Natural Language Processing for Breast Imaging: A Systematic Review
Abstract
1. Introduction
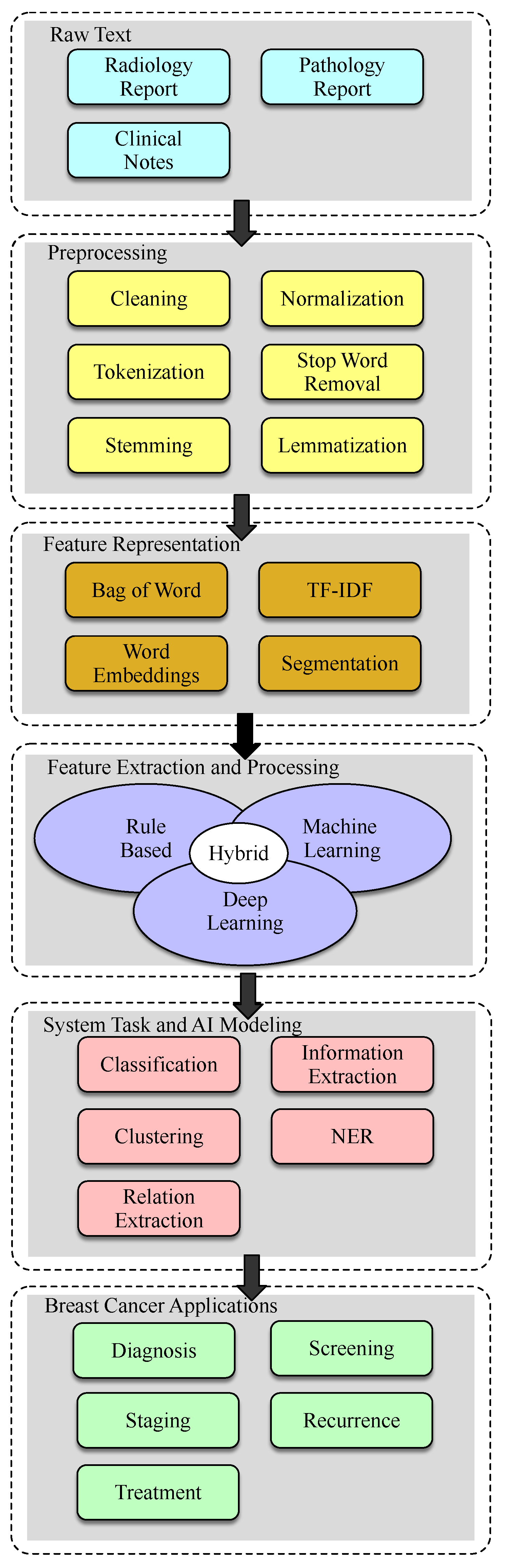
2. Materials and Methods
3. Background
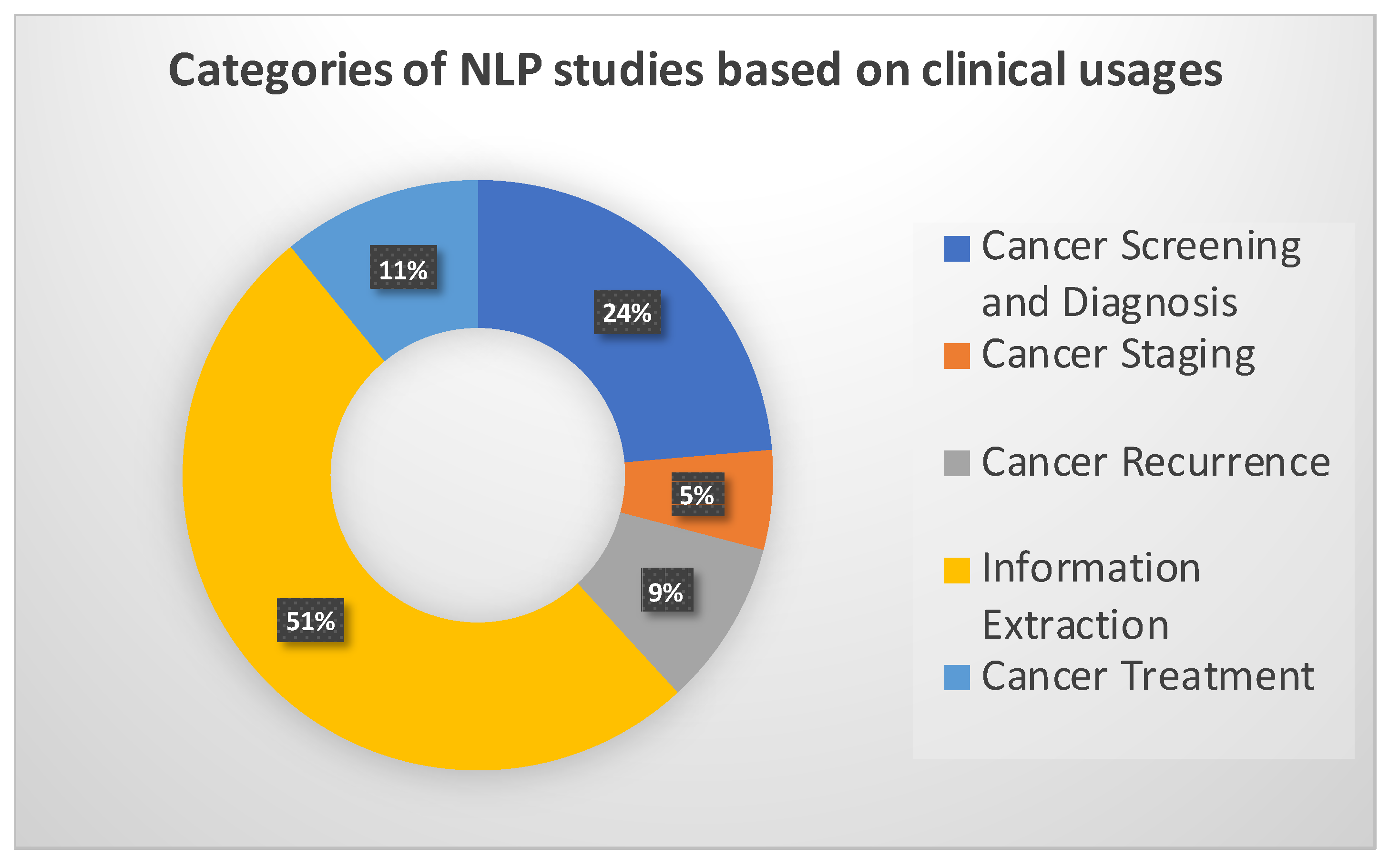
4. Clinical Applications and NLP Methods in Breast Imaging
4.1. Breast Cancer Screening and Diagnoses
4.2. Breast Cancer Staging
4.3. Breast Cancer Recurrence
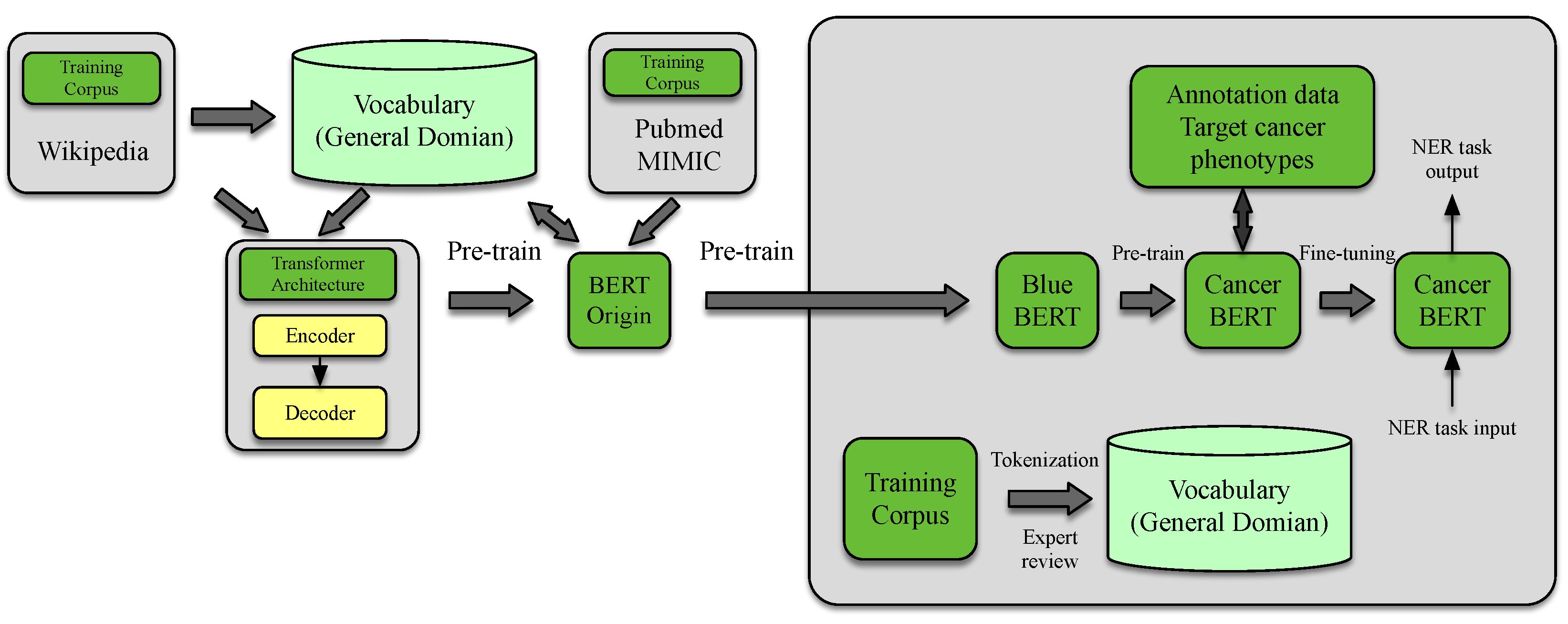
4.4. Information Extraction
4.5. Breast Cancer Treatment
5. Discussion
5.1. NLP Applications in Breast Imaging
5.2. Open Research and Clinician Interests
5.2.1. Clinical Decision Support
5.2.2. Computer Assist Coding
5.2.3. Computer Assist Reporting
5.2.4. Improved Classification of High Risk Breast Lesions
5.2.5. Lesion Detection and Classification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pons, E.; Braun, L.M.; Hunink, M.M.; Kors, J.A. Natural language processing in radiology: A systematic review. Radiology 2016, 279, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Johanna Johnsi Rani, G.; Gladis, D.; Manipadam, M.T.; Ishitha, G. Breast cancer staging using Natural Language Processing. In Proceedings of the 2015 International Conference on Advances in Computing, Communications and Informatics (ICACCI), Kochi, India, 10–13 August 2015; pp. 1552–1558. [Google Scholar] [CrossRef]
- Shen, Y.; Heacock, L.; Elias, J.; Hentel, K.D.; Reig, B.; Shih, G.; Moy, L. ChatGPT and Other Large Language Models Are Double-edged Swords. Radiology 2013, 267, 230163. