Atrophic Gastritis and Autoimmunity: Results from a Prospective, Multicenter Study
Abstract
1. Introduction
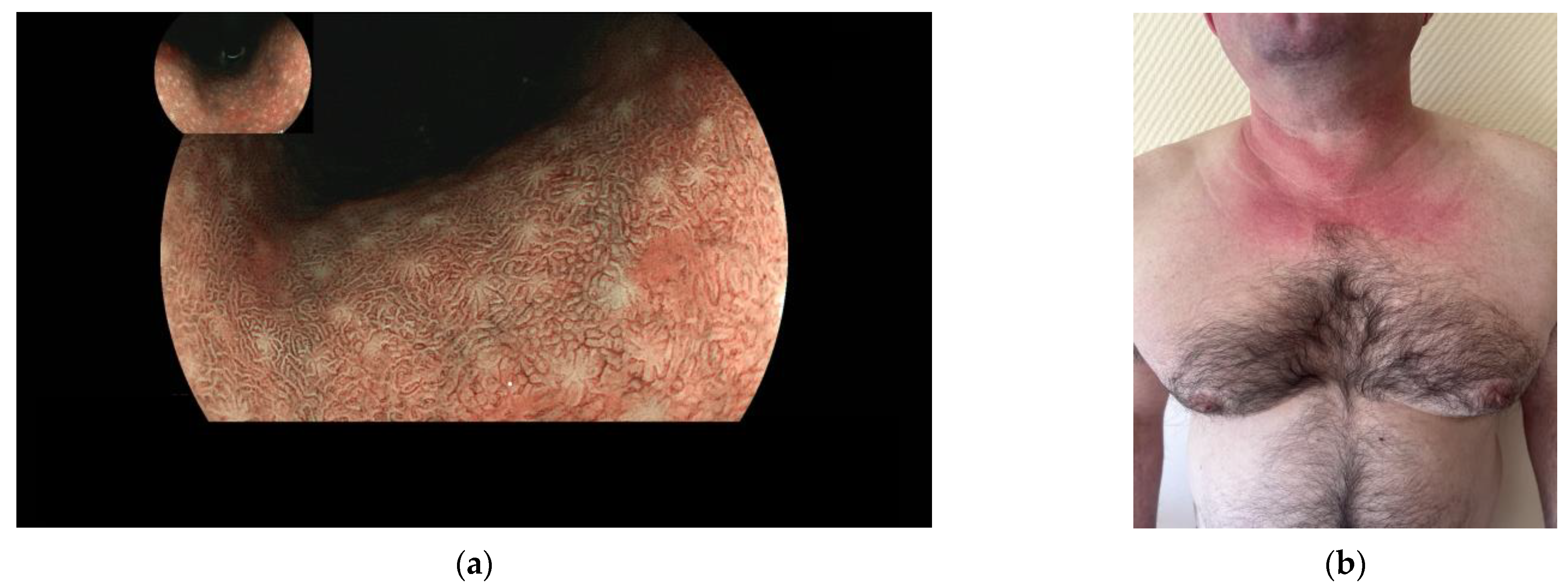
2. Materials and Methods
2.1. Design of the Study
2.2. Antibodies
2.3. Statistical Analysis
3. Results
3.1. Descriptive Analysis of the Study Population
3.2. Autoantibodies
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric Cancer |
| GPL | Gastric Precancerous Lesions |
| H. pylori | Helicobacter pylori |
| CAG | Chronic Atrophic Gastritis |
| AIG | Autoimmune Gastritis |
| NAIG | Non-autoimmune Gastritis |
| ELISA | Enzyme-Linked Immunoabsorbent Assay |
| ANA | Anti-Nuclear Antibodies |
| APCA | Anti-Parietal Cell Antibody |
| AIFA | Anti-Intrinsic Factor Antibody |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. A Human Model of Gastric Carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar] [PubMed]
- Leung, W.K.; Lin, S.R.; Ching, J.Y.L.; To, K.; Ng, E.; Chan, F.; Lau, J.; Sung, J. Factors predicting progression of gastric intestinal metaplasia: Results of a randomised trial on Helicobacter pylori eradication. Gut 2004, 53, 1244–1249. [Google Scholar] [CrossRef]
- Fukase, K.; Kato, M.; Kikuchi, S.; Inoue, K.; Uemura, N.; Okamoto, S.; Terao, S.; Amagai, K.; Hayashi, S.; Asaka, M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet 2008, 372, 392–397. [Google Scholar] [CrossRef]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.; Genta, R.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Prim. 2020, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Coati, I.; Fassan, M.; Farinati, F.; Graham, D.; Genta, R.; Rugge, M. Autoimmune gastritis: Pathologist’s viewpoint. World J. Gastroenterol. 2015, 21, 12179–12189. [Google Scholar] [CrossRef]
- Miceli, E.; Lenti, M.V.; Padula, D.; Luinetti, O.; Vattiato, C.; Monti, C.; Di Stefano, M.; Corazza, G. Common Features of Patients with Autoimmune Atrophic Gastritis. Clin. Gastroenterol. Hepatol. 2012, 10, 812–814. [Google Scholar] [CrossRef]
- Rustgi, S.D.; Bijlani, P.; Shah, S.C. Autoimmune gastritis, with or without pernicious anemia: Epidemiology, risk factors, and clinical management. Ther. Adv. Gastroenterol. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Massironi, S.; Zilli, A.; Elvevi, A.; Invernizzi, P. The changing face of chronic autoimmune atrophic gastritis: An updated comprehensive perspective. Autoimmun. Rev. 2019, 18, 215–222. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Anderson, W.F.; Rabkin, C.S.; Turner, N.; Fraumeni, J.; Rosenberg, P.; Camargo, M. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J. Natl. Cancer Inst. 2018, 110, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Chen, Y. A new gastric cancer among US. J. Natl. Cancer Inst. 2018, 110, 549–550. [Google Scholar] [CrossRef]
