediction | Demographic laboratory | LR DT RF XGM SVM | Further studies should be conducted and consider the inclusion of more variables that may be relevant in predicting AMI mortality, such as socioeconomic factors, systolic blood pressure, heart rate, and electrocardiogram results | |
| 2021 | Bai, Z. [41] | Prediction for CS risk in patients with STEMI | Demographic ECG laboratory in-hospital events | LR SVM XGB LASSO | Future studies with other risk factor analyses; for example, glycemia is not routinely recorded in our patients and therefore could not be tested as a potential predictor of CS. Further investigations using larger populations are warranted to fully evaluate the applicability of this model | |
| 2024 | Azdaki, N. [191] | Predicting CAD in hospital patients | Demographic Clinical comorbidities | ANN | Larger studies on ethnic groups | |
| Pericardial Disease | 2024 | Zhan, W. [192] | Prognostic model based on malignant pericardial effusion (PE) | genes | LASSO | Future studies to discover immunotherapy drugs |
| 2022 | Liu, Y.-L. [193] | Diagnosis of acute pericarditis and differentiate it from STEMI in the ED | ECG | XGB | Prospective, multicenter study; large-scale, further external validation | |
| 2023 | Cheng, C.-Y. [194] | Detecting and measurement of PE | TTE | CNN | Future studies should utilize a multicenter design with greater heterogeneity in the dataset. Further research should evaluate the collapsibility of the cardiac chambers and the presence of tamponade signs | |
| 2022 | Wilder-Smith, A. J. [195] | Detection and segmentation of PE | CT images | CNN | Future studies to include CT images of older machines, as this study was performed on state-of-the-art CT | |
| 2022 | Piccini, J. P. [196] | Predicting PE after leadless pacemaker implantation | Demographic clinical comorbidities | LASSO LR | A larger number of patients | |
| Peripheral Arterial Disease | 2024 | McBane, R. D. [197] | Diagnosis patients with peripheral artery disease (PAD) at greatest risk for major adverse events | Demographic Comorbidities Ankle-brachial index | CNN | Future studies to assess the tolerance of such signal acquisition would be important. Further validation studies are required to assess test accuracy and reproducibility in community settings outside of a large-volume academic vascular laboratory |
| 2024 | Rusinovich, Y. [198] | Identifying and classifying the anatomical patterns of PAD | Angiograms | ML | A larger number of patients | |
| 2024 | Sasikala, P. [199] | Prediction of PAD and accurate categorization of its severity levels | Demographic clinical angiograms | DT | Future studies for validation | |
| 2024 | Li, B. [200] | Prognosis of PAD | Laboratory, Demographic, Comorbidities Treatment | ML | Future validation at other institutions is needed to demonstrate the generalizability of this model | |
| 2024 | Masoumi Shahrbabak, S. [201] | Diagnosis of PAD | Arterial pulse waveforms ankle-brachial index BP | DL | External validation. | |
| 2024 | McBane, R. D., II [202] | Prediction of major adverse outcomes among patients with diabetes mellitus and PAD | Posterior tibial arterial Doppler waveforms | DNN | Larger studies | |
| 2024 | Li, B. [203] | Predict outcomes after infra-inguinal bypass in patients with PAD | Demographic Clinical complications | XGB LR | Larger studies | |
| 2024 | Liu, L. [204] | Prediction of PAD in diabetes mellitus (T2DM) | demographic diagnoses, biochemical index test | DT LR RF SVM XGB | Future studies in other regions also need to address the lack of collected and analyzed data on patients’ subjective descriptions, such as their duration of diabetes and smoking habits, which have been reported as associated with PVD in T2DM | |
| Thromboembolic Disease | 2024 | Nassour, N. [205] | Predicting venous thromboembolism (VTE) in ankle fracture patients | Demographic CXR CT images ultrasonograpy | RF | Studies on larger groups of patients |
| 2024 | Chen, R. [206] | Predicting diagnosis and 1-year risk of VTE | Demographics, laboratory, medications | ML | Future research should focus on refining and validating these models in different healthcare settings as well as informing personalized treatment strategies and exploring their potential utility in predicting VTE recurrence | |
| 2024 | Pan, S. [207] | VTE risk prediction for surgical patients | Demographic clinical laboratory comorbidities | ANN LR | Validation studies | |
| 2024 | Grdinic, A. G. [208] | Bleeding prediction in patients with cancer-associated thrombosis | Clinical biochemistry diagnosis | LR RF XGB | Validation studies | |
| 2023 | Chiasakul, T. [209] | Prediction of VTE | Ultrasonography | LR | Future studies should focus on transparent reporting, external validation, and clinical application of these models | |
| 2023 | Wang, X. [210] | Predicting the risk of DVT after knee/hip arthroplasty | Demographic clinical comorbidities | NLP XGB RF SVM RL | In future studies, model accuracy will be further evaluated by performing prospective and real-time predictions of DVT | |
| 2023 | Wang, K. Y. [211] | Identification of patients at risk of VTE following posterior lumbar fusion | Demographic, clinical comorbidities | LR XGB | Future studies should seek to externally validate these predictive tools and should examine the potential cost savings provided by predictive analytics models which can accurately identify patients at risk of VTE following spine surgery | |
| 2023 | Muñoz, A. J. [212] | Predicting 6-month VTE recurrence in patients with cancer | Demographic, clinical comorbidities | LR DT RF NLP | Future studies are needed to assess the validity of these results | |
