Ultrasonographic Evaluation of Entheseal Fibrocartilage in Patients with Psoriatic Arthritis, Athletes and Healthy Controls: A Comparison Study
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Age < 50 years-old.
- (2)
- Satisfying CASPAR criteria for PsA [21].
- (3)
- At least 5 years of competitive activity and at least 10 h of weekly training.
- (4)
- Absence of any musculoskeletal complaints in recent years.
2.1. Clinical Assessment of PsA Patients
2.2. Ultrasound Assessment
2.3. Ultrasound Assessment of EF
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lubrano, E.; Scriffignano, S.; Perrotta, F.M. Psoriatic Arthritis, Psoriatic Disease, or Psoriatic Syndrome? J. Rheumatol. 2019, 46, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, I.; Padula, A.; D’Angelo, S.; Cutro, M.S. Psoriatic Arthritis sine Psoriasis. J. Rheumatol. Suppl. 2009, 83, 28–29. [Google Scholar] [CrossRef]
- Mander, M.; Simpson, J.M.; McLellan, A.; Walker, D.; Goodacre, J.A.; Dick, W.C. Studies with an enthesis index as a method of clinical assessment in anky-losing spondylitis. Ann. Rheum. Dis. 1987, 46, 197–202. [Google Scholar] [CrossRef]
- Mandl, P.; Navarro-Compán, V.; Terslev, L.; Aegerter, P.; van der Heijde, D.; D’Agostino, M.A.; Baraliakos, X.; Pedersen, S.J.; Jurik, A.G.; Naredo, E.; et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef]
- Delle Sedie, A.; Riente, L. Psoriatic arthritis: What ultrasound can provide us. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S5), S60–S65. [Google Scholar]
- Felbo, S.K.; Østergaard, M.; Sørensen, I.J.; Terslev, L. Ultrasound of the Heel Improves Diagnosis—Tender Entheses in the Heel Region Rarely Corresponds to Inflammatory Enthesitis in Patients with Peripheral Spondyloarthritis. J. Clin. Med. 2022, 11, 2325. [Google Scholar] [CrossRef]
- Elnady, B.; El Shaarawy, N.K.; Dawoud, N.M.; Elkhouly, T.; Desouky, D.E.-S.; ElShafey, E.N.; El Husseiny, M.S.; Rasker, J.J. Subclinical synovitis and enthesitis in psoriasis patients and controls by ultrasonography in Saudi Arabia; incidence of psoriatic arthritis during two years. Clin. Rheumatol. 2019, 38, 1627–1635. [Google Scholar] [CrossRef]