- Song, M.; Camargo, M.C.; Katki, H.A.; Weinstein, S.; Männistö, S.; Albanes, D.; Surcel, H.; Rabkin, C. Association of Antiparietal Cell and Anti-Intrinsic Factor Antibodies with Risk of Gastric Cancer. JAMA Oncol. 2022, 8, 268–274. [Google Scholar] [CrossRef]
- Song, M.; Latorre, G.; Ivanovic-Zuvic, D.; Camargo, M.C.; Rabkin, C.S. Autoimmune diseases and gastric cancer risk: A systematic review and meta-analysis. Cancer Res. Treat. 2019, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Zádori, N.; Szakó, L.; Váncsa, S.; Vörhendi, N.; Oštarijaš, E.; Kiss, S.; Frim, L.; Hegyi, P.; Czimmer, J. Six Autoimmune Disorders Are Associated with Increased Incidence of Gastric Cancer: A Systematic Review and Meta-Analysis of Half a Million Patients. Front. Immunol. 2021, 12, 750533. [Google Scholar] [CrossRef] [PubMed]
- Landgren, A.M.; Landgren, O.; Gridley, G.; Dores, G.; Linet, M.; Morton, L. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million U.S. male Veterans. Cancer 2011, 117, 1163–1171. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014, 73, 17–23. [Google Scholar] [CrossRef]
- Chapelle, N.; Petryszyn, P.; Blin, J.; Leroy, M.; Le Berre-Scoul, C.; Jirka, I.; Neunlist, M.; Moussata, D.; Lamarque, D.; Olivier, R.; et al. A panel of stomach-specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: A prospective, multicenter study in a low gastric cancer incidence area. Helicobacter 2020, 25, e12727. [Google Scholar] [CrossRef]
- Chapelle, N.; Osmola, M.; Martin, J.; Blin, J.; Leroy, M.; Jirka, I.; Moussata, D.; Lamarque, D.; Olivier, R.; Tougeron, D.; et al. Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics 2022, 12, 695. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P.; Batts, K.; Dahms, B.; Filipe, M.; Haggitt, R.; Haot, J. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Burnham, T. Antinuclear antibodies in patients with malignancies. Lancet 1972, 300, 436–437. [Google Scholar] [CrossRef]
- Monroy-Iglesias, M.J.; Crescioli, S.; Beckmann, K.; Le, N.; Karagiannis, S.N.; Van Hemelrijck, M.; Aida, S. Antibodies as biomarkers for cancer risk: A systematic review. Clin. Exp. Immunol. 2022, 209, 46–63. [Google Scholar] [CrossRef]
- Shmerling, R.H. Autoantibodies in Systemic Lupus Erythematosus—There before You Know It. N. Engl. J. Med. 2003, 349, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Chan, E.K.L.; Ho, L.A.; Rose, K.M.; Parks, C.G.; Cohn, R.D.; Jusko, T.A.; Walker, N.J.; Germolec, D.R.; Whitt, I.Z.; et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012, 64, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Solans-Laque, R.; Perez-Bocanegra, C.; Salud-Salvia, A.; Fonollosa-Pla, V.; Rodrigo, M.J.; Armadans, L.; Simeon-Aznar, C.P.; Vilardell-Tarres, M. Clinical significance of antinuclear antibodies in malignant diseases: Association with rheumatic and connective tissue paraneoplastic syndromes. Lupus 2004, 13, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Eck, M.; Schmausser, B.; Kerkau, T.; Greiner, A.; Kraus, M.; Fischbach, W.; Müller-Hermelink, H.K. Autoantibodies in gastric MALT-type lymphoma. Ann. Oncol. 2003, 14, 1153–1154. [Google Scholar] [CrossRef]
