SDS Electrophoresis on Gradient Polyacrylamide Gels as a Semiquantitative Tool for the Evaluation of Proteinuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Samples
- -
- A total of 93 samples from patients with normal urinalysis results and urine albumin/creatinine ratio (ACR) < 30 mg/g were used to verify the limit of detection of the studied method and to observe a normal SDS-PAGE protein pattern;
- -
- In total, 300 samples from patients with proteinuria of varying severity, detectable in routine urinalysis, and with a broad range of eGFR, were used to study glomerular and tubular patterns of proteinuria; from this group, selected samples were also used to identify proteins present in SDS-PAGE fractions;
- -
- Ten samples from patients with hemoglobinuria detected with urinalysis were used to study the SDS-PAGE protein pattern associated with hemoglobinuria.
2.2. Ethical Statement
2.3. SDS-PAGE
2.4. Western Blot
2.5. Liquid Chromatography and Tandem Mass Spectrometry (LC-MS/MS)
2.6. Albumin and Immunoglobulin Light Chain Concentration in Urine
2.7. Statistical Analysis
3. Results
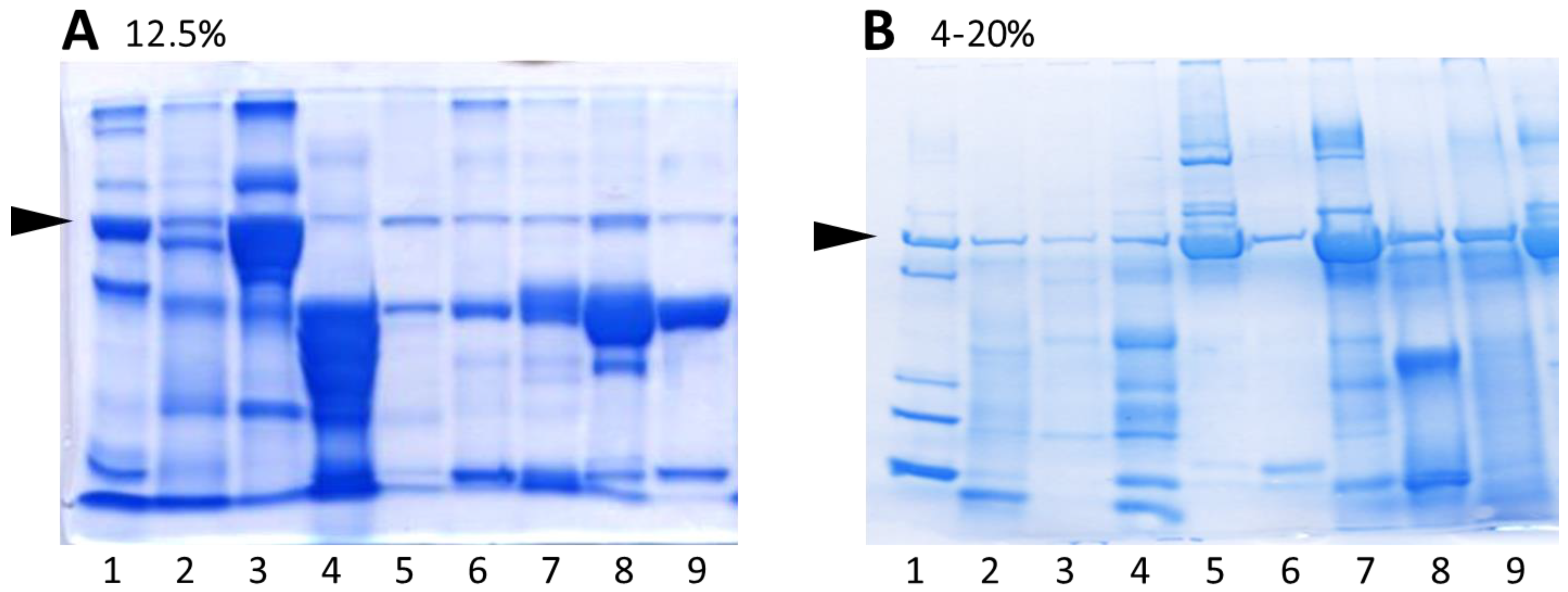
3.1. Comparison of Urine Protein Separation Using Commercially Available 4–20% Gradient Polyacrylamide Gels with Self-Cast Gels
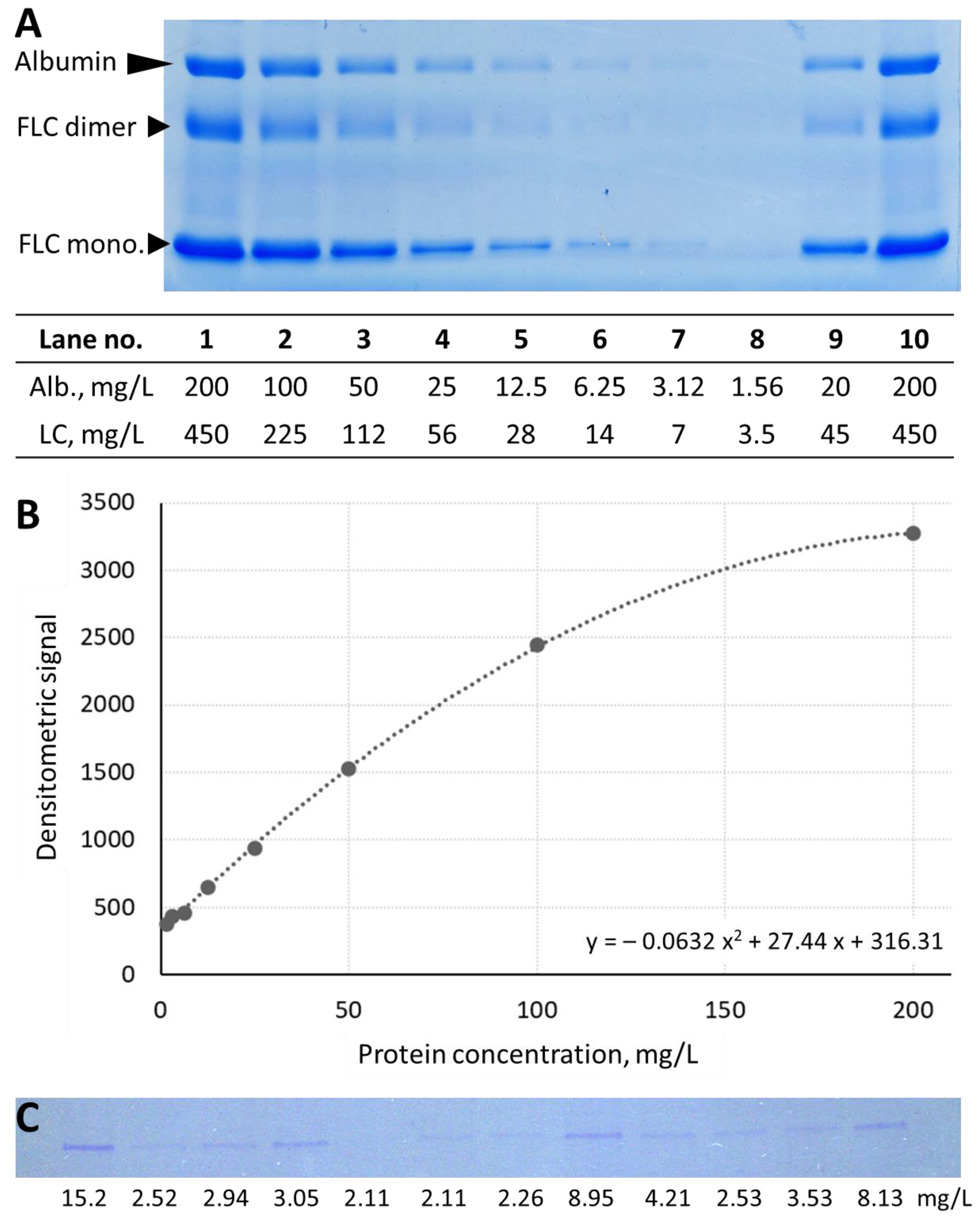
3.2. Analytical Performance of Urine Protein Separation Using SDS-PAGE
3.3. Identification of Urine Protein Fractions Obtained with SDS-PAGE Separation Using 4–20% Gradient Gels
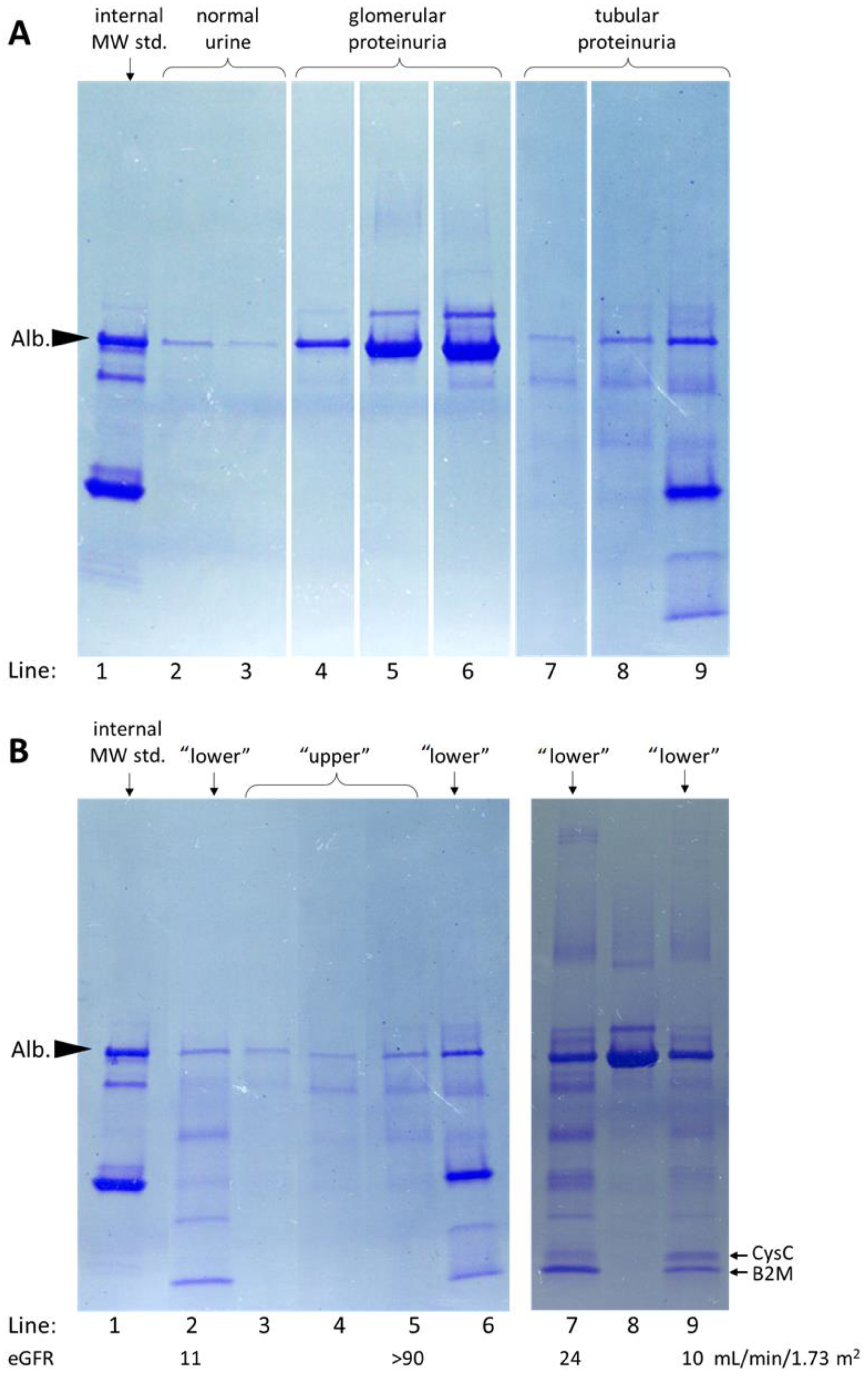
3.4. Urine Protein Patterns Obtained Using SDS-PAGE on 4–20% Gradient Gels in Patients’ Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burrows, R.N.; Koyama, A.; Pavkov, M.E. Reported cases of end-stage kidney disease—United States, 2000–2019. Am. J. Transplant. 2022, 22, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Ikemiya, Y.; Iseki, C.; Takishita, S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003, 63, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Decramer, S.; de Peredo, A.G.; Breuil, B.; Mischak, H.; Monsarrat, B.; Bascands, J.L.; Schanstra, J.P. Urine in clinical proteomics. Mol. Cell. Proteom. 2008, 7, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Jafar, T.H.; Stark, P.C.; Schmid, C.H.; Landa, M.; Maschio, G.; Marcantoni, C.; De Jong, P.E.; De Zeeuw, D.; Shahinfar, S.; Ruggenenti, P.; et al. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int. 2001, 60, 1131–1140. [Google Scholar] [CrossRef]
