Performance Evaluation of Microscanner Plus, an Automated Image-Based Cell Counter, for Counting CD4+ T Lymphocytes in HIV Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Study Design
2.2. Flow Cytometry
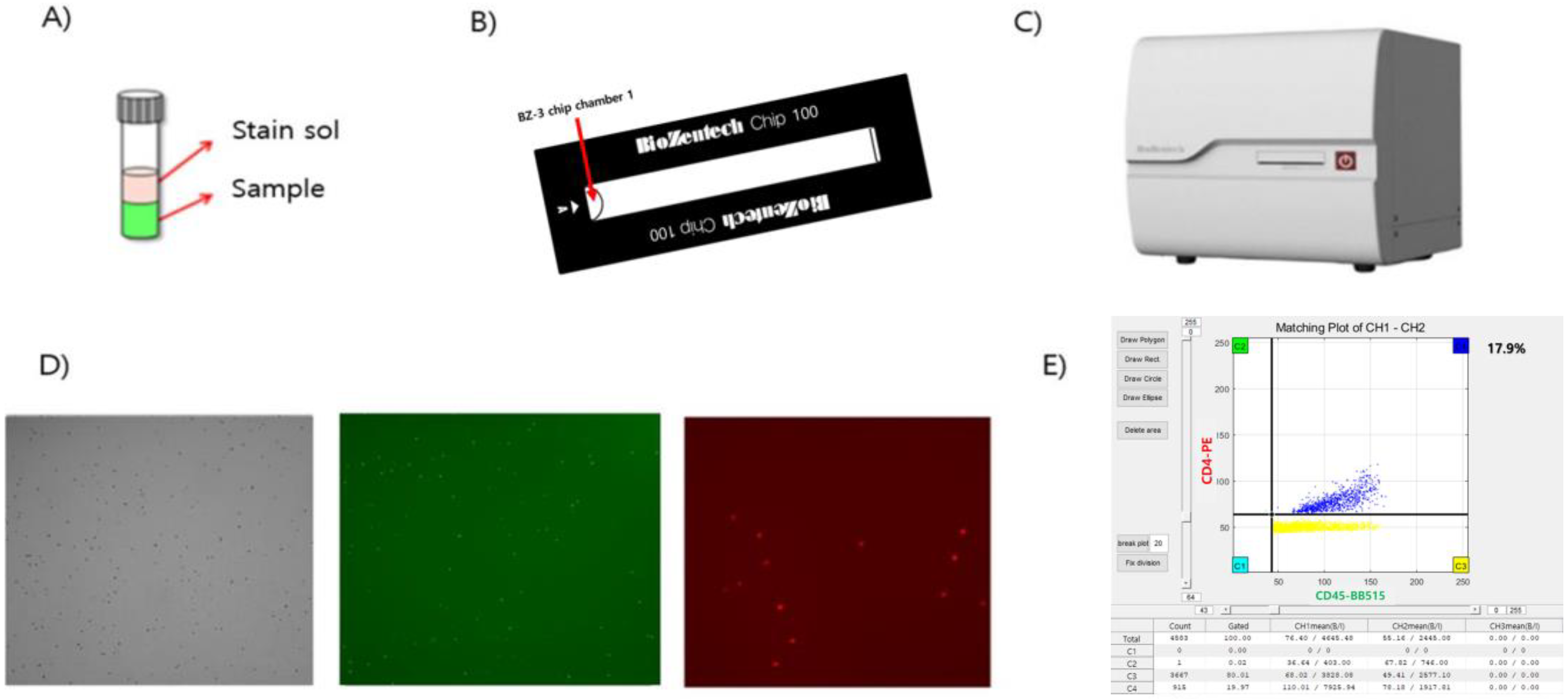
2.3. Microscanner Plus
2.4. Counting CD4+ T Lymphocytes
2.5. Evaluation of the Performance of Microscanner Plus
2.6. Statistical Analysis
3. Results
3.1. Precision
3.2. Reproducibility
3.3. Limit of Detection
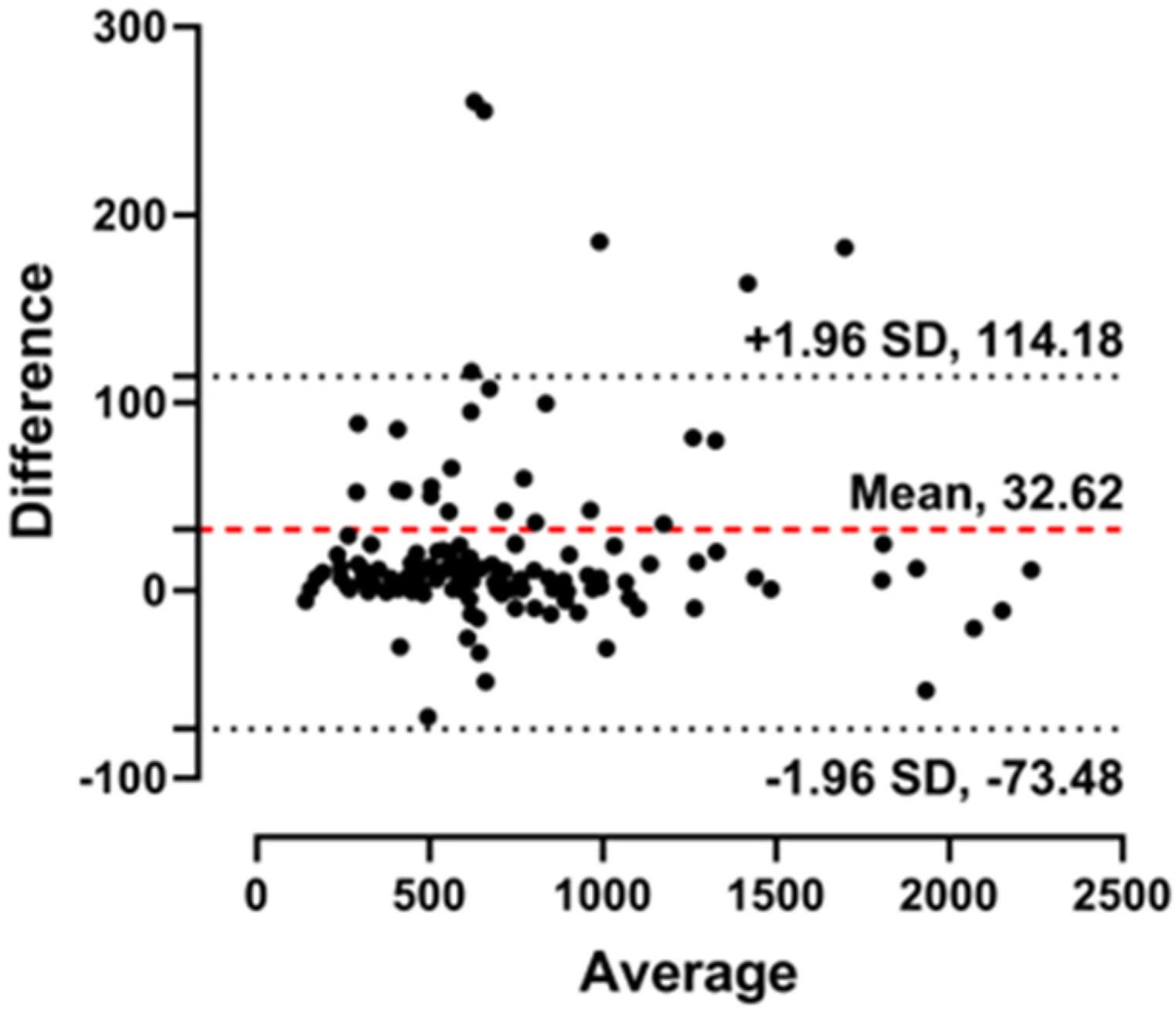
3.4. Clinical Performance of the Microscanner Plus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS. UNAIDS DATA 2020. Joint United Nations Programme on HIV/AIDS (UNAIDS); UNAIDS: Geneva, Switzerland, 2020. [Google Scholar]
- Valdiserri, R.O. Achieving an AIDS-Free Generation: It’s the Details That Matter. Public Health Rep. 2012, 127, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2014–2018; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020; Volume 25. [Google Scholar]
- Rutherford, G.W.; Lifson, A.R.; A Hessol, N.; Darrow, W.W.; O’Malley, P.M.; Buchbinder, S.P.; Barnhart, J.L.; Bodecker, T.W.; Cannon, L.; Doll, L.S. Course of HIV-I infection in a cohort of homosexual and bisexual men: An 11 year follow up study. BMJ 1990, 301, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.S.; Satcher Johnson, A.; Pembleton, E.S.; Stephenson, R.; Justice, A.C.; Althoff, K.N.; Bradley, H.; Castel, A.D.; Oster, A.M.; Rosenberg, E.S.; et al. Epidemiology of HIV in the USA: Epidemic burden, inequities, contexts, and responses. Lancet 2021, 397, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A. The human immunodeficiency virus: Infectivity and mechanisms of pathogenesis. Science 1988, 239, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Auld, A.F.; Shiraishi, R.W.; Oboho, I.; Ross, C.; Bateganya, M.; Pelletier, V.; Dee, J.; Francois, K.; Duval, N.; Antoine, M.; et al. Trends in Prevalence of Advanced HIV Disease at Antiretroviral Therapy Enrollment—10 Countries, 2004–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 558–563. [Google Scholar] [CrossRef]
- Thompson, M.A.; Aberg, J.A.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Treatment of Adult HIV Infection. JAMA 2012, 308, 387–402. [Google Scholar] [CrossRef]
- WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Pandori, M.W.; Hackett, J.; Louie, B.; Vallari, A.; Dowling, T.; Liska, S.; Klausner, J.D. Assessment of the Ability of a Fourth-Generation Immunoassay for Human Immunodeficiency Virus (HIV) Antibody and p24 Antigen to Detect both Acute and Recent HIV Infections in a High-Risk Setting. J. Clin. Microbiol. 2009, 47, 2639–2642. [Google Scholar] [CrossRef]
- Pallela, F.J., Jr.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef]
- World Health Organization. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Parekh, B.S.; Ou, C.-Y.; Fonjungo, P.N.; Kalou, M.B.; Rottinghaus, E.; Puren, A.; Alexander, H.; Cox, M.H.; Nkengasong, J.N. Diagnosis of Human Immunodeficiency Virus Infection. Clin. Microbiol. Rev. 2018, 32, e00064-18. [Google Scholar] [CrossRef]
- Mcbride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef]
- Barnett, D.; Walker, B.; Landay, A.; Denny, T.N. CD4 immunophenotyping in HIV infection. Nat. Rev. Microbiol. 2008, 6, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Okoye, A.A.; Picker, L.J. CD4+ T-cell depletion in HIV infection: Mechanisms of immunological failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Saracino, A.; Monno, L.; Angarano, G. The Revival of an “Old” Marker: CD4/CD8 Ratio. AIDS Rev. 2017, 19, 81–88. [Google Scholar] [PubMed]
- Peeling, R.W.; Smith, P.G.; Bossuyt, P.M.M. A guide for diagnostic evaluations. Nat. Rev. Microbiol. 2006, 4, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Petti, C.A.; Polage, C.R.; Quinn, T.C.; Ronald, A.R.; Sande, M.A. Laboratory Medicine in Africa: A Barrier to Effective Health Care. Clin. Infect. Dis. 2006, 42, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Dunne, M.; Kumarasamy, N.; Crowe, S.; Subbulakshmi, G.; Ganesh, A.K.; Cecelia, A.J.; Roth, P.; Mayer, K.H.; Thyagarajan, S.P.; et al. An Inexpensive, Simple, and Manual Method of CD4 T-Cell Quantitation in HIV-Infected Individuals for Use in Developing Countries. JAIDS J. Acquir. Immune Defic. Syndr. 2004, 36, 1006–1010. [Google Scholar] [CrossRef]
- WHO. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Guidelines for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Janossy, G.; Jani, I.V.; Bradley, N.J.; Bikoue, A.; Pitfield, T.; Glencross, D.K. Affordable CD4(+)-T-cell counting by flow cytometry: CD45 gating for volumetric analysis. Clin. Diagn. Lab. Immunol. 2002, 9, 1085–1094. [Google Scholar] [CrossRef]