- Zádori, N.; Németh, D.; Szakó, L.; Váncsa, S.; Vörhendi, N.; Szakács, Z.; Frim, L.; Hegyi, P.; Czimmer, J. Prevalence of Autoimmune-phenomena behind Chronic Gastritis of Unknown Origin, and their Role in the Poor Histological Outcome of the Stomach: A Single-centre, Retrospective Cross-sectional Study. J. Gastrointestin. Liver Dis. 2022, 31, 168–175. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Anapliotou, M.; Vlachoyiannopoulos, P.; Moutsopoulos, H.M. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann. Rheum. Dis. 2004, 63, 1159–1161. [Google Scholar] [CrossRef]
- Siriwardhane, T.; Krishna, K.; Ranganathan, V.; Jayaraman, V.; Wang, T.H.; Bei, K.; Rajasekaran, J.J.; Krishnamurthy, H. Exploring systemic autoimmunity in thyroid disease subjects. J. Immunol. Res. 2018, 2018, 6895146. [Google Scholar] [CrossRef]
- Meier, H.C.S.; Miller, F.W.; Dinse, G.E.; Weinberg, C.R.; Cho, C.C.; Parks, C.G. Helicobacter pylori seropositivity is associated with antinuclear antibodies in US adults, NHANES 1999–2000. Epidemiol. Infect. 2020, 148, e20. [Google Scholar] [CrossRef]
- Satoh, M.; Tanaka, S.; Ceribelli, A.; Calise, S.J.; Chan, E.K.L. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin. Rev. Allergy Immunol. 2017, 52, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hanke, K.; Brückner, C.S.; Dähnrich, C.; Huscher, D.; Komorowski, L.; Meyer, W.; Janssen, A.; Backhaus, M.; Becker, M.; Kill, A. Antibodies against PM/Scl-75 and PM/Scl-100 are independent markers for different subsets of systemic sclerosis patients. Arthritis Res. Ther. 2009, 11, R22. [Google Scholar] [CrossRef] [PubMed]
- Wielosz, E.; Dryglewska, M.; Majdan, M. The prevalence and significance of anti-pm/scl antibodies in systemic sclerosis. Ann. Agric. Environ. Med. 2021, 28, 189–192. [Google Scholar] [CrossRef]
- DeWane, M.E.; Waldman, R.; Lu, J. Dermatomyositis: Clinical features and pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 267–281. [Google Scholar] [CrossRef]
- Azuma, K.; Yamada, H.; Ohkubo, M.; Yamasaki, Y.; Yamasaki, M.; Mizushima, M.; Ozaki, S. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod. Rheumatol. 2011, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Rusak, E.; Chobot, A.; Krzywicka, A.; Wenzlau, J. Anti-parietal cell antibodies—Diagnostic significance. Adv. Med. Sci. 2016, 61, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Miceli, E.; Vanoli, A.; Klersy, C.; Corazza, G.R.; Di Sabatino, A. Time course and risk factors of evolution from potential to overt autoimmune gastritis. Dig. Liver Dis. 2022, 54, 642–644. [Google Scholar] [CrossRef]