- Bandinelli, F.; Prignano, F.; Bonciani, D.; Bartoli, F.; Collaku, L.; Candelieri, A.; Lotti, T.; Matucci-Cerinic, M. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Ann. Rheum. Dis. 2013, 31, 219–224. [Google Scholar]
- Perrotta, F.M.; Astorri, D.; Zappia, M.; Reginelli, A.; Brunese, L.; Lubrano, E. An ultrasonographic study of enthesis in early psoriatic arthritis patients naive to traditional and biologic DMARDs treatment. Rheumatol. Int. 2016, 36, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, P.; Salvarani, C.; Possemato, N.; Gutierrez, M.; Grassi, W.; Gasparini, S.; Perricone, C.; Perrotta, F.M.; Grembiale, R.D.; Bruno, C.; et al. Ultrasonographic and Clinical Assessment of Peripheral Enthesitis in Patients with Psoriatic Arthritis, Psoriasis, and Fibromyalgia Syndrome: The ULISSE Study. J. Rheumatol. 2019, 46, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Balint, P.V.; Terslev, L.; Aegerter, P.; Bruyn, G.A.W.; Chary-Valckenaere, I.; Gandjbakhch, F.; Iagnocco, A.; Jousse-Joulin, S.; Möller, I.; Naredo, E.; et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann. Rheum. Dis. 2018, 77, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Huang, Y.-H.; Lin, C.-H.; Kuo, C.-F.; Huang, Y.-J. Ultrasound Can Be Usefully Integrated with the Clinical Assessment of Nail and Enthesis Involvement in Psoriasis and Psoriatic Arthritis. J. Clin. Med. 2022, 11, 6296. [Google Scholar] [CrossRef]
- Iagnocco, A.; Spadaro, A.; Marchesoni, A.; Cauli, A.; De Lucia, O.; Gabba, A.; Takanen, S.; Montepaone, M.; Perrotta, F.M.; D’Agostino, M.A.; et al. Power Doppler ultrasonographic evaluation of enthesitis in psoriatic arthritis. A multi-center study. Jt. Bone Spine 2012, 79, 324–325. [Google Scholar] [CrossRef]
- Spadaro, A.; Iagnocco, A.; Perrotta, F.M.; Modesti, M.; Scarno, A.; Valesini, G. Clinical and ultrasonography assessment of peripheral enthesitis in an-kylosing spondylitis. Rheumatology 2011, 50, 2080–2086. [Google Scholar] [CrossRef]