| 2021 | Razzaq, M. [213] | Predicting the risk of recurrent VTE by biomarkers | Laboratory | ANN | Further experimental validations for these biomarkers | |
| 2023 | Valente Silva, B. [95] | Acute pulmonary embolism (PE) diagnosis | ECG laboratory | DNN | Further application in larger cohorts and external validation of the deep learning model are essential to fully validate its performance. These results should be validated in an external sample from other centers in the future, as well as in high-risk patient subgroups, for example, patients with hemodynamic instability | |
| 2022 | Contreras-Luján, E. E [214] | Early diagnosis of DVT | Clinical ultrasonography | KNN DT SVM RF | External validation | |
| 2023 | Seo, J. W. [215] | Detecting iliofemoral DVT | CT angiography | CNN | Future studies to extend the detection ranges to the infrapopliteal vein for investigating DVT | |
| Valvular Heart Disease | 2023 | Alhwiti, T. [216] | Predicting in-hospital mortality post-TAVR | Demographic disease | LR | Future studies looking at long-term mortality post-TAVR |
| 2023 | Strange, G. [217] | Identifying severe aortic stenosis (AS) phenotypes associated with high mortality | TTE | ML | Future studies in other geographic regions/health systems or specific ethnic groups | |
| 2023 | Ueda, D. [218] | Screening for heart valve disease | CXR TTE | CNN | Further validation with prospectively acquired test datasets from cohorts with various disease prevalences | |
| 2024 | Singh, S. [219] | Valvular heart disease screening | TTE | SVM LR CNN | Future studies to validate these data | |
| 2024 | Brown, K. [220] | Screening for rheumatic heart disease (RHD) in children | TTE | CNN | Future work needs to be pursued incorporating other features of RHD into our current AI algorithms as well as feasibility testing for field implementation in RHD endemic regions | |
| 2024 | Toggweile, S. [221] | TAVR planning and implantation | Cardiac CT | CNN | Further studies are required to demonstrate if these results can be reproduced by other centers using different scanners and different protocols for image acquisition (external validation) | |
| 2021 | Solomun M.D. [222] | Screening of AS | ECG | NLP | Future studies leveraging NLP-derived data to evaluate the association between the severity of AS and clinical outcomes, along with identifying predictors of AS progression | |
| 2022 | Aoyama, G. [223] | Diagnosis of AS | Cardiac CT | DNN | In future work, we should compare the intraoperative direct observation and the manual measurements by physicians with the automatic measurements by our proposed method to more concretely verify the clinical effectiveness and risks of the proposed method | |
| 2024 | Dasi, A. [224] | Predicting post-transcatheter aortic valve replacement | TTE Cardiac CT | ANN SVR | Future studies can focus on building upon these AI models to account for the nonlinear and complex relationships among postoperative AV pressure gradient, AVA, and patient outcomes | |
| 2023 | Krishna, H. [225] | Assessment of AS | ETT | ANN | Future work will aim to utilize AI to categorize AS severity, incorporating multiple echocardiographic variables | |
| 2024 | Xie, L.-F. [226] | Predicting postoperative adverse outcomes following surgical treatment of acute type A aortic dissection | Demographic, clinical | LASSO XGB | Multicenter study; external validation data are needed to test the clinical utility of the model | |
| 2024 | Zhou, M. [227] | Screening of aortic dissection | ECG | CNN | Larger studies; future prospective studies could enhance the efficacy of this AI model | |
| 2023 | Irtyuga, O [228] | Screening of AS in patients with and without bicuspid aortic valve | TTE | SVM ANN RF DR | Future research efforts should focus on early diagnostics and strategies to delay the progression of degenerative aortic valve disease | |
| 2023 | Kennedy, L. [229] | Prediction of the mechanical function of thoracic aortic aneurysm (TAA) tissue | TTE medical history genetic paneling | SVM GPR | Larger studies and an investigation are needed to compare the accuracy of risk levels assigned by the integrated ML approach compared to that of diameter thresholding using histopathological classification of tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamate, E.; Piraianu, A.-I.; Ciobotaru, O.R.; Crassas, R.; Duca, O.; Fulga, A.; Grigore, I.; Vintila, V.; Fulga, I.; Ciobotaru, O.C. Revolutionizing Cardiology through Artificial Intelligence—Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment—A Comprehensive Review of the Past 5 Years. Diagnostics 2024, 14, 1103. https://doi.org/10.3390/diagnostics14111103
Stamate E, Piraianu A-I, Ciobotaru OR, Crassas R, Duca O, Fulga A, Grigore I, Vintila V, Fulga I, Ciobotaru OC. Revolutionizing Cardiology through Artificial Intelligence—Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment—A Comprehensive Review of the Past 5 Years. Diagnostics. 2024; 14(11):1103. https://doi.org/10.3390/diagnostics14111103
Chicago/Turabian StyleStamate, Elena, Alin-Ionut Piraianu, Oana Roxana Ciobotaru, Rodica Crassas, Oana Duca, Ana Fulga, Ionica Grigore, Vlad Vintila, Iuliu Fulga, and Octavian Catalin Ciobotaru. 2024. "Revolutionizing Cardiology through Artificial Intelligence—Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment—A Comprehensive Review of the Past 5 Years" Diagnostics 14, no. 11: 1103. https://doi.org/10.3390/diagnostics14111103
APA StyleStamate, E., Piraianu, A.-I., Ciobotaru, O. R., Crassas, R., Duca, O., Fulga, A., Grigore, I., Vintila, V., Fulga, I., & Ciobotaru, O. C. (2024). Revolutionizing Cardiology through Artificial Intelligence—Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment—A Comprehensive Review of the Past 5 Years. Diagnostics, 14(11), 1103. https://doi.org/10.3390/diagnostics14111103