- Peeling, R.W.; Sollis, K.A.; Glover, S.; Crowe, S.M.; Landay, A.L.; Cheng, B.; Barnett, D.; Denny, T.N.; Spira, T.J.; Stevens, W.S.; et al. CD4 Enumeration Technologies: A Systematic Review of Test Performance for Determining Eligibility for Antiretroviral Therapy. PLoS ONE 2015, 10, e0115019. [Google Scholar] [CrossRef]
- Grishagin, I.V. Automatic cell counting with ImageJ. Anal. Biochem. March 2015, 473, 63–65. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Singh, S.; Carpenter, A.E. Applications in image-based profiling of perturbations. Curr. Opin. Biotechnol. 2016, 39, 134–142. [Google Scholar] [CrossRef]
- Shearer, W.T.; Pahwa, S.; Read, J.S.; Chen, J.; Wijayawardana, S.R.; Palumbo, P.; Abrams, E.J.; Nesheim, S.R.; Yin, W.; Thompson, B.; et al. CD4/CD8 T-cell ratio predicts HIV infection in infants: The National Heart, Lung, and Blood Institute P2C2 Study. J. Allergy Clin. Immunol. 2007, 120, 1449–1456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shearer, W.T.; Easley, K.A.; Goldfarb, J.; Rosenblatt, H.M.; Jenson, H.B.; Kovacs, A.; McIntosh, K. Prospective 5-year study of peripheral blood CD4+, CD8+, and CD19+/CD20+ lymphocytes and serum Igs in children born to HIV-1+ women. J. Allergy Clin. Immunol. 2000, 106, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kestens, L.; Mandy, F. Thirty-five years of CD4 T-cell counting in HIV infection: From flow cytometry in the lab to point-of-care testing in the field. Cytom. Part B Clin. Cytom. 2017, 92, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Spacek, L.A.; Shihab, H.M.; Lutwama, F.; Summerton, J.; Mayanja, H.; Kamya, M.; Ronald, A.; Margolick, J.B.; Nilles, T.L.; Quinn, T.C. Evaluation of a low-cost method, the Guava EasyCD4 assay, to enumerate CD4-positive lymphocyte counts in HIV-infected patients in the United States and Uganda. J. Acquir. Immune Defic. Syndr. 2006, 41, 607–610. [Google Scholar] [CrossRef]
- Kratz, A.; Bengtsson, H.-I.; Casey, J.E.; Jenson, H.B.; Rosenblatt, H.M.; Kovacs, A.; McIntosh, K. P2C2 HIV Study GroupPerformance. Evaluation of the CellaVision DM96 System. Ann. N. Y. Acad. Sci. 2005, 124, 770–781. [Google Scholar]