- Cabrera de León, A.; Almeida González, D.; Almeida, A.A.; Hernandez, A.G.; Perez, M.C.; Perez, M.D.R.; Guillen, V.G.; Diaz, B.B. Factors associated with parietal cell autoantibodies in the general population. Immunol. Lett. 2012, 147, 63–66. [Google Scholar] [CrossRef]
- Tiberti, C.; Panimolle, F.; Borghini, R.; Montuori, M.; Trovato, C.M.; Filardi, T.; Lenzi, A.; Picarelli, A. Type 1 diabetes, thyroid, gastric and adrenal humoral autoantibodies are present altogether in almost one third of adult celiac patients at diagnosis, with a higher frequency than children and adolescent celiac patients. Scand. J. Gastroenterol. 2020, 55, 549–554. [Google Scholar] [CrossRef]
- Goldenring, J. No H. pylori, no adenocarcinoma for patients with autoimmune gastritis. Gut 2023, 72, 1–2. [Google Scholar] [CrossRef]
- Waldum, H.L. Conclusion that autoimmune gastritis does not predispose to gastric cancer is unproven. Gut 2023. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.; Lehtinen, M.; Öhman, H.; Waterboer, T.; Epplein, M. Association of Helicobacter pylori and Autoimmune Gastritis with Stomach Cancer in a Cohort of Young Finnish Women. Gastroenterology 2022, 163, 305–307.e4. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Bricca, L.; Guzzinati, S.; Sacchi, D.; Pizzi, M.; Savarino, E.; Farinati, F.; Zorzi, M.; Fassan, M.; Dei Tos, A.P. Autoimmune gastritis: Long-Term natural history in naïve Helicobacter pylori-negative patients. Gut. 2022, 72, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.L.; Dooley, C.P.; Dehesa, M.; Cohen, H.; Carmel, R.; Fitzgibbons, P.L.; Perez-Perez, G.I.; Blaser, M.J. Helicobacter pylori infection in pernicious anemia: A prospective controlled study. Gastroenterology 1991, 100, 328–332. [Google Scholar] [PubMed]
- Presotto, F.; Sabini, B.; Cecchetto, A.; Plebani, M.; De Lazzari, F.; Pedini, B.; Betterle, C. Helicobacter pylori Infection and Gastric Autoimmune Diseases: Is There a Link? Helicobacter 2003, 8, 578–584. [Google Scholar] [CrossRef]
- Kotera, T.; Nishimi, Y.; Kushima, R.; Haruma, K. Regression of Autoimmune Gastritis after Eradication of Helicobacter pylori. Case Rep. Gastroenterol. 2023, 17, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Haruma, K.; Kaya, S.; Kamada, T.; Kim, S.; Sasaki, A.; Sumii, M.; Tanaka, S.; Yoshihara, M.; Chayama, K. Role of Anti-Parietal Cell Antibody in Helicobacter pylori-associated Atrophic Gastritis: Evaluation in a Country of High Prevalence of Atrophic Gastritis. Scand. J. Gastroenterol. 2002, 37, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.M.; Zhang, L.L.; Dai, X.; Liu, Z.; Zeng, Y.; Li, X.; Wu, Q.; Lv, W. Helicobacter pylori and Autoimmune Diseases: Involving Multiple Systems. Front. Immunol. 2022, 13, 833424. [Google Scholar] [CrossRef]
- Hasni, S.A. Role of Helicobacter pylori infection in autoimmune diseases. Curr. Opin. Rheumatol. 2012, 24, 429–434. [Google Scholar] [CrossRef]
