Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Newly Developed Ag-RDT Measurement Principle
2.3. Testing Procedures
2.4. Statistical Analyses
3. Results
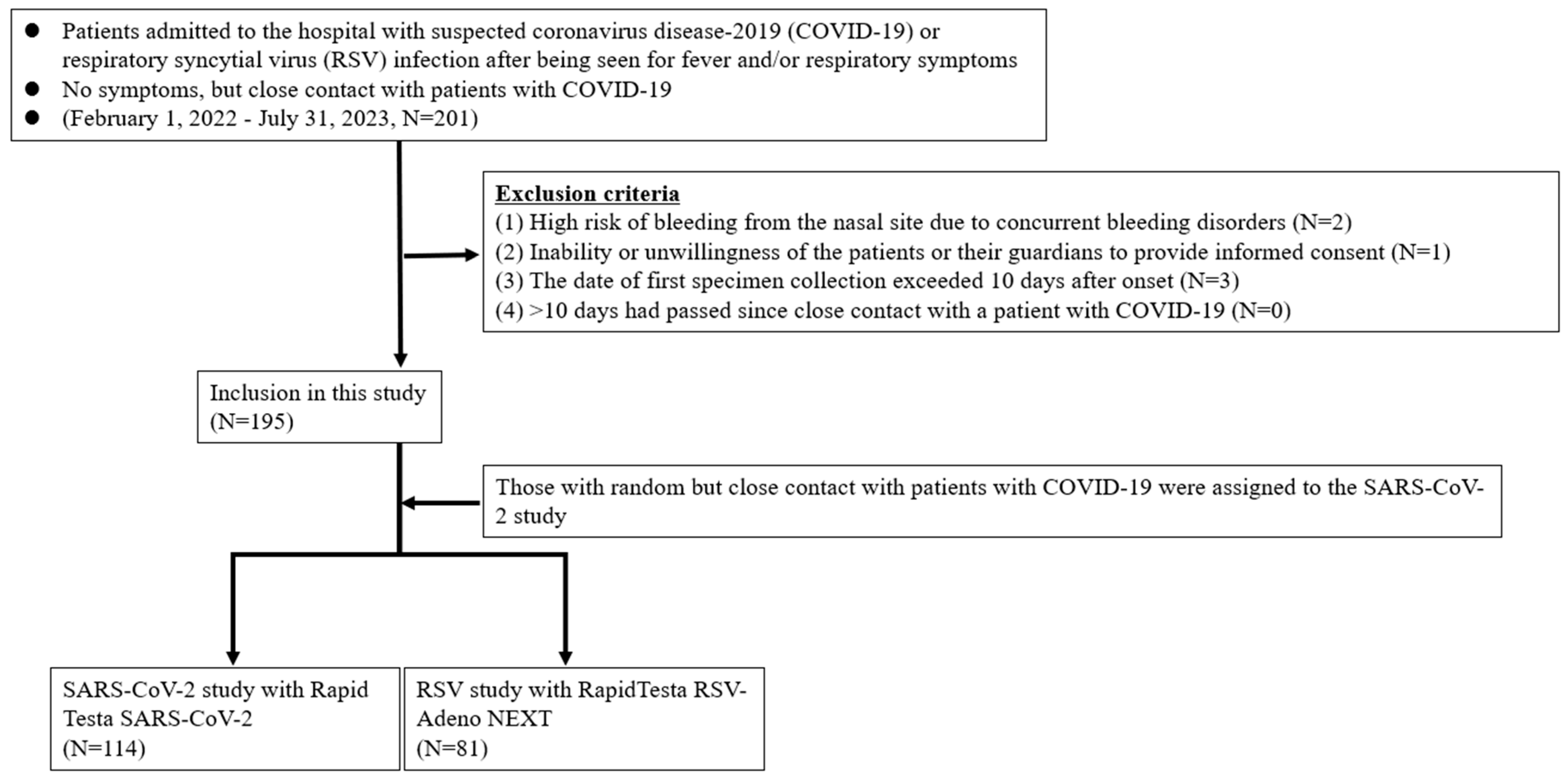
3.1. Participants
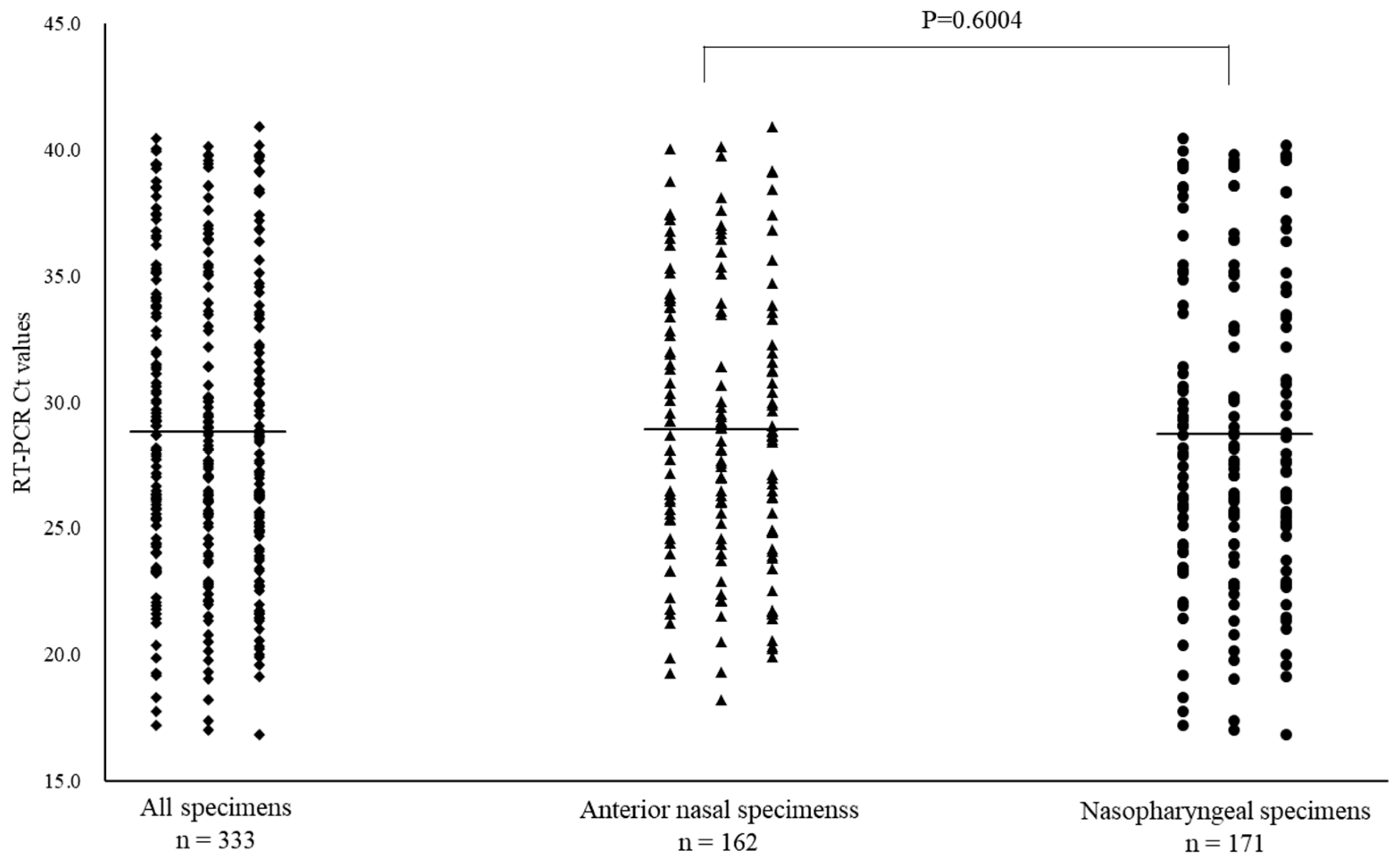
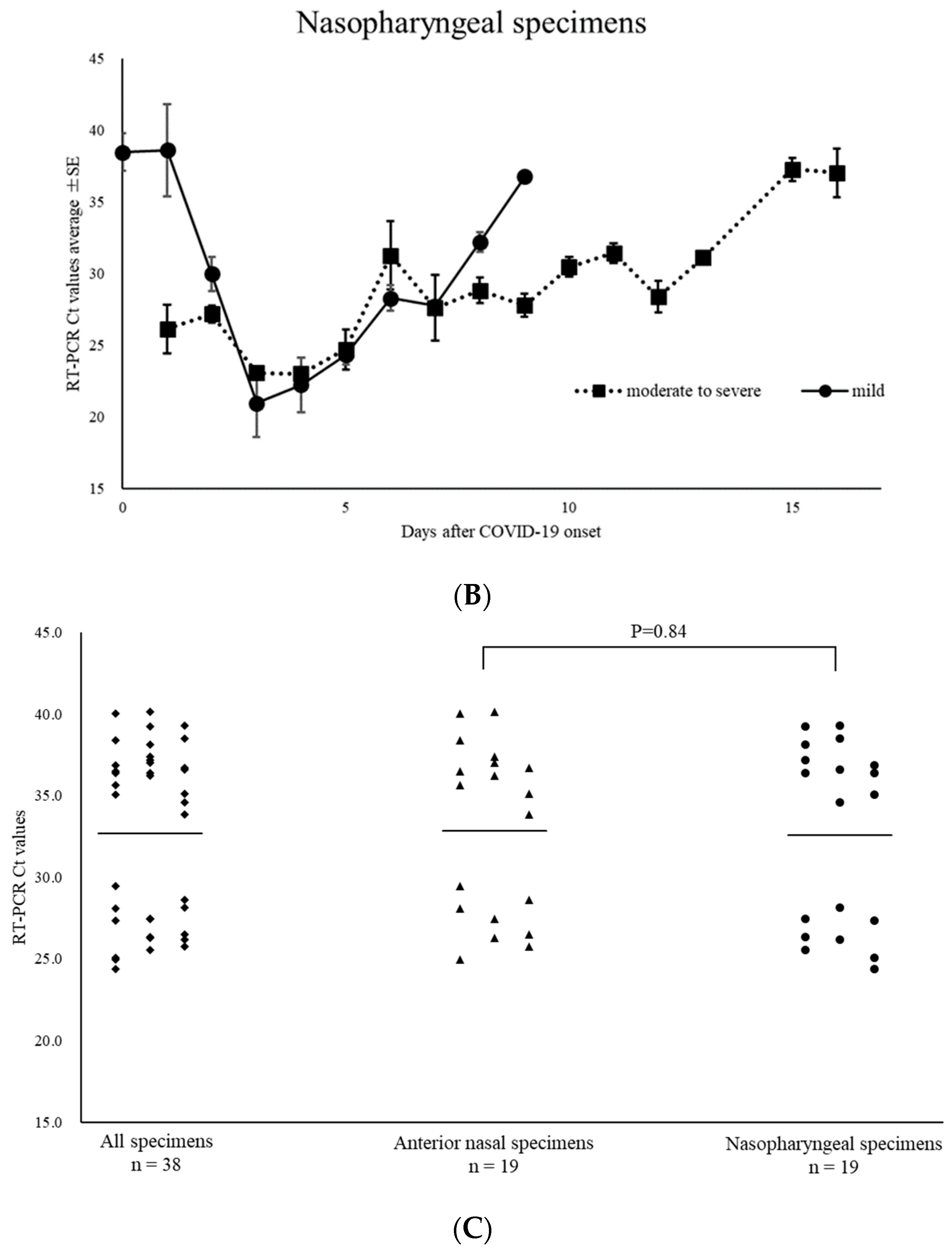
3.2. SARS-CoV-2 Positivity
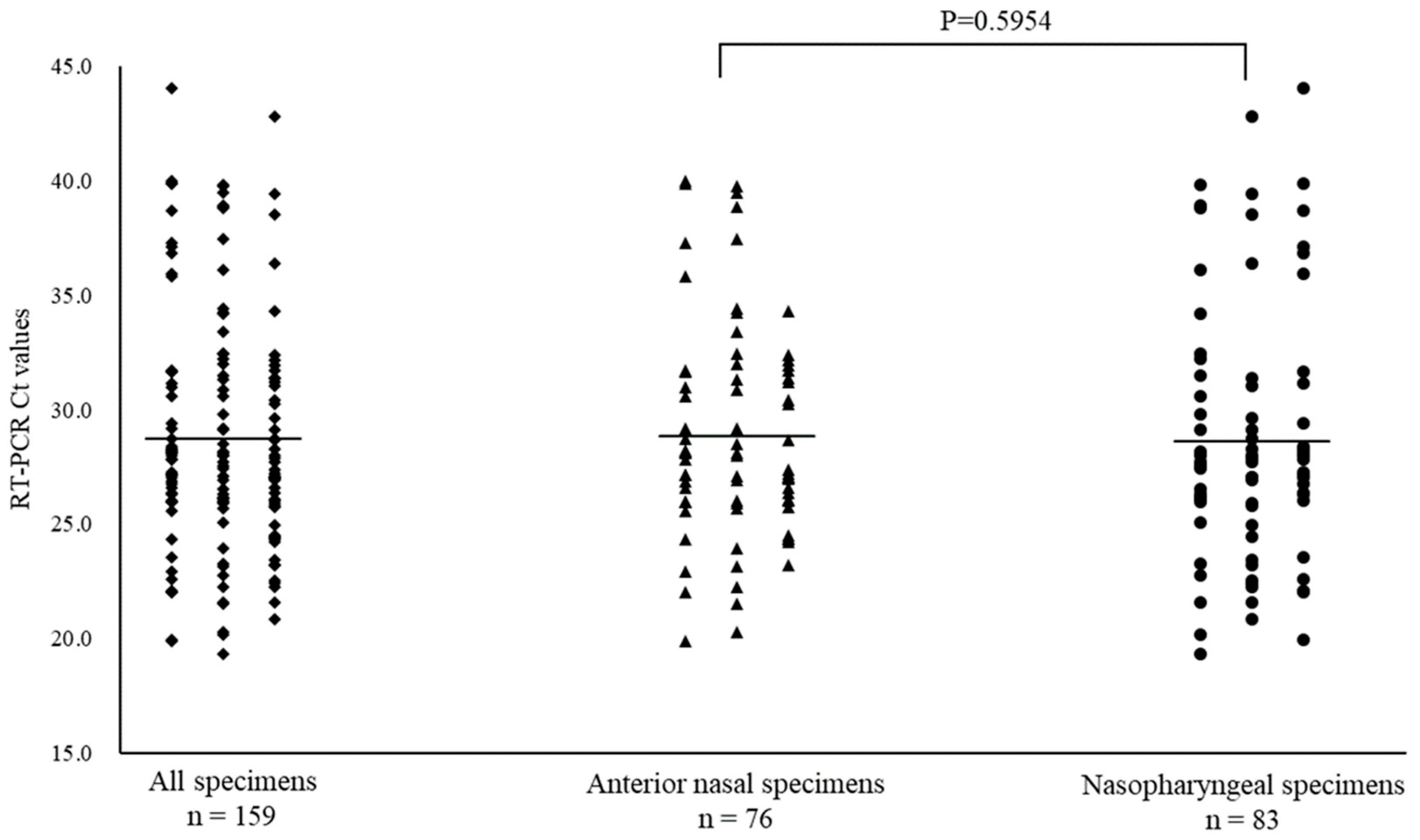
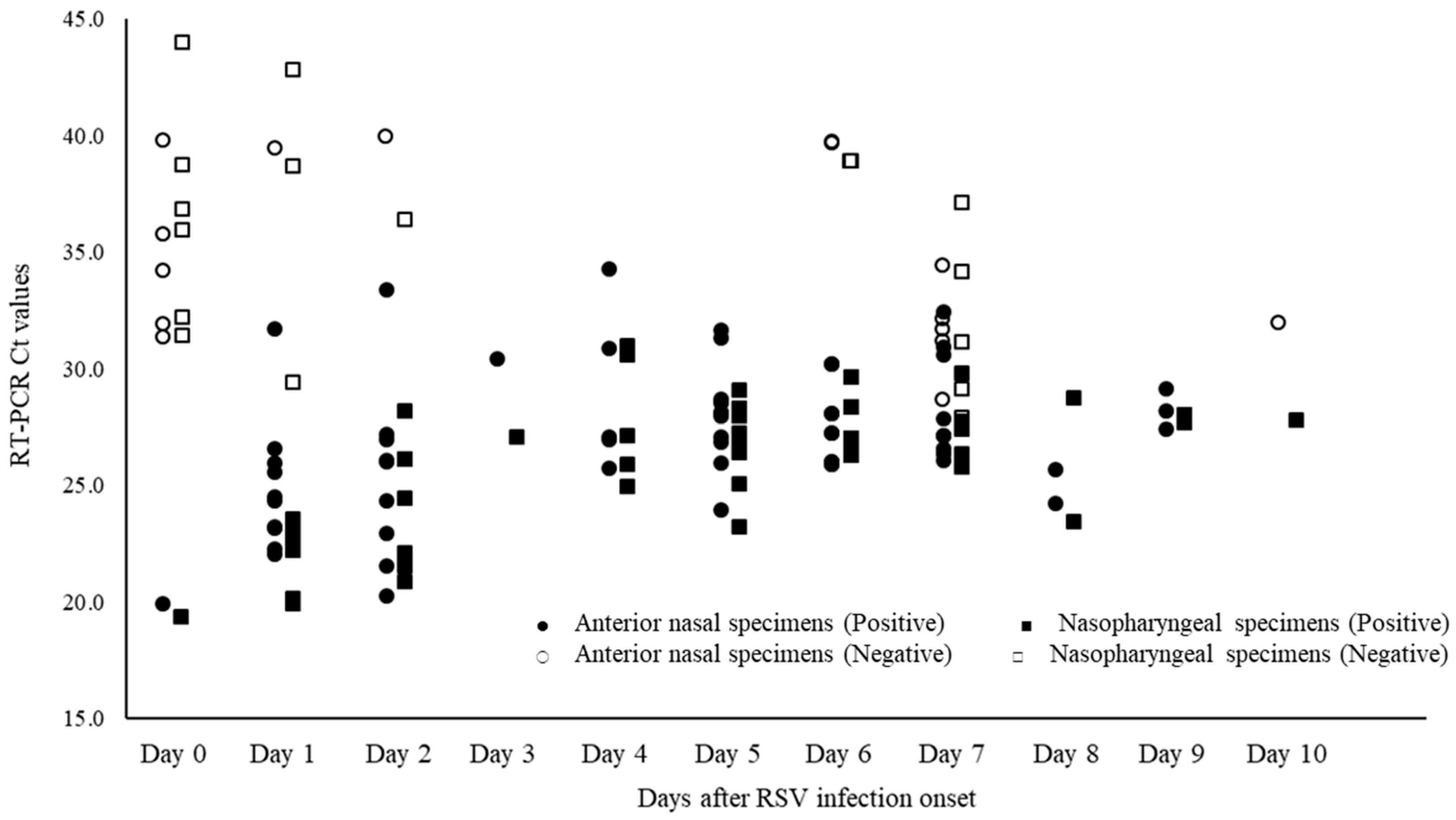
3.3. RSV Positivity
