Prediction of Chemotherapy Efficacy in Patients with Colorectal Cancer Ovarian Metastases: A Preliminary Study Using Contrast-Enhanced Computed-Tomography-Based Radiomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Image Processing and Radiomics Features Extraction
2.3. Model Construction and Evaluation
2.4. Calibration and Decision Curve Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Radiomics Features’ Extraction and Validation
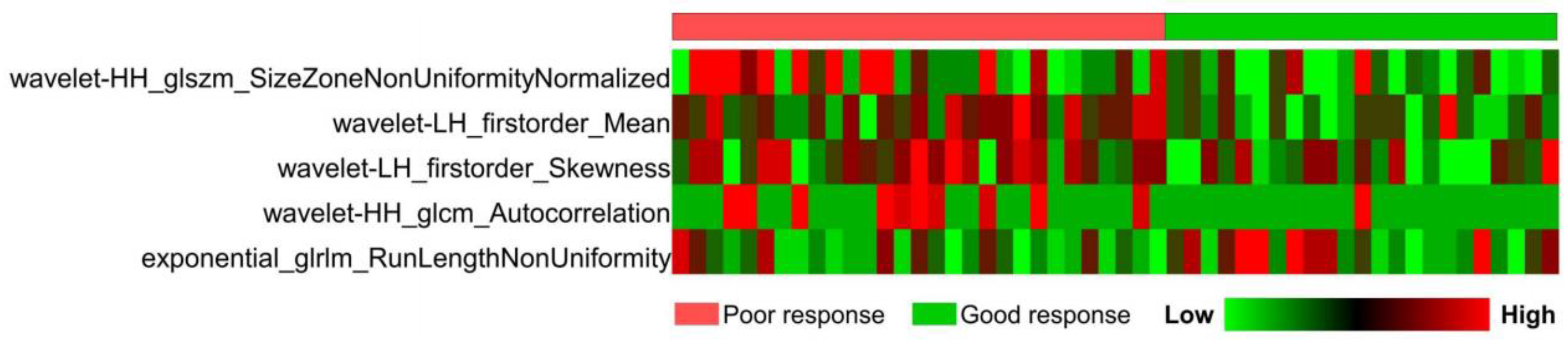
3.2.1. Selection of Radiomics Features
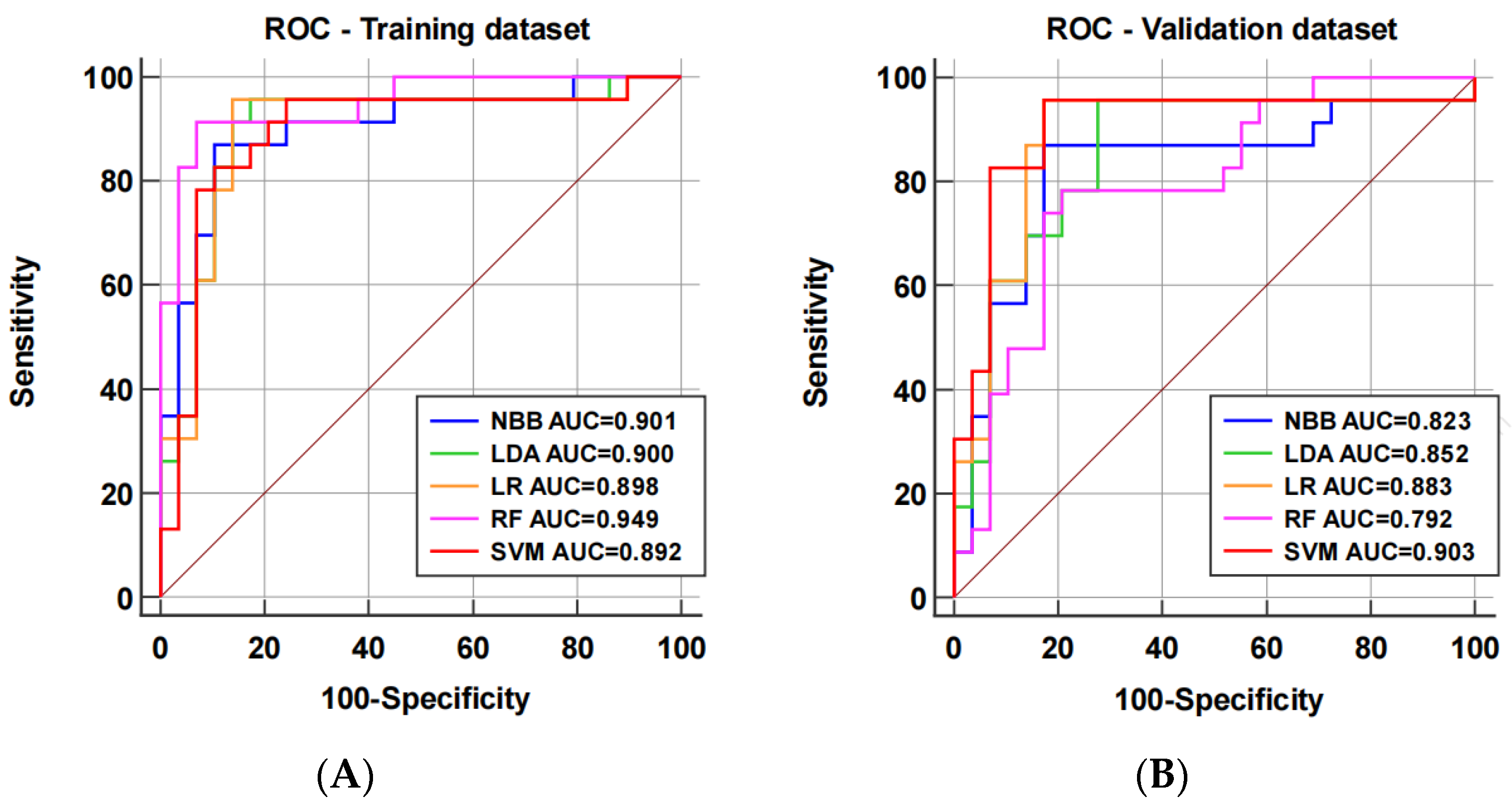
3.2.2. Model Performance
3.2.3. Clinical Utilities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Thornblade, L.W.; Han, E.; Fong, Y. Colorectal cancer ovarian metastases. Int. J. Gynecol. Cancer 2021, 31, 1137–1144. [Google Scholar] [CrossRef]
- Kurokawa, R.; Nakai, Y.; Gonoi, W.; Mori, H.; Tsuruga, T.; Makise, N.; Ushiku, T.; Abe, O. Differentiation between ovarian metastasis from colorectal carcinoma and primary ovarian carcinoma: Evaluation of tumour markers and “mille-feuille sign” on computed tomography/magnetic resonance imaging. Eur. J. Radiol. 2020, 124, 108823. [Google Scholar] [CrossRef]
- Bourgioti, C.; Konidari, M.; Moulopoulos, L.A. Manifestations of Ovarian Cancer in Relation to Other Pelvic Diseases by MRI. Cancers 2023, 15, 2106. [Google Scholar] [CrossRef]
- Al-Busaidi, I.S.; Bailey, T.; Dobbs, B.; Eglinton, T.W.; Wakeman, C.J.; Frizelle, F.A. Complete resection of colorectal cancer with ovarian metastases combined with chemotherapy is associated with improved survival. ANZ J. Surg. 2019, 89, 1091–1096. [Google Scholar] [CrossRef]
- Goéré, D.; Daveau, C.; Elias, D.; Boige, V.; Tomasic, G.; Bonnet, S.; Pocard, M.; Dromain, C.; Ducreux, M.; Lasser, P.; et al. The differential response to chemotherapy of ovarian metastases from colorectal carcinoma. Eur. J. Surg. Oncol. 2008, 34, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, J.; Lim, H.Y.; Kang, W.K.; Choi, C.H.; Lee, J.W.; Kim, T.J.; Kim, B.G.; Bae, D.S.; Cho, Y.B.; et al. Survival benefit from ovarian metastatectomy in colorectal cancer patients with ovarian metastasis: A retrospective analysis. Cancer Chemother. Pharmacol. 2010, 66, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, Z.; Yang, Y.; Wang, J.; Dai, X.; Wang, L.; Lu, Y.; Xue, F. Preoperative diagnosis of malignant pulmonary nodules in lung cancer screening with a radiomics nomogram. Cancer Commun. 2020, 40, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Duggento, A.; Indovina, I.; Guerrisi, M.; Toschi, N. Radiomics in breast cancer classification and prediction. Semin. Cancer Biol. 2021, 72, 238–250. [Google Scholar] [CrossRef]