- Chapelle, N.; Péron, M.; Mosnier, J.F.; Quénéhervé, L.; Coron, E.; Bourget, A.; Cauchin, E.; Touchefeu, Y.; Matysiak-Budnik, T. Prevalence, Characteristics and Endoscopic Management of Gastric Premalignant Lesions in France. Dig. Dis. 2020, 38, 286–292. [Google Scholar] [CrossRef]
- Altayar, O.; Davitkov, P.; Shah, S.C.; Gawron, A.; Morgan, D.; Turner, K.; Mustafa, R. AGA Technical Review on Gastric Intestinal Metaplasia—Epidemiology and Risk Factors. Gastroenterology 2020, 158, 732–744.e16. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Negative | Equivocal | Positive |
|---|---|---|---|
| APCA, AIFA [U/mL] | <7 | 7–10 | >10 |
| ANA | <1:80 | 1:80 | ≥1:160 |
| Myositis-associated antibodies | ≤10 | >10 | >25 |
| Parameter | CAG (n = 154) | Control (n = 201) | p-Value | Total (n = 355) |
|---|---|---|---|---|
| Age (year) mean (±SD) | 61.5 (±13.8) | 56.4 (±14.2) | <0.001 | 58.6 (±14.2) |
| Range (year) | 22–89 | 18–82 | 18–89 | |
| Sex | 0.09 | |||
| Female n (%) | 76 (49.4) | 117 (58.2) | 193 (54.4) | |
| Male n (%) | 78 (50.6) | 84 (41.8) | 162 (45.6) | |
| H. pylori status | 0.006 | |||
| Histology positive n (%) | 25 (16.2) | 22 (10.9) | 47 (13.2) | |
| Serology positive n (%) | 35 (22.7) | 27 (13.4) | 62 (17.5) | |
| Any H. pylori positive n (%) | 42 (27.3) | 31 (15.4) | 73 (20.6) | |
| APCA n (%) | 41 (27.0) | 8 (4.0) | <0.001 | 49 (13.9) |
| AIFA n (%) | 20 (13.5) | 0 | <0.001 | 20 (5.8) |
| ANA n (%) | 52 (34.2) | 54 (27.0) | 0.1 | 106 (30.1) |
| Myositis-associated antibodies | 0.6 | |||
| At least one antibody equivocal or positive n (%) | 22 (14.5) | 26 (12.9) | 59 (13.8) | |
| At least one positive antibody n (%) | 9 (5.9) | 9 (4.4) | 19 (5.3) |
| Parameter | AIG (n = 45) | NAIG (n = 109) | Control (n = 201) | p-Value | Total (n = 355) |
|---|---|---|---|---|---|
| Age (year) mean (±SD) | 58.9 (±15.7) | 62.5 (±12.8) | 56.4 (±14.2) | 0.001 | 58.6 (±14.2) |
| Range (year) | 23–89 | 22–87 | 18–82 | 18–89 | |
| Sex | 0.059 | ||||
| Female n (%) | 27 (60.0) | 49 (45.0) | 117 (58.2) | 193 (54.4) | |
| Male n (%) | 18 (40.0) | 60 (55.0) | 84 (41.8) | 162 (45.6) | |
| H. pylori status | <0.001 | ||||
| Histology positive n (%) | 0 | 25 (22.9) | 22 (10.9) | 47 (13.2) | |
| Serology positive n (%) | 5 (11.1) | 30 (27.5) | 27 (13.4) | 62 (17.5) | |
| Any H. pylori positive n (%) | 5 (11.1) | 37 (33.9) | 31 (15.4) | 73 (20.6) | |
| APCA n (%) | 33 (73.3) | 8 (7.5) | 8 (4.0) | <0.001 | 49 (13.9) |
| AIFA n (%) | 17 (40.5) | 3 (2.8) | 0 | <0.001 | 20 (5.8) |
| ANA n (%) | 21 (46.7) | 31 (29.0) | 54 (27.0) | 0.03 | 106 (30.1) |
| Myositis antibodies | 0.8 | ||||
| At least one antibody equivocal or positive n (%) | 7 (14.3) | 15 (15.6) | 26 (12.9) | 59 (13.8) | |
| At least one positive antibody n (%) | 4 (8.9) | 6 (5.5) | 9 (4.4) | 19 (5.3) |