3.4. Evaluation of the Newly Developed Ag-RDT for SARS-CoV-2 and RSV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 4 February 2023).
- Duong, B.V.; Larpruenrudee, P.; Fang, T.; Hossain, S.I.; Saha, S.C.; Gu, Y.; Islam, M.S. Is the SARS CoV-2 Omicron Variant Deadlier and More Transmissible than Delta Variant? Int. J. Environ. Res. Public Health 2022, 19, 4586. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Update on Omicron. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron (accessed on 29 May 2023).
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Jacquérioz, F.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious Viral Load in Unvaccinated and Vaccinated Individuals Infected with Ancestral, Delta or Omicron SARS-CoV-2. Nat. Med. 2022, 28, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ito, J.; Uriu, K.; Zahradnik, J.; Kida, I.; Anraku, Y.; Nasser, H.; Shofa, M.; Oda, Y.; Lytras, S.; et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat. Commun. 2023, 14, 2800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 Variant Biology: Immune Escape, Transmission and Fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Tamura, D.; Kawaoka, Y. Omicron Proliferation in the Nasal Cavity May Explain Its High Infectivity. J. Infect. 2023, 86, 584–587. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Respiratory Syncytial Virus Infection in Adults. Clin. Microbiol. Rev. 2000, 13, 371–384. [Google Scholar] [CrossRef]
- World Health Organization. Antigen Detection for the Diagnosis of SARS-CoV-2 Infection. 2021. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 4 January 2023).
- World Health Organization. Diagnostic Testing for SARS-CoV-2. 2020. Available online: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 (accessed on 4 January 2023).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, D.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 4 January 2023).