- Ots, M.; Pechter, Ü.; Tamm, A. Characteristics of progressive renal disease. Clin. Chim. Acta 2000, 297, 29–41. [Google Scholar] [CrossRef]
- Abbate, M.; Zoja, C.; Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 2006, 17, 2974–2984. [Google Scholar] [CrossRef]
- Schanstra, J.P.; Zürbig, P.; Alkhalaf, A.; Argiles, A.; Bakker, S.J.L.; Beige, J.; Bilo, H.J.G.; Chatzikyrkou, C.; Dakna, M.; Dawson, J.; et al. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J. Am. Soc. Nephrol. 2015, 26, 1999–2010. [Google Scholar] [CrossRef]
- Keane, W.F. Proteinuria: Its clinical importance and role in progressive renal disease. Am. J. Kidney Dis. 2000, 35, 97–105. [Google Scholar] [CrossRef]
- Wahl, T.S.; Graham, L.A.; Morris, M.S.; Richman, J.S.; Hollis, R.H.; Jones, C.E.; Itani, K.M.; Wagner, T.H.; Mull, H.J.; Whittle, J.C.; et al. Association between preoperative proteinuria and postoperative acute kidney injury and readmission. JAMA Surg. 2018, 153, e182009. [Google Scholar] [CrossRef]
- Flammia, R.S.; Tufano, A.; Proietti, F.; Gerolimetto, C.; de Nunzio, C.; Franco, G.; Leonardo, C. Renal surgery for kidney cancer: Is preoperative proteinuria a predictor of functional and survival outcomes after surgery? A systematic review of the literature. Minerva Urol. Nephrol. 2022, 74, 255–264. [Google Scholar] [CrossRef]
- Barnett, L.M.A.; Cummings, B.S. Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicol. Sci. 2018, 164, 379–390. [Google Scholar] [CrossRef]
- Magalhães, P.; Pejchinovski, M.; Markoska, K.; Banasik, M.; Klinger, M.; Švec-Billá, D.; Rychlík, I.; Rroji, M.; Restivo, A.; Capasso, G.; et al. Association of kidney fibrosis with urinary peptides: A path towards non-invasive liquid biopsies? Sci. Rep. 2017, 7, 16915. [Google Scholar] [CrossRef]
- Bergón, E.; Granados, R.; Fernández-Segoviano, P.; Miravalles, E.; Bergón, M. Classification of renal proteinuria: A simple algorithm. Clin. Chem. Lab. Med. 2002, 40, 1143–1150. [Google Scholar] [CrossRef]
- Julian, B.A.; Suzuki, H.; Suzuki, Y.; Tomino, Y.; Spasovski, G.; Novak, J. Sources of urinary proteins and their analysis by urinary proteomics for the detection of biomarkers of disease. Proteom. Clin. Appl. 2009, 3, 1029–1043. [Google Scholar] [CrossRef]