- McGONAGLE, D.; Aydin, S.Z.; Tan, A.L. The Synovio-entheseal Complex and Its Role in Tendon and Capsular Associated Inflammation. J. Rheumatol. Supppl. 2012, 89, 11–14. [Google Scholar] [CrossRef]
- Benjamin, M.; McGonagle, D. Entheses: Tendon and ligament attachment sites. Scand. J. Med. Sci. Sports 2009, 19, 520–527. [Google Scholar] [CrossRef]
- Aydin, S.Z.; Bas, E.; Basci, O.; Filippucci, E.; Wakefield, R.J.; Çelikel, C.; Karahan, M.; Atagündüz, M.P.; Benjamin, M.; Direskeneli, H.; et al. Validation of ultrasound imaging for Achilles entheseal fibrocartilage in bovines and description of changes in humans with spondyloarthritis. Ann. Rheum. Dis. 2010, 69, 2165–2168. [Google Scholar] [CrossRef]
- De Miguel, E. New perspectives in enthesis ultrasound: Validation of enthesis fibrocartilage. Int. J. Clin. Rheum. 2011, 6, 11. [Google Scholar] [CrossRef]
- Killian, M.L. Growth and mechanobiology of the tendon-bone enthesis. Semin. Cell Dev. Biol. 2022, 123, 64–73. [Google Scholar] [CrossRef] [PubMed]
- He, L.R.; Prado, P.S.D.A. An evaluation of the relationship between the degree of entheseal changes and the severity of osteodegenerative processes at fibrocartilaginous entheses. Anat. Rec. 2021, 304, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; Caspar Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Healy, P.J.; Helliwell, P.S. Measuring clinical enthesitis in psoriatic arthritis: Assessment of existing measures and develop-ment of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008, 59, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, E.; Perrotta, F.M.; Parsons, W.J.; Marchesoni, A. Patient’s Global Assessment as an Outcome Measure for Psoriatic Arthritis in Clinical Practice: A Surrogate for Measuring Low Disease Activity? J. Rheumatol. 2015, 42, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; Fransen, J.; Helliwell, P.S. Defining minimal disease activity in psoriatic arthritis: A proposed objective target for treatment. Ann. Rheum. Dis. 2010, 69, 48–53. [Google Scholar] [CrossRef]