- Yadav, H.; Shah, D.; Sayed, S.; Horton, S.; Schroeder, L.F. Availability of Essential Diagnostics in Ten Low-Income and Middle-Income Countries: Results from National Health Facility Surveys. Lancet Glob. Health 2021, 9, e1553–e1560. [Google Scholar] [CrossRef]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.Y.; Chan, K.G.; Yean, C.Y.; Yu, C.Y. Lateral Flow Immunoassays for SARS-CoV-2. Diagnostics 2022, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Karlafti, E.; Tsavdaris, D.; Kotzakioulafi, E.; Kaiafa, G.; Savopoulos, C.; Netta, S.; Michalopoulos, A.; Paramythiotis, D. The Diagnostic Accuracy of SARS-CoV-2 Nasal Rapid Antigen Self-Test: A Systematic Review and Meta-analysis. Life 2023, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Medoro, A.; Davinelli, S.; Voccola, S.; Cardinale, G.; Passarella, D.; Marziliano, N.; Intrieri, M. Assessment of the Diagnostic Performance of a Novel SARS-CoV-2 Antigen Sealing Tube Test Strip (Colloidal Gold) as Point-of-Care Surveillance Test. Diagnostics 2022, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Galliez, R.M.; Bomfim, L.; Mariani, D.; Leitão, I.C.; Castiñeiras, A.C.P.; Gonçalves, C.C.A.; Ortiz da Silva, B.; Cardoso, P.H.; Arruda, M.B.; Alvarez, P.; et al. Evaluation of the Panbio COVID-19 Antigen Rapid Diagnostic Test in Subjects Infected with Omicron Using Different Specimens. Microbiol Spectr. 2022, 10, e0125022. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, C.; Tremblay, N.; Renaud, C.; Papenburg, J. Diagnostic Accuracy of Rapid Antigen Detection Tests for Respiratory Syncytial Virus Infection: Systematic Review and Meta-analysis. J. Clin. Microbiol. 2015, 53, 3738–3749. [Google Scholar] [CrossRef]
- World Health Organization. Use of SARS-CoV-2 Antigen-Detection Rapid Diagnostic Tests for COVID-19 Self-Testing. 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Ag-RDTs-Self_testing-2022.1 (accessed on 4 January 2023).
- Ministry of Health, Labour and Welfare. Coronavirus (COVID-19). Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00079.html (accessed on 4 January 2023).
- Wright, C.; Oliver, K.C.; Fenwick, F.I.; Smith, N.M.; Toms, G.L. A monoclonal antibody pool for routine immunohistochemical detection of human respiratory syncytial virus antigens in formalin-fixed, paraffin-embedded tissue. J. Pathol. 1997, 182, 238–244. [Google Scholar] [CrossRef]
- Tamura, D.; Yamagishi, H.; Morisawa, Y.; Mato, T.; Nunomiya, S.; Maehara, Y.; Ochiai, Y.; Okuyama, S.; Ohmika, N.; Yamagata, T.; et al. Diagnostic Accuracy of a Novel SARS CoV-2 Rapid Antigen Test and Usefulness of Specimens Collected from the Anterior Nasal Cavity. Int. J. Infect. Dis. 2022, 124, 199–205. [Google Scholar] [CrossRef]
- Sekisui Medical Co., Ltd.; Next, R.T. R.-A. Package Insert. Available online: https://www.info.pmda.go.jp/tgo/pack/30200EZX00014000_B_01_03/ (accessed on 2 December 2023).
- National Institute of Infectious Disease, Laboratory Manuals for Pathogen Detection. Manual for the Detection of Pathogen, 2019–.nCoV, ver.2.6. Vol. 2020. Available online: https://www.niid.go.jp/niid/images/epi/corona/2019-nCoVmanual20200217-en.pdf (accessed on 4 January 2023).
- Yokoi, H.; Kobayashi, K.; Mizumura, A.; Tanaka, T. Detection of RS Virus and hMPV Genes by Real-Time RT-PCR. In Chiba City Environmental Health Research Institute Annual Report; 2012; Volume 19, pp. 39–43. Available online: https://www.city.chiba.jp/hokenfukushi/iryoeisei/khoken/kkagaku/documents/19tyousakennkyuu1-1.pdf (accessed on 27 December 2023). (In Japanese).