- Yousef Yengej, F.A.; Jansen, J.; Rookmaaker, M.B.; Verhaar, M.C.; Clevers, H. Kidney Organoids and Tubuloids. Cells 2020, 9, 1326. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar]
- Zhang, H.; Wang, F.; Xiao, H.; Yao, Y. The ratio of urinary α1-microglobulin to microalbumin can be used as a diagnostic criterion for tubuloproteinuria. Intractable Rare Dis. Res. 2018, 7, 46–50. [Google Scholar] [CrossRef]
- Beetham, R.; Cattell, W.R. Proteinuria: Pathophysiology, significance and recommendations or measurement in clinical practice. Ann. Clin. Biochem. 1993, 30, 425–434. [Google Scholar] [CrossRef]
- Norden, A.G.W.; Scheinman, S.J.; Deschodt-Lanckman, M.M.; Lapsley, M.; Nortier, J.L.; Thakker, R.V.; Unwin, R.J.; Wrong, O. Tubular proteinuria defined by a study of Dent’s (CLCN5 mutation) and other tubular diseases. Kidney Int. 2000, 57, 240–249. [Google Scholar] [CrossRef]
- Parikh, C.R.; Lu, J.C.; Coca, S.G.; Devarajan, P. Tubular proteinuria in acute kidney injury: A critical evaluation of current status and future promise. Ann. Clin. Biochem. 2010, 47, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Song, S.H.; Kim, I.J.; Jeon, Y.K.; Kim, B.H.; Kwak, I.S.; Lee, E.K.; Kim, Y.K. Urinary cystatin c and tubular proteinuria predict progression of diabetic nephropathy. Diabetes Care 2013, 36, 656–661. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.M.; Woo, K.T.; Tan, H.K.; Wong, K.S. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of urinary protein in acute kidney injury. Saudi J. Kidney Dis. Transplant. 2011, 22, 739–745. [Google Scholar]
- Brocklebank, T.; Cooper, E.H.; Richmond, K. Sodium dodecyl sulphate polyacrylamide gel electrophoresis patterns of proteinuria in various renal diseases of childhood. Pediatr. Nephrol. 1991, 5, 371–375. [Google Scholar] [CrossRef]
- Wai-Hoe, L.; Wing-Seng, L.; Ismail, Z.; Lay-Harn, G. SDS-PAGE-based quantitative assay for screening of kidney stone disease. Biol. Proced. Online 2009, 11, 145–160. [Google Scholar] [CrossRef]
- Pabis, M.; Termathe, M.; Ravichandran, K.E.; Kienast, S.D.; Krutyhołowa, R.; Sokołowski, M.; Jankowska, U.; Grudnik, P.; Leidel, S.A.; Glatt, S. Molecular basis for the bifunctional Uba4–Urm1 sulfur-relay system in tRNA thiolation and ubiquitin-like conjugation. EMBO J. 2020, 39, e105087. [Google Scholar] [CrossRef]