- Wang, W.; Peng, Y.; Feng, X.; Zhao, Y.; Seeruttun, S.R.; Zhang, J.; Cheng, Z.; Li, Y.; Liu, Z.; Zhou, Z. Development and Validation of a Computed Tomography-Based Radiomics Signature to Predict Response to Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer. JAMA Netw. Open 2021, 4, e2121143. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Nalbantov, G.; Carvalho, S.; van Elmpt, W.J.; Troost, E.G.; Boellaard, R.; Aerts, H.J.; Gillies, R.J.; Lambin, P. The effect of SUV discretization in quantitative FDG-PET Radiomics: The need for standardized methodology in tumor texture analysis. Sci. Rep. 2015, 5, 11075. [Google Scholar] [CrossRef]
- Finazzi, S.; Poole, D.; Luciani, D.; Cogo, P.E.; Bertolini, G. Calibration belt for quality-of-care assessment based on dichotomous outcomes. PLoS ONE 2011, 6, e16110. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, C.; van der Meer, R.; Roumen, R.M.; Lurvink, R.J.; Lemmens, V.E.; van Erning, F.N.; de Hingh, I.H. Incidence, risk factors, treatment, and survival of ovarian metastases of colorectal origin: A Dutch population-based study. Int. J. Color. Dis. 2020, 35, 1035–1044. [Google Scholar] [CrossRef]
- Kim, D.D.; Park, I.J.; Kim, H.C.; Yu, C.S.; Kim, J.C. Ovarian metastases from colorectal cancer: A clinicopathological analysis of 103 patients. Color. Dis. 2009, 11, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.E.; Pittman, B.; Wan, J.Y.; Fleming, M. Colon cancer with metastasis to the ovary at time of initial diagnosis. Gynecol. Oncol. 1997, 66, 368–371. [Google Scholar] [CrossRef]
- Megibow, A.J.; Hulnick, D.H.; Bosniak, M.A.; Balthazar, E.J. Ovarian metastases: Computed tomographic appearances. Radiology 1985, 156, 161–164. [Google Scholar] [CrossRef]
- Maeda, Y.; Minagawa, N.; Shoji, H.; Kobayashi, T.; Yamamoto, K. Giant ovarian tumor with colorectal cancer: Suggestion concerning the need for colonoscopy screening in cases with large ovarian tumor-a report of three cases. BMC Surg. 2022, 22, 111. [Google Scholar] [CrossRef]