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.; Davidson, E.; Poon, M.; Dong, H.; Duma, D.; Grivas, A.; Grover, C.; Suárez-Paniagua, V.; Tobin, R.; Whiteley, W.; et al. A systematic review of natural language processing applied to radiology reports. BMC Med. Inform. Decis. Mak. 2021, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [CrossRef] [PubMed]
- Abedian, S.; Sholle, E.T.; Adekkanattu, P.M.; Cusick, M.M.; Weiner, S.E.; Shoag, J.E.; Hu, J.C.; Campion, T.R.J. Automated Extraction of Tumor Staging and Diagnosis Information from Surgical. JCO Clin. Cancer Inform. 2021, 1054–1061. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Phalnikar, R. Information extraction for prognostic stage prediction from breast cancer medical records using NLP and ML. Med. Biol. Eng. Comput. 2021, 59, 1751–1772. [Google Scholar] [CrossRef]
- Carrell, D.; Halgrim, S.; Tran, d.T.; Buist, D.S.M.; Chubak, J.; Chapman, W.W.; Savova, G. Weakly supervised temporal model for prediction of breast cancer reccurence. Sci. Rep. 2021, 11, 9461. [Google Scholar] [CrossRef]
- Banerjee, I.; Bozkurt, S.; Caswell-Jin, J.L.; Kurian, A.W.; Rubin, D.L. Natural Language Processing Approaches to Detect the Timeline of Metastatic Recurrence of Breast Cancer. JCO Clin. Cancer Inform. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Kaka, H.; Michalopoulos, G.; Subendran, S.; Decker, K.; Lambert, P.; Pitz, M.; Singh, H.; Chen, H. pre-trained Neural Networks Accurately Identify Cancer Recurrence in Medical. Stud. Health Technol. Inform. 2022, 294, 93–97. [Google Scholar] [CrossRef]