- Rabaan, A.A.; Tirupathi, R.; Sule, A.A.; Aldali, J.; Mutair, A.A.; Alhumaid, S.; Muzaheed, G.N.; Gupta, N.; Koritala, T.; Adhikari, R.; et al. Viral Dynamics and Real-Time RT-PCR CT Values Correlation with Disease Severity in COVID-19. Diagnostics 2021, 11, 1091. [Google Scholar] [CrossRef]
- Syed, A.M.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; Chen, P.Y.; Kumar, G.R.; Silva, I.; Milbes, B.; Kojima, N.; Hess, V.; et al. Omicron Mutations Enhance Infectivity and Reduce Antibody Neutralization of SARS-CoV-2 Virus-Like Particles. medRxiv 2022. [Google Scholar] [CrossRef]
- Kim, K.S.; Ejima, K.; Iwanami, S.; Fujita, Y.; Ohashi, H.; Koizumi, Y.; Asai, Y.; Nakaoka, S.; Watashi, K.; Aihara, K.; et al. A Quantitative Model Used to Compare Within-Host SARS-CoV-2, MERS-CoV, and SARS-CoV Dynamics Provides Insights into the Pathogenesis and Treatment of SARS-CoV-2. PLOS Biol. 2021, 19, e3001128. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.C.; Ng, K.C.; Ching, R.H.H.; Lai, K.L.; Kam, T.T.; Gu, H.; Sit, K.Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron Variant Replication in Human Bronchus and Lung Ex Vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.M.; Mak, T.M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; et al. Virological and Serological Kinetics of SARS-CoV-2 Delta Variant Vaccine Breakthrough Infections: A Multicentre Cohort Study. Clin. Microbiol. Infect. 2022, 28, 612.e1–612.e7. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Waning of SARS-CoV-2 Booster Viral-Load Reduction Effectiveness. Nat. Commun. 2022, 13, 1237. [Google Scholar] [CrossRef]
- Becker, S.; Soukup, J.; Yankaskas, J.R. Respiratory syncytial virus infection of human primary nasal and bronchial epithelial cell cultures and bronchoalveolar macrophages. Am. J. Respir. Cell Mol. Biol. 1992, 6, 369–374. [Google Scholar] [CrossRef]


| Clinical Severity | COVID–19 (n = 56) | RSV (n = 47) |
|---|---|---|
| Moderate to Severe Age average (y) Age range (y) | 10 21.9 0–79 | 13 3.4 0–15 |
| Mild Age average (y) Age range (y) | 28 31.3 2–71 | 34 26.4 0–71 |
| Asymptomatic Age average (y) Age range (y) | 18 28.9 6–72 | – – – |
| (A) | ||||||
|---|---|---|---|---|---|---|
| Sensitivity (%) | 95%CI | Specificity (%) | 95%CI | Detection limit by Ct value | ||
| RapidTesta RSV & SARS-CoV-2 (visual judgement) | Anterior nasal specimens | 92.0% (149/162) | 86.7–95.7% | 100% (171/171) | 97.9–100% | 39.8 |
| Nasopharyngeal specimens | 85.4% (146/171) | 79.2–90.3% | 100% (162/162) | 97.7–100% | 39.9 | |
| RapidTesta RSV & SARS-CoV-2 (RapidTesta Reader) | Anterior nasal specimens | 92.6% (150/162) | 87.4–96.1% | 99.4% (169/170) | 96.8–100% | 39.8 |
| Nasopharyngeal specimens | 86.5% (148/171) | 80.5–91.3% | 100% (161/161) | 97.7–100% | 40.2 | |
| RapidTesta SARS-CoV-2 (visual judgement) | Anterior nasal specimens | 92.1% (140/152) | 86.6–95.9% | 100% (66/66) | 94.6–100% | 38.4 |
| Nasopharyngeal specimens | 86.0% (141/164) | 79.7–90.9% | 100% (59/59) | 93.9–100% | 39.3 | |
| RapidTesta SARS-CoV-2 (RapidTesta Reader) | Anterior nasal specimens | 92.2% (130/141) | 86.5–96.0% | 100% (65/65) | 94.5–100% | 37.6 |
| Nasopharyngeal specimens | 88.5% (131/148) | 82.2–93.2% | 100% (58/58) | 93.8–100% | 38.8 | |
| (B) | ||||||
| Sensitivity (%) | 95%CI | Specificity (%) | 95%CI | Detection limit by Ct value | ||
| RapidTesta RSV & SARS-CoV-2 (visual judgement) | Anterior nasal specimens | 78.6% (55/70) | 67.1–87.5% | 100% (139/139) | 97.4–100% | 34.3 |