- Schoels, M.; Aletaha, D.; Funovits, J.; Kavanaugh, A.; Baker, D.; Smolen, J.S. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann. Rheum. Dis. 2010, 69, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, E.; De Socio, A.; Perrotta, F.M. Comparison of Composite Indices Tailored for Psoriatic Arthritis Treated with csDMARD and bDMARD: A Cross-sectional Analysis of a Longitudinal Cohort. J. Rheumatol. 2017, 44, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, E.; Scriffignano, S.; Azuaga, A.B.; Ramirez, J.; Cañete, J.D.; Perrotta, F.M. Assessment of the Patient Acceptable Symptom State (PASS) in psoriatic arthritis: Association with disease activity and quality of life indices. RMD Open 2020, 6, e001170. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; de Wit, M.; Kiltz, U.; Braun, J.; Kalyoncu, U.; Scrivo, R.; Maccarone, M.; Carton, L.; Otsa, K.; Sooäär, I.; et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: Elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann. Rheum. Dis. 2014, 73, 1012–1019. [Google Scholar] [CrossRef]
- McGonagle, D.; Lories, R.; Tan, A.L.; Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007, 56, 2482–2491. [Google Scholar] [CrossRef]
- Benjamin, M.; Mcgonagle, D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv. Exp. Med. Biol. 2009, 649, 57–70. [Google Scholar]
- Gracey, E.; Burssens, A.; Cambré, I.; Schett, G.; Lories, R.; McInnes, I.B.; Asahara, H.; Elewaut, D. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.M.; Lories, R.; Lubrano, E. To move or not to move: The paradoxical effect of physical exercise in axial spondyloarthritis. RMD Open 2021, 7, e001480. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Filippucci, E.; Atagündüz, M.P.; Yavuz, S.; Grassi, W.; Direskeneli, H.; Aydın, S.Z. Sonographic measurement of Achilles tendon thickness in seronegative spondyloarthropathies. Eur. J. Rheumatol. 2014, 1, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, G.; Groves, C.; Chandramohan, M.; Beltran, A.; Valle, R.; Reyes, B.; Healy, P.; Harrison, A.; Helliwell, P.S. Clinical and ultrasound examination of the leeds enthesitis index in psoriatic arthritis and rheumatoid arthritis. ISRN Rheumatol. 2011, 2011, 731917. [Google Scholar] [CrossRef]