- Zeng, Z.; Espino, S.; Roy, A.; Li, X.; Khan, S.A.; Clare, S.E.; Jiang, X.; Neapolitan, R.; Yuan, L. Identifying Breast Cancer Distant Recurrences from Electronic Health Records. J. Healthc. Inform. Res. 2019, 3, 283–299. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Han, C.; Zhang, X.; Wang, X. The implementation of natural language processing to extract index lesions from breast magnetic resonance imaging reports. BMC Med. Inform. Decis. Mak. 2019, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami Bejnordi, B.; Veta, M.; Johannes van Diest, P.; van Ginneken, B.; Karssemeijer, N.; Litjens, G.; van der Laak, J.A.W.M.; The CAMELYON16 Consortium. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA 2017, 318, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Hammami, L.; Paglialonga, A.; Pruneri, G.; Torresani, M.; Sant, M.; Bono, C.; Caiani, E.G.; Baili, P. Automated classification of cancer morphology from Italian pathology reports using Natural Language Processing techniques: A rule-based approach. J. Biomed. Inform. 2021, 116, 103712. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Ouyang, L.; Li, C.; He, Y.; Griffin, M.; Taghian, A.; Smith, B.; Yala, A.; Barzilay, R.; Hughes, K. Machine learning to parse breast pathology reports in Chinese. Breast Cancer Res. Treat. 2018, 169, 243–250. [Google Scholar] [CrossRef]
- Wieneke, A.E.; Bowles, E.J.; Cronkite, D.; Wernli, K.J.; Gao, H.; Carrell, D.; Buist, D.S. Validation of natural language processing to extract breast cancer pathology procedures and results. J. Pathol. Inform. 2015, 6, 38. [Google Scholar] [CrossRef]
- Magge, A.; Scotch, M