- Miller, A.J.; Roman, B.; Norstrom, E. A method for easily customizable gradient gel electrophoresis. Anal. Biochem. 2016, 509, 12–14. [Google Scholar] [CrossRef]
- Lau, Y.K.; Woo, K.T. SDS-PAGE Is Underutilised as a Tool for Investigating Renal Patients. Nephron 2002, 90, 227–229. [Google Scholar] [CrossRef]
- Giori, L.; Tricomi, F.M.; Zatelli, A.; Roura, X.; Paltrinieri, S. High-resolution gel electrophoresis and sodium dodecyl sulphate-agarose gel electrophoresis on urine samples for qualitative analysis of proteinuria in dogs. J. Vet. Diagn. Investig. 2011, 23, 682–690. [Google Scholar] [CrossRef]
- Maeda, H.; Sogawa, K.; Sakaguchi, K.; Abe, S.; Sagizaka, W.; Mochizuki, S.; Horie, W.; Watanabe, T.; Shibata, Y.; Satoh, M.; et al. Urinary albumin and transferrin as early diagnostic markers of chronic kidney disease. J. Vet. Med. Sci. 2015, 77, 937–943. [Google Scholar] [CrossRef]
- Ladner, C.L.; Yang, J.; Turner, R.J.; Edwards, R.A. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal. Biochem. 2004, 326, 13–20. [Google Scholar] [CrossRef]
- Scheller, C.; Krebs, F.; Wiesner, R.; Wätzig, H.; Oltmann-Norden, I. A comparative study of CE-SDS, SDS-PAGE, and Simple Western—Precision, repeatability, and apparent molecular mass shifts by glycosylation. Electrophoresis 2021, 42, 1521–1531. [Google Scholar] [CrossRef]
- Weber, K.; Osborn, M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969, 244, 4406–4412. [Google Scholar] [CrossRef]
- Neville, D.M. Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J. Biol. Chem. 1971, 246, 6328–6334. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. Biomed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]
- Llames, L.; Gómez-Lucia, E.; Doménech, A.; Suárez, G.; Goyache, J. Analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot of nonspecific and specific viral proteins frequently detected in different antigen preparations of bovine leukemia virus. J. Vet. Diagn. Investig. 2000, 12, 337–344. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Eckermann, N.; Fettke, J.; Steup, M. Identification of polysaccharide binding proteins by affinity electrophoresis in inhomogeneous polyacrylamide gels and subsequent SDS-PAGE/matrix-assisted laser desorption ionization-time of flight analysis. Anal. Biochem. 2002, 304, 180–192. [Google Scholar] [CrossRef]