- Bystryak, S.; Bandwar, R.P.; Santockyte, R. A flow-through cell counting assay for point-of-care enumeration of CD4 T-cells. J. Virol. Methods 2019, 271, 113672. [Google Scholar] [CrossRef]

| Sample | Mean (Cells/µL) | SD (Cells/µL) | Repeatability (CV%) | Total Imprecision (CV%) |
|---|---|---|---|---|
| 1 | 999.40 | 31.52 | 3.3 | 3.1 |
| 2 | 498.78 | 19.93 | 6.6 | 6.3 |
| 3 | 168.15 | 3.77 | 2.4 | 2.2 |
| Sample | Tester | Mean (Cells/µL) | SD (Cells/µL) | CV (%) |
|---|---|---|---|---|
| 1 | A | 1499.40 | 11.90 | 0.79 |
| B | 1487.50 | 20.61 | 1.39 | |
| C | 1483.53 | 24.77 | 1.67 | |
| D | 1495.43 | 13.74 | 0.92 | |
| 2 | A | 740.17 | 4.75 | 0.64 |
| B | 746.13 | 3.55 | 0.48 | |
| C | 741.77 | 6.87 | 0.93 | |
| D | 741.77 | 6.87 | 0.93 | |
| 3 | A | 314.57 | 2.46 | 0.78 |
| B | 314.20 | 2.08 | 0.66 | |
| C | 317.73 | 3.55 | 1.12 | |
| D | 309.80 | 8.75 | 2.82 |
| Sample | Mean (Cells/µL) | SD (Cells/µL) | CV (%) |
|---|---|---|---|
| 1 | 1002.52 | 42.16 | 4.21 |
| 2 | 746.52 | 34.67 | 4.64 |
| 3 | 488.10 | 24.06 | 4.89 |
| 4 | 166.62 | 3.91 | 2.35 |
| 5 | 82.37 | 3.90 | 4.73 |
| 6 | 15.27 | 0.47 | 3.09 |
| 7 | 6.20 | 0.42 | 6.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.S.; Lee, J.; Park, S.; Lim, C.S.; Kim, J. Performance Evaluation of Microscanner Plus, an Automated Image-Based Cell Counter, for Counting CD4+ T Lymphocytes in HIV Patients. Diagnostics 2024, 14, 73. https://doi.org/10.3390/diagnostics14010073
Jang WS, Lee J, Park S, Lim CS, Kim J. Performance Evaluation of Microscanner Plus, an Automated Image-Based Cell Counter, for Counting CD4+ T Lymphocytes in HIV Patients. Diagnostics. 2024; 14(1):73. https://doi.org/10.3390/diagnostics14010073
Chicago/Turabian StyleJang, Woong Sik, Junmin Lee, Seoyeon Park, Chae Seung Lim, and Jeeyong Kim. 2024. "Performance Evaluation of Microscanner Plus, an Automated Image-Based Cell Counter, for Counting CD4+ T Lymphocytes in HIV Patients" Diagnostics 14, no. 1: 73. https://doi.org/10.3390/diagnostics14010073
APA StyleJang, W. S., Lee, J., Park, S., Lim, C. S., & Kim, J. (2024). Performance Evaluation of Microscanner Plus, an Automated Image-Based Cell Counter, for Counting CD4+ T Lymphocytes in HIV Patients. Diagnostics, 14(1), 73. https://doi.org/10.3390/diagnostics14010073