- Sekine, K.; Hamaguchi, T.; Shoji, H.; Takashima, A.; Honma, Y.; Iwasa, S.; Kato, K.; Takahashi, K.; Kato, T.; Kanemitsu, Y.; et al. Retrospective Analyses of Systemic Chemotherapy and Cytoreductive Surgery for Patients with Ovarian Metastases from Colorectal Cancer: A Single-Center Experience. Oncology 2018, 95, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Guvenal, T.; Salman, M.C.; Ozyuncu, O.; Sakinci, M.; Basaran, M. The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol. Oncol. 2005, 98, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, Y.; Wang, Y.; Huo, X.; Zhu, J.; Zhang, T. Clinicopathological characteristics and prognosis analysis of ovarian metastases in colorectal cancer: A single-center experience. Int. J. Clin. Oncol. 2020, 25, 1822–1829. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.Y.; Gao, H.; Lian, Z.J.; Ding, L.; Li, M.; Gu, J. Clinical analysis of Krukenberg tumours in patients with colorectal cancer—A review of 57 cases. World J. Surg. Oncol. 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis. Colon Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kapur, S.; Moran, B.J. The role of prophylactic oophorectomy in women undergoing surgery for colorectal cancer. Color. Dis. 2005, 7, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; Kritz-Silverstein, D.; von Mühlen, D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000, 85, 645–651. [Google Scholar]
- Christensen, T.D.; Palshof, J.A.; Larsen, F.O.; Poulsen, T.S.; Høgdall, E.; Pfeiffer, P.; Jensen, B.V.; Yilmaz, M.K.; Nielsen, D. Associations between primary tumor RAS, BRAF and PIK3CA mutation status and metastatic site in patients with chemo-resistant metastatic colorectal cancer. Acta Oncol. 2018, 57, 1057–1062. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, B.; Wang, Z.; Li, W.; Xiu, J.; Liu, Z.; Han, M. Radiomics signature of brain metastasis: Prediction of EGFR mutation status. Eur. Radiol. 2021, 31, 4538–4547. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Yi, X.; Chen, C.; Pang, P.; Fu, Y.; Lei, G.; Chen, C.; Tan, F.; Gong, G.; Li, Q.; et al. Pre-treatment CT-based radiomics nomogram for predicting microsatellite instability status in colorectal cancer. Eur. Radiol. 2022, 32, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Kirienko, M.; Sollini, M.; Corbetta, M.; Voulaz, E.; Gozzi, N.; Interlenghi, M.; Gallivanone, F.; Castiglioni, I.; Asselta, R.; Duga, S.; et al. Radiomics and gene expression profile to characterise the disease and predict outcome in patients with lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3643–3655. [Google Scholar] [CrossRef]