- Gharahdaghi, F.; Weinberg, C.R.; Meagher, D.A.; Imai, B.S.; Mische, S.M. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: A method for the removal of silver ions to enhance sensitivity. Electrophoresis 1999, 20, 601–605. [Google Scholar] [CrossRef]
- Hogan, J.J.; Alexander, M.P.; Leung, N. Dysproteinemia and the Kidney: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients: Diagnostic accuracy of urinary β-trace protein. Am. J. Physiol. Ren. Physiol. 2010, 299, 1407–1423. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hossain, D.; Bostwick, D.; Herrera, G.; Ballester, B.; Zhang, P. Urinary β2-Microglobulin is a Sensitive Indicator for Renal Tubular Injury. SAJ Case Rep. 2014, 1, 492838. [Google Scholar] [CrossRef]
- Nejat, M.; Pickering, J.W.; Walker, R.J.; Westhuyzen, J.; Shaw, G.M.; Frampton, C.M.; Endre, Z.H. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit. Care 2010, 14, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Kuleš, J.; Bilić, P.; Beer Ljubić, B.; Gotić, J.; Crnogaj, M.; Brkljačić, M.; Mrljak, V. Glomerular and tubular kidney damage markers in canine babesiosis caused by Babesia canis. Ticks Tick-Borne Dis. 2018, 9, 1508–1517. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Sonneveld, P.; Leung, N.; Merlini, G.; Ludwig, H.; Kastritis, E.; Goldschmidt, H.; Joshua, D.; Orlowski, R.Z.; Powles, R.; et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J. Clin. Oncol. 2016, 34, 1544–1557. [Google Scholar] [CrossRef]
- Vávrová, J.; Maisnar, V.; Tichý, M.; Friedecký, B.; Čermáková, Z.; Dastych, M.; Gottwaldová, J.; Kučera, P.; Krotká, J.; Racek, J.; et al. Interlaboratory study of free monoclonal immunoglobulin light chain quantification. Clin. Chem. Lab. Med. 2011, 49, 89–92. [Google Scholar] [CrossRef]
- Doshi, M.; Lahoti, A.; Danesh, F.R.; Batuman, V.; Sanders, P.W. Paraprotein-related kidney disease: Kidney injury from paraproteins—What determines the site of injury? Clin. J. Am. Soc. Nephrol. 2016, 11, 2288–2294. [Google Scholar] [CrossRef]
- Veerreddy, P. Hemoglobinuria misidentified as hematuria: Review of discolored urine and paroxysmal nocturnal hemoglobinuria. Clin. Med. Insights Blood Disord. 2013, 6, 7–17. [Google Scholar] [CrossRef]