| Parameter | ANA Negative | ANA Positive | OR (Univariate) | OR (Multivariate) | |
|---|---|---|---|---|---|
| Age n (%) | ≤50 | 70 (72.2) | 27 (27.8) | ||
| >50 | 176 (69.0) | 79 (31.0) | 1.16 (0.70–1.97, p = 0.5) | 1.23 (0.73–2.11, p = 0.4) | |
| Sex n (%) | Female | 122 (63.5) | 70 (36.5) | ||
| Male | 124 (77.5) | 36 (22.5) | 0.51 (0.31–0.81, p = 0.005) | 0.50 (0.31–0.80, p = 0.004) | |
| H. Pylori n (%) | Negative | 199 (71.1) | 81 (28.9) | ||
| Positive | 47 (65.3) | 25 (34.7) | 1.31 (0.75–2.25, p = 0.3) | 1.31 (0.74–2.27, p = 0.3) |
| Parameter | APCA Negative | APCA Positive | OR (Univariate) | OR (Multivariate) | AIFA Negative | AIFA Positive | OR (Univariate) | OR (Multivariate) | |
|---|---|---|---|---|---|---|---|---|---|
| Age n (%) | ≤50 | 80 (82.5) | 17 (17.5) | 87 (90.6) | 9 (9.4) | ||||
| >50 | 223 (87.5) | 32 (12.5) | 0.68 (0.36–1.31, p = 0.2) | 0.69 (0.37–1.34, p = 0.3) | 240 (95.6) | 11 (4.4) | 0.44 (0.18–1.13, p = 0.08) | 0.46 (0.18–1.12, p = 0.09) | |
| Sex n (%) | Female | 163 (84.9) | 29 (15.1) | 176 (93.6) | 12 (6.4) | ||||
| Male | 140 (87.5) | 20 (12.5) | 0.80 (0.43–1.47, p = 0.5) | 0.83 (0.44–1.52, p = 0.5) | 151 (95.0) | 8 (5.0) | 0.78 (0.30–1.93, p = 0.6) | 0.85 (0.34–2.09, p = 0.7) | |
| H. Pylori n (%) | Neg. | 239 (85.4) | 41 (14.6) | 258 (92.8) | 20 (7.2) | ||||
| Pos. | 64 (88.9) | 8 (11.1) | 0.73 (0.30–1.56, p = 0.4) | 0.74 (0.31–1.58, p = 0.5) | 69 (100.0) | 0 | - | 0.09 (0.006–1.5, p = 0.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmola, M.; Hemont, C.; Chapelle, N.; Vibet, M.-A.; Tougeron, D.; Moussata, D.; Lamarque, D.; Bigot-Corbel, E.; Masson, D.; Blin, J.; et al. Atrophic Gastritis and Autoimmunity: Results from a Prospective, Multicenter Study. Diagnostics 2023, 13, 1599. https://doi.org/10.3390/diagnostics13091599
Osmola M, Hemont C, Chapelle N, Vibet M-A, Tougeron D, Moussata D, Lamarque D, Bigot-Corbel E, Masson D, Blin J, et al. Atrophic Gastritis and Autoimmunity: Results from a Prospective, Multicenter Study. Diagnostics. 2023; 13(9):1599. https://doi.org/10.3390/diagnostics13091599
Chicago/Turabian StyleOsmola, Malgorzata, Caroline Hemont, Nicolas Chapelle, Marie-Anne Vibet, David Tougeron, Driffa Moussata, Dominique Lamarque, Edith Bigot-Corbel, Damien Masson, Justine Blin, and et al. 2023. "Atrophic Gastritis and Autoimmunity: Results from a Prospective, Multicenter Study" Diagnostics 13, no. 9: 1599. https://doi.org/10.3390/diagnostics13091599
APA StyleOsmola, M., Hemont, C., Chapelle, N., Vibet, M.-A., Tougeron, D., Moussata, D., Lamarque, D., Bigot-Corbel, E., Masson, D., Blin, J., Leroy, M., Josien, R., Mosnier, J.-F., Martin, J., & Matysiak-Budnik, T. (2023). Atrophic Gastritis and Autoimmunity: Results from a Prospective, Multicenter Study. Diagnostics, 13(9), 1599. https://doi.org/10.3390/diagnostics13091599