- Florescu, A.; Vere, C.C.; Florescu, L.M.; Mușetescu, A.E.; Bondari, A.; Ciurea, P.L. Clinical and Ultrasound Assessment of Enthesis in Psoriatic Arthritis in a Romanian Cohort. Curr. Health Sci J. 2018, 44, 347–351. [Google Scholar] [CrossRef]
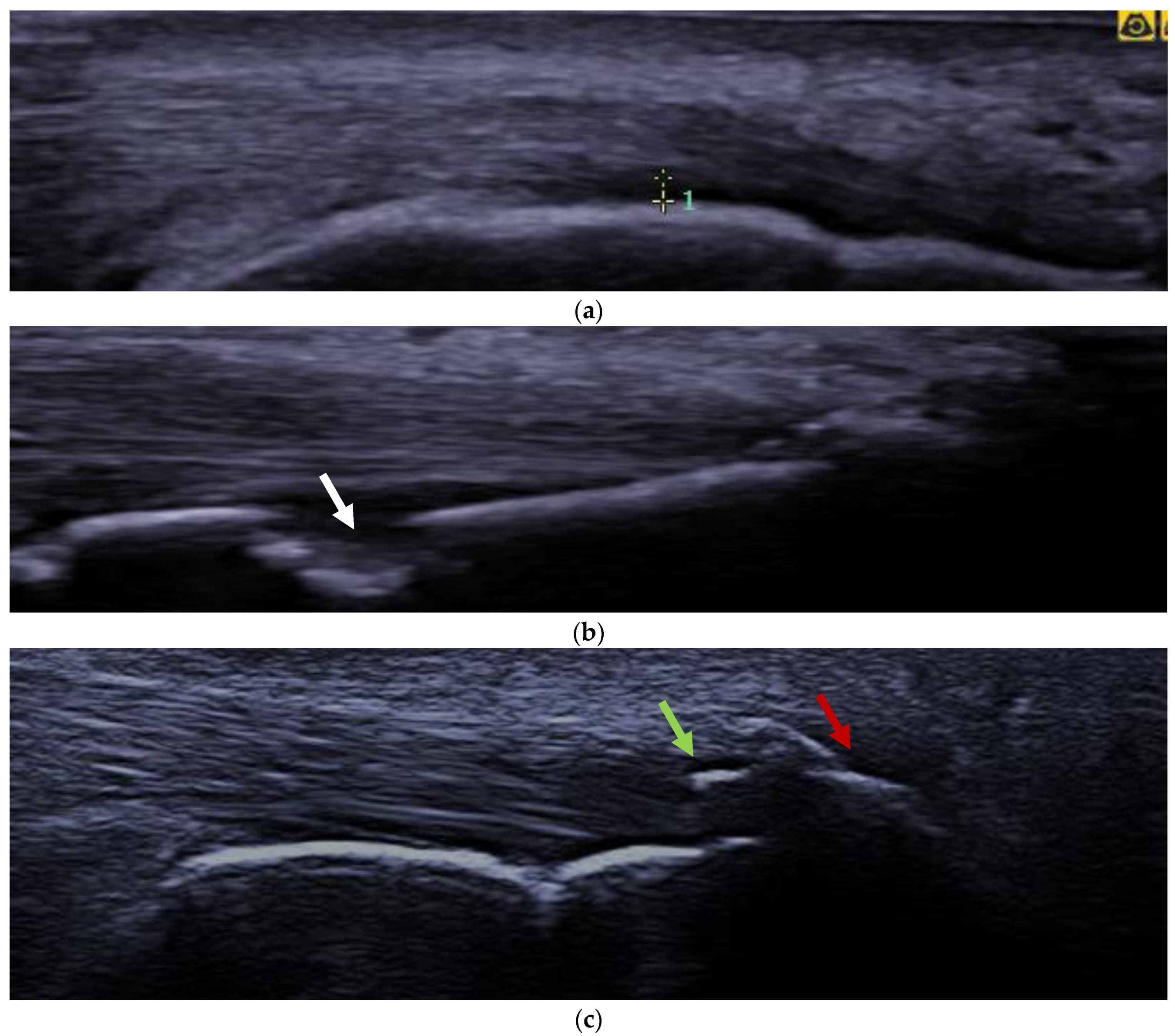
| PsA Patients n. 30 | Athletes n. 40 | Healthy Control n. 20 | |
|---|---|---|---|
| Sex (F), n. (%) | 7 (23.3) | 12 (30) | 10 (50) |
| Age, median (range) | 48 (22–50) | 24 (20–41) | 25.3 (21–40) |
| Weight, Kg, median (IQR) | 80 (72–88) | 70 (65–74) | 63.5 (58–70) |
| Height, m, median (IQR) | 1.74 (1.72–1.76) | 1.75 (1.69–1.80) | 1.67 (1.63–1.75) |
| BMI, median (IQR) | 26.12 (23.70–29.22) | 23.10 (22.18–24.55) | 21.71 (19.59–23.26) |
| Smoke (yes), n. (%) | 4 (14.3) | 7 (18.4) | 7 (31.8) |
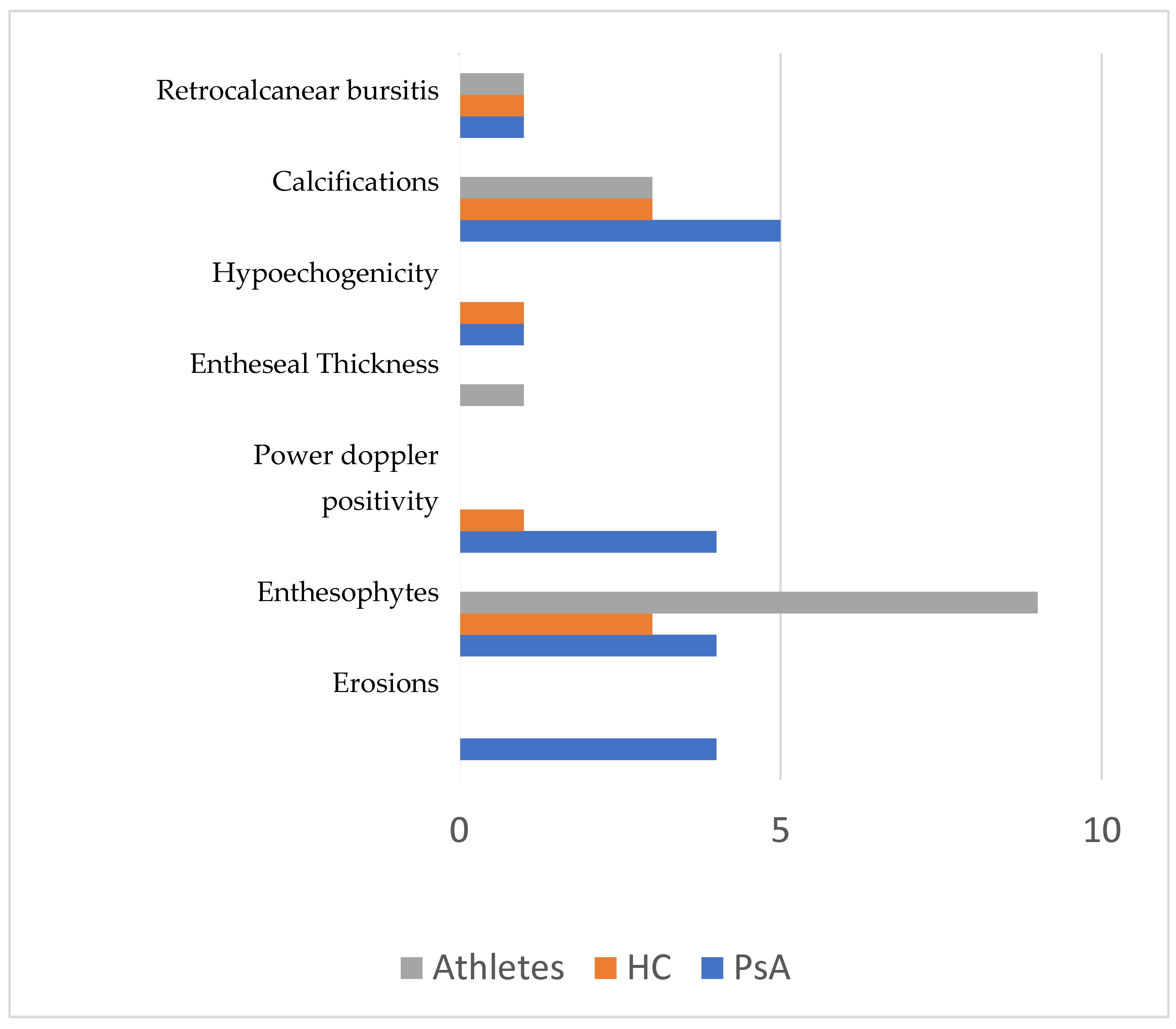
| Enthesopathy (yes), n. (%) | 6/26 (23.1) | 13 (34.2) | 6 (27.3) |
| Entheseal calcifications (yes), n. (%) | 8/28 (28.6) | 11 (28.9) | 7 (31.8) |
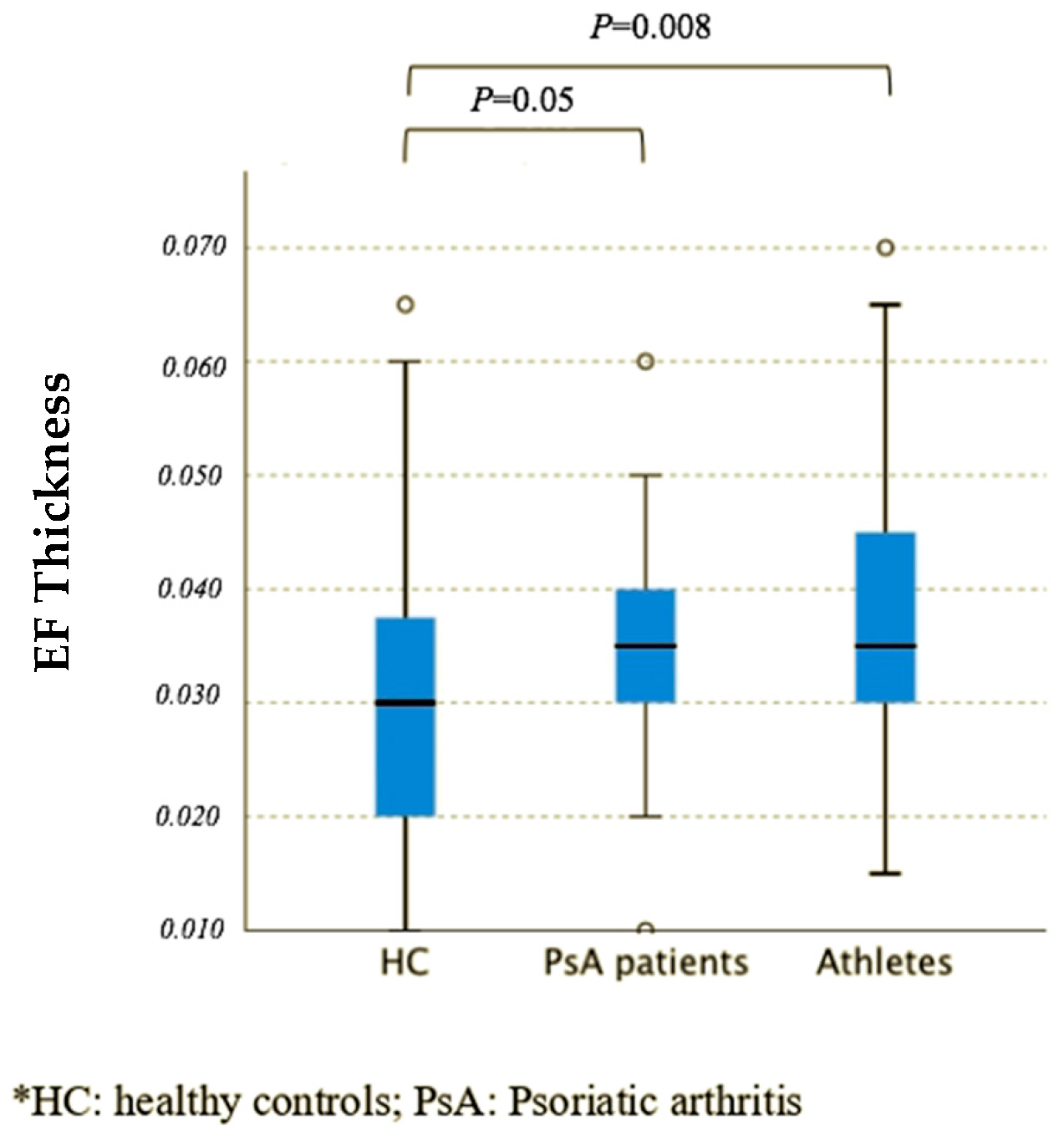
| EF thickness (right), cm, median (IQR) | 0.0350 (0.0300–0.0400) | 0.0350 (0.0300–0.0413) | 0.0300 (0.0200–0.0400) |
| EF thickness (left), cm, median (IQR) | 0.0350 (0.0263–0.0400) | 0.0375 (0.0200–0.0450) | 0.0300 (0.0200–0.0363) |
| DAPSA, median (IQR) | 5.75 (4.00–14.26) | - | - |
| LEI, median (IQR) | 0 (0–0) | - | - |
| Disease duration (months), median (IQR) | 48 (12.5–79) | - | - |
| BSA, median, (IQR) | 0 (0–1) | - | - |
| Hours of training (per week), median (IQR) | - | 12 (8–15) |
| EC Thickness (Right) | Single Measures | p Value | Average Measures | p Value |
|---|---|---|---|---|
| Intra-reader | 0.910 (0.880–0.950) | <0.001 | 0.950 (0.890–0.970) | <0.001 |
| Inter-reader | 0.802 (0.713–0.866) | <0.001 | 0.890 (0.833–0.928) | <0.001 |
| EC thickness (left) | ||||
| Intra-reader | 0.910 (0.880–0.950) | <0.001 | 0.920 (0.890–0.960) | <0.001 |