| Characteristics of Patients and Primary Tumors | Total (n = 52), n (%) | Chemo-Benefit Group (n = 25), n (%) | No-Chemo-Benefit Group (n = 27), n (%) | p Value |
|---|---|---|---|---|
| Age at diagnosis of OM (years, mean ± SD) | 46.0 ± 11.7 | 47.1 ± 13.6 | 45.0 ± 9.8 | 0.58 |
| Menopausal status | 0.40 | |||
| Premenopausal | 29 (55.8) | 13 (52.0) | 16 (59.3) | |
| Postmenopausal | 23 (44.2) | 12 (48.0) | 11 (40.7) | |
| Primary tumor location | 0.58 | |||
| proximal colon | 12 (23.0) | 7 (28.0) | 5 (18.5) | |
| distal colon | 20 (38.5) | 8 (32.0) | 12 (44.4) | |
| rectum | 20 (38.5) | 10 (40.0) | 10 (37.0) | |
| Histological type of primary tumor | 0.18 | |||
| Adenocarcinoma | 37 (71.2) | 16 (64.0) | 21 (77.8) | |
| Mucinous adenocarcinoma | 13 (25) | 8 (32.0) | 5 (18.5) | |
| Signet ring cell carcinoma | 2 (3.8) | 1 (4.0) | 1 (3.7) | |
| T stage of primary tumor | 0.95 | |||
| T2 | 7 (13.5) | 3 (12.0) | 4 (14.8) | |
| T3 | 10 (19.2) | 5(20.0) | 5 (18.5) | |
| T4 | 35 (67.3) | 17 (68.0) | 18 (66.7) | |
| N stage of primary tumor | 0.38 | |||
| N0 | 4 (7.7) | 3 (12.0) | 1 (3.7) | |
| N1 | 21 (40.4) | 11 (44.0) | 10 (37.0) | |
| N2 | 27 (51.9) | 11 (44.0) | 16 (59.3) | |
| Distal metastasis | 0.23 | |||
| M1a | 2 (3.8) | 2 (8.0) | 0 | |
| M1b | 27 (51.9) | 14 (56.0) | 13 (48.1) | |
| M1c | 23 (44.2) | 9 (36.0) | 14 (51.8) | |
| Ascites | 0.48 | |||
| Yes | 7 (13.5) | 2 (8.0) | 5 (18.5) | |
| No | 45 (86.5) | 23 (92.0) | 22 (81.5) | |
| CEA (ng/mL, mean ± SD) | 116.1 ± 212.7 | 26.7 ± 20.8 | 198.8 ± 271.2 | 0.008 |
| CA125 (U/mL, mean ± SD) | 87.2 ± 171.6 | 47.9 ± 42.3 | 123.5 ± 230.7 | 0.053 |
| CA19-9 (U/mL, mean ± SD) | 331.0 ± 524.4 | 393.1 ± 695.1 | 273.5 ± 293.6 | 0.86 |
| RAS mutation | 0.84 | |||
| Mutant | 18 (34.6) | 9 (36.0) | 9 (33.3) | |
| BRAF mutation | 0.51 | |||
| Mutant | 1 (1.9) | 0 | 1 (3.7) | |
| PIK3CA mutation | 0.51 | |||
| Mutant | 1 (1.9) | 0 | 1 (3.7) | |
| Timing of metastases | 0.60 | |||
| Synchronous | 22 (42.3) | 9 (36.0) | 13 (48.1) | |
| Metachronous | 30 (57.7) | 16 (64.0) | 14 (51.9) | |
| Ovarian metastases laterality | 0.79 | |||
| Bilateral | 24 (46.2) | 12 (48.0) | 12 (44.4) | |
| Unilateral | 28 (53.8) | 13 (52.0) | 15 (55.6) | |
| Longest diameter of OM (cm, mean ± SD) | 5.9 ± 3.9 | 5.5 ± 3.6 | 6.2 ± 4.2 | 0.43 |