- Chen-Levy, Z.; Wener, M.H.; Toivola, B.; Daum, P.; Reyes, M.; Fine, J.S. Factors affecting urinary myoglobin stability in vitro. Am. J. Clin. Pathol. 2005, 123, 432–438. [Google Scholar] [CrossRef]
- Osserman, E.F.; Lawlor, D.P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J. Exp. Med. 1966, 124, 921–952. [Google Scholar] [CrossRef]
- Nakayama, A.; Kubota, R.; Sakatsume, M.; Suzuki, H.; Katayama, A.; Kanamori, K.; Shiba, K.; Iijima, S. Cellulose Acetate Membrane Electrophoresis Based Urinary Proteomics for the Identification of Characteristic Proteins. J. Clin. Lab. Anal. 2016, 30, 359–367. [Google Scholar] [CrossRef]
- Jenkins, M.A. Serum and urine electrophoresis for detection and identification of monoclonal proteins. Clin. Biochem. Rev. 2009, 30, 119–122. [Google Scholar]
- Aitekenov, S.; Gaipov, A.; Bukasov, R. Review: Detection and quantification of proteins in human urine. Talanta 2021, 223, 121718. [Google Scholar] [CrossRef]
- Booth, R.A.; McCudden, C.R.; Balion, C.M.; Blasutig, I.M.; Bouhtiauy, I.; Rodriguez-Capote, K.; Catomeris, P.; Chan, P.C.; Chen, Y.; Collier, C.; et al. Candidate recommendations for protein electrophoresis reporting from the Canadian Society of Clinical Chemists Monoclonal Gammopathy Working Group. Clin. Biochem. 2018, 51, 10–20. [Google Scholar] [CrossRef]
- Moss, M.A. Moving towards harmonized reporting of serum and urine protein electrophoresis. Clin. Chem. Lab. Med. 2016, 54, 973–979. [Google Scholar] [CrossRef]
- Tate, J.R.; Keren, D.F.; Mollee, P. A global call to arms for clinical laboratories—Harmonised quantification and reporting of monoclonal proteins. Clin. Biochem. 2018, 51, 4–9. [Google Scholar] [CrossRef]


| Protein Fraction Number | Protein Identification | ||
|---|---|---|---|
| Protein | PSMs | Molecular Weight [kDa] | |
| 1 a | 1. Ceruloplasmin 2. Complement factor H | 28 17 | 122.13 139.01 |
| 2 | 1. Plasminogen 2. Complement component C6 3. Complement component C7 4. Inter- α-trypsin inhibitor heavy chain H | 29 17 12 11 | 90.51 104.72 93.46 103.29 |
| 3 | 1. Serotransferrin b 2. Coagulation factor XIII B chain 3. Kininogen 4. Afamin 5. Protein disulfide-isomerase A4 | 550 104 65 34 18 | 77.01 75.46 71.91 69.02 72.89 |
| 4 | 1. Albumin 2. Complement component C9 | 1073 55 | 69.23 63.13 |
| 5 | 1. Albumin 2. Hemopexin 3. Antithrombin-III | 2756 308 104 | 69.23 51.64 52.57 |