| Inter-reader | 0.787 (0.693–0.855) | <0.001 | 0.881 (0.819–0.922) | <0.001 |
| EF Thickness (Right) | ||
|---|---|---|
| rho | p value | |
| All (PsA + HCs + Athletes) | ||
| Age | −0.144 | 0.177 |
| Weight | 0.193 | 0.072 |
| BMI | 0.117 | 0.277 |
| PsA patients | ||
| DAPSA | 0.194 | 0.364 |
| Disease duration (months) | −0.171 | 0.375 |
| PsAID | 0.200 | 0.240 |
| LEI | 0.020 | 0.780 |
| Athletes | ||
| Hours of training per week | 0.210 | 0.120 |
| EF thickness (left) | ||
| rho | p value | |
| All (PsA + HCs + Athletes) | ||
| Age | −0.091 | 0.398 |
| Weight | 0.156 | 0.148 |
| BMI | 0.149 | 0.165 |
| PsA patients | ||
| DAPSA | 0.036 | 0.868 |
| Disease duration (months) | −0.094 | 0.635 |
| PsAID | 0.210 | 0.300 |
| LEI | 0.140 | 0.460 |
| Athletes | ||
| Hours of training per week | 0.150 | 0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrotta, F.M.; Ronga, M.; Scriffignano, S.; Lubrano, E. Ultrasonographic Evaluation of Entheseal Fibrocartilage in Patients with Psoriatic Arthritis, Athletes and Healthy Controls: A Comparison Study. Diagnostics 2023, 13, 1446. https://doi.org/10.3390/diagnostics13081446
Perrotta FM, Ronga M, Scriffignano S, Lubrano E. Ultrasonographic Evaluation of Entheseal Fibrocartilage in Patients with Psoriatic Arthritis, Athletes and Healthy Controls: A Comparison Study. Diagnostics. 2023; 13(8):1446. https://doi.org/10.3390/diagnostics13081446
Chicago/Turabian StylePerrotta, Fabio Massimo, Mario Ronga, Silvia Scriffignano, and Ennio Lubrano. 2023. "Ultrasonographic Evaluation of Entheseal Fibrocartilage in Patients with Psoriatic Arthritis, Athletes and Healthy Controls: A Comparison Study" Diagnostics 13, no. 8: 1446. https://doi.org/10.3390/diagnostics13081446
APA StylePerrotta, F. M., Ronga, M., Scriffignano, S., & Lubrano, E. (2023). Ultrasonographic Evaluation of Entheseal Fibrocartilage in Patients with Psoriatic Arthritis, Athletes and Healthy Controls: A Comparison Study. Diagnostics, 13(8), 1446. https://doi.org/10.3390/diagnostics13081446