| Morphology | 0.68 | |||
| Solid and cystic | 19 (36.5) | 10 (40.0) | 9(33.3) | |
| Multicystic without nodules | 25 (48.1) | 10 (40.0) | 15 (55.6) | |
| Multicystic with nodules | 5 (9.6) | 3 (12.0) | 2 (7.4) | |
| Cystic | 3 (5.8) | 2 (8.0) | 1 (3.7) | |
| Chemotherapy regimens | 0.77 | |||
| XELOX | 19 (36.5) | 10 (40.0) | 9 (33.3) | |
| FOLFOX | 16 (30.7) | 7 (28.0) | 9 (33.3) | |
| FOLFIRI | 17 (32.7) | 8 (32.0) | 9 (33.3) | |
| Targeted Drugs | 0.84 | |||
| No | 17 (32.7) | 8 (32.0) | 9 (33.3) | |
| Bevacizumab | 23 (44.2) | 12 (48.0) | 11 (40.7) | |
| Cetuximab | 12 (23.1) | 5 (20.0) | 7 (25.9) |
| Model | AUC (95% CI) | p Value | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| NBB | 0.823 (0.692–0.915) | 0.024 | 84.6 | 87.0 | 82.8 | 80.0 | 88.9 |
| LDA | 0.852 (0.726–0.935) | 0.045 | 82.7 | 95.7 | 72.4 | 73.3 | 95.5 |
| LR | 0.883 (0.764–0.955) | 0.282 | 88.5 | 95.7 | 82.8 | 81.5 | 96.0 |
| RF | 0.792 (0.656–0.892) | 0.051 | 78.8 | 78.3 | 79.3 | 75.0 | 82.1 |
| SVM | 0.903 (0.788–0.967) | reference | 88.5 | 95.7 | 82.8 | 81.5 | 96.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Li, X.; Zeng, H.; Yin, H.; Wang, Y.; Wang, B.; Qiu, M.; Wu, B. Prediction of Chemotherapy Efficacy in Patients with Colorectal Cancer Ovarian Metastases: A Preliminary Study Using Contrast-Enhanced Computed-Tomography-Based Radiomics. Diagnostics 2024, 14, 6. https://doi.org/10.3390/diagnostics14010006
Yu J, Li X, Zeng H, Yin H, Wang Y, Wang B, Qiu M, Wu B. Prediction of Chemotherapy Efficacy in Patients with Colorectal Cancer Ovarian Metastases: A Preliminary Study Using Contrast-Enhanced Computed-Tomography-Based Radiomics. Diagnostics. 2024; 14(1):6. https://doi.org/10.3390/diagnostics14010006
Chicago/Turabian StyleYu, Jinghan, Xiaofen Li, Hanjiang Zeng, Hongkun Yin, Ya Wang, Bo Wang, Meng Qiu, and Bing Wu. 2024. "Prediction of Chemotherapy Efficacy in Patients with Colorectal Cancer Ovarian Metastases: A Preliminary Study Using Contrast-Enhanced Computed-Tomography-Based Radiomics" Diagnostics 14, no. 1: 6. https://doi.org/10.3390/diagnostics14010006
APA StyleYu, J., Li, X., Zeng, H., Yin, H., Wang, Y., Wang, B., Qiu, M., & Wu, B. (2024). Prediction of Chemotherapy Efficacy in Patients with Colorectal Cancer Ovarian Metastases: A Preliminary Study Using Contrast-Enhanced Computed-Tomography-Based Radiomics. Diagnostics, 14(1), 6. https://doi.org/10.3390/diagnostics14010006