| 6 | 1. Albumin 2. Pigment epithelium-derived factor 3. Vitamin D-binding protein 4. Hemopexin 5. Apolipoprotein A-IV 6. Antithrombin-III | 1135 411 295 147 140 135 | 69.23 46.28 52.88 51.64 45.34 52.57 |
| 7 | 1. Apolipoprotein A-IV 2. Pigment epithelium-derived factor 3. Vitamin D-binding protein 4. EGF-containing fibulin-like extracellular matrix protein 1 | 471 163 124 22 | 45.34 46.28 52.88 54.60 |
| 8 | 1. Protein AMBP c 2. Zinc-alpha-2-glycoprotein 3. β2-glycoprotein 4. Insulin-like growth factor-binding protein 2 | 276 62 51 22 | 38.97 34.24 38.27 34.79 |
| 9 | 1. Protein AMBP c 2. CD-5 antigen like 3. Mimecan 4. Apolipoprotein A-I 5. Corticotropin-releasing factor-binding protein | 933 42 31 29 28 | 38.97 38.06 33.90 30.76 36.12 |
| 10 | 1. Complement factor D 2. Apolipoprotein A-I 3. Immunoglobulin κ light chain 4. Phosphatidylethanolamine-binding protein 4 5. Prostaglandin-H2 D-isomerase | 532 438 280 130 87 | 27.02 30.76 23.36 25.72 21.02 |
| 11 | 1. Immunoglobulin κ light chain 2. Retinol-binding protein 4 3. Apolipoprotein A-I 4. Neutrophil gelatinase-associated lipocalin | 499 132 58 56 | 23.36 22.99 30.76 22.57 |
| 12 | 1. Retinol-binding protein 4 2. Protein FAM3C 3. Tetranectin 4. Neutrophil gelatinase-associated lipocalin 5. Transgelin 6. Ganglioside GM2 activator 7. Metalloproteinase inhibitor 2 | 777 56 27 19 18 16 16 | 22.99 24.67 22.52 22.57 22.60 20.83 24.38 |
| 13 | 1. Retinol-binding protein 4 2. Retinoic acid receptor responder protein 2 3. Myoglobin 4. Peptidyl-prolyl cis-trans isomerase A 5. Transthyretin | 234 96 82 52 33 | 22.99 18.61 17.17 18.00 15.88 |
| 14 | 1. Cystatin C 2. Lysozyme C 3. Fatty acid-binding protein, adipocyte 4. Proflin-1 5. Fatty acid-binding protein, liver 6. Hemoglobin subunit β | 608 288 107 51 38 27 | 15.79 16.53 14.71 15.05 14.20 15.99 |
| 15 | 1. β2-microglobulin 2. Cystatin C 3. Cystatin M | 251 82 34 | 13.71 15.79 16.50 |
| ICD-10 Code | Name of Disorder | All Samples, n (%) | Tubular/Mixed Pattern, n (%) | Pure Glomerular Pattern, n (%) | p-Value |
|---|---|---|---|---|---|
| Hematologic lymphoid disorders: | 117 (39) | 91 (45) | 26 (27) | 0.004 | |
| C90.0 | Multiple myeloma | 112 (37) | 87 (43) | 25 (26) | 0.006 |
| D47.2 | MGUS | 4 (1) | 3 (1) | 1 (1) | 1.0 |
| C82.9 | Follicular lymphoma | 1 (0.3) | 1 (0.5) | 0 | 1.0 |
| Renal disorders: | 133 (44) | 83 (41) | 50 (52) | 0.064 | |