| Nasopharyngeal specimens | 77.0% (57/74) | 65.8–86.0% | 100% (135/135) | 97.3–100% | 31.0 | |
| RapidTesta RSV & SARS-CoV-2 (RapidTesta Reader) | Anterior nasal specimens | 80.0% (56/70) | 68.7–88.6% | 100% (139/139) | 97.4–100% | 34.3 |
| Nasopharyngeal specimens | 79.7% (59/74) | 68.8–88.2% | 99.3% (134/135) | 95.9–100% | 34.2 | |
| RapidTesta RSV-Adeno NEXT (visual judgement) | Anterior nasal specimens | 74.6% (50/67) | 62.5–84.5% | 100% (43/43) | 91.8–100% | 34.3 |
| Nasopharyngeal specimens | 74.6% (53/71) | 62.9–84.2% | 100% (39/39) | 91.0–100% | 31.0 | |
| RapidTesta RSV-Adeno NEXT (RapidTesta Reader) | Anterior nasal specimens | 80.6% (54/67) | 69.1–89.2% | 100% (43/43) | 91.8–100% | 34.3 |
| Nasopharyngeal specimens | 77.5% (55/71) | 66.0–86.5% | 100% (39/39) | 91.0–100% | 31.0 | |
| ≤3 days | 4–6 days | 7–10 days | ≥11 days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | 95%CI | Sensitivity (%) | 95%CI | Sensitivity (%) | 95%CI | Sensitivity (%) | 95%CI | ||
| RapidTesta RSV & SARS-CoV-2 (visual judgement) | Anterior nasal specimens | 97.6% (41/42) | (87.4–99.9%) | 93.3% (28/30) | (77.9–99.2%) | 94.9% (37/39) | (82.7–99.4%) | 80.0% (24/30) | (61.4–92.3%) |
| Nasopharyngeal specimens | 80.4% (41/51) | (66.9–90.2%) | 90.3% (28/31) | (74.2–98.0%) | 97.4% (37/38) | (86.2–99.9%) | 70.0% (21/30) | (50.6–85.3%) | |
| RapidTesta RSV & SARS-CoV-2 (RapidTesta Reader) | Anterior nasal specimens | 97.6% (41/42) | (87.4–99.9%) | 93.3% (28/30) | (77.9–99.2%) | 94.9% (37/39) | (82.7–99.9%) | 83.3% (25/30) | (65.3–94.4%) |
| Nasopharyngeal specimens | 80.4% (41/51) | (66.9–90.2%) | 90.3% (28/31) | (74.2–98.0%) | 97.4% (37/38) | (86.2–99.9%) | 76.3% (23/30) | (57.7–90.1%) | |
| RapidTesta SARS-CoV-2 (visual judgement) | Anterior nasal specimens | 95.1% (39/41) | (83.5–99.9%) | 100% (24/24) | (85.8–100%) | 91.4% (32/35) | (76.9–98.2%) | 73.3% (22/30) | (54.1–87.7%) |
| Nasopharyngeal specimens | 85.1% (40/47) | (71.1–93.8%) | 96.3% (26/27) | (81.8–99.9%) | 91.7% (33/36) | (77.5–98.2%) | 66.7% (20/30) | (47.2–82.7%) | |
| RapidTesta SARS-CoV-2 (RapidTesta Reader) | Anterior nasal specimens | 95.1% (39/41) | (88.4–100%) | 100% (24/24) | (85.8–100%) | 92.6% (25/27) | (75.5–99.1%) | 77.8% (21/27) | (57.7–91.4%) |
| Nasopharyngeal specimens | 87.2% (41/47) | (74.3–95.2%) | 96.2% (25/26) | (86.8–100%) | 93.3% (28/30) | (77.9–99.2%) | 66.7% (18/27) | (46.0–83.5%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, D.; Morisawa, Y.; Mato, T.; Nunomiya, S.; Yoshihiro, M.; Maehara, Y.; Ito, S.; Ochiai, Y.; Yamagishi, H.; Tajima, T.; et al. Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test. Diagnostics 2024, 14, 119. https://doi.org/10.3390/diagnostics14010119
Tamura D, Morisawa Y, Mato T, Nunomiya S, Yoshihiro M, Maehara Y, Ito S, Ochiai Y, Yamagishi H, Tajima T, et al. Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test. Diagnostics. 2024; 14(1):119. https://doi.org/10.3390/diagnostics14010119
Chicago/Turabian StyleTamura, Daisuke, Yuji Morisawa, Takashi Mato, Shin Nunomiya, Masaki Yoshihiro, Yuta Maehara, Shizuka Ito, Yasushi Ochiai, Hirokazu Yamagishi, Toshihiro Tajima, and et al. 2024. "Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test" Diagnostics 14, no. 1: 119. https://doi.org/10.3390/diagnostics14010119
APA StyleTamura, D., Morisawa, Y., Mato, T., Nunomiya, S., Yoshihiro, M., Maehara, Y., Ito, S., Ochiai, Y., Yamagishi, H., Tajima, T., Yamagata, T., & Osaka, H. (2024). Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test. Diagnostics, 14(1), 119. https://doi.org/10.3390/diagnostics14010119