| N18.x | Chronic kidney disease | 59 (20) | 37 (18) | 22 (23) | 0.3 |
| Z94.0 | Kidney transplant status | 18 (6) | 12 (6) | 6 (6) | 0.9 |
| N03.x-N06.x | Glomerular diseases | 36 (12) | 20 (10) | 16 (17) | 0.087 |
| N17.x | Acute kidney injury | 9 (3) | 7 (3) | 2 (2) | 0.7 |
| N19.x | Unspecified kidney failure | 6 (2) | 2 (1) | 4 (4) | 0.085 |
| E10.2, E11.2 | Type 1/type 2 diabetes mellitus with renal complications | 3 (1) | 3 (1) | 0 | 0.6 |
| N28.x | Other disorders of the kidney and ureter | 2 (0.7) | 2 (1) | 0 | 1.0 |
| Other potentially relevant disorders: | 16 (5) | 9 (4) | 7 (7) | 0.3 | |
| M31.3 | Wegener granulomatosis | 2 (0.7) | 0 | 2 (2) | 0.1 |
| M35.0 | Sjögren syndrome | 1 (0.3) | 1 (0.5) | 0 | 1.0 |
| Cx (other than C90.x) | Malignant neoplasms | 9 (3) | 2 (1) | 7 (7) | 0.006 |
| Ix | Diseases of the circulatory system | 5 (2) | 5 (2) | 0 | 1.0 |
| Uninformative, irrelevant, or missing codes: | 33 (11) | 22 (11) | 11 (11) | 0.9 | |
| Type of Tubular Proteinuria | Number of Samples | eGFR Value <60 mL/min/1.73 m2, n (%) | Median (Q1; Q3) eGFR, mL/min/1.73 m2 | p-Value |
|---|---|---|---|---|
| “upper” | 73 | 22 (30) | 71 (55; 90) | <0.001 |
| “lower” | 131 | 114 (87) | 31 (13; 39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, P.; Dumnicka, P.; Tisończyk, J.; Ząbek-Adamska, A.; Drożdż, R. SDS Electrophoresis on Gradient Polyacrylamide Gels as a Semiquantitative Tool for the Evaluation of Proteinuria. Diagnostics 2023, 13, 1513. https://doi.org/10.3390/diagnostics13091513
Mazur P, Dumnicka P, Tisończyk J, Ząbek-Adamska A, Drożdż R. SDS Electrophoresis on Gradient Polyacrylamide Gels as a Semiquantitative Tool for the Evaluation of Proteinuria. Diagnostics. 2023; 13(9):1513. https://doi.org/10.3390/diagnostics13091513
Chicago/Turabian StyleMazur, Paulina, Paulina Dumnicka, Joanna Tisończyk, Anna Ząbek-Adamska, and Ryszard Drożdż. 2023. "SDS Electrophoresis on Gradient Polyacrylamide Gels as a Semiquantitative Tool for the Evaluation of Proteinuria" Diagnostics 13, no. 9: 1513. https://doi.org/10.3390/diagnostics13091513
APA StyleMazur, P., Dumnicka, P., Tisończyk, J., Ząbek-Adamska, A., & Drożdż, R. (2023). SDS Electrophoresis on Gradient Polyacrylamide Gels as a Semiquantitative Tool for the Evaluation of Proteinuria. Diagnostics, 13(9), 1513. https://doi.org/10.3390/diagnostics13091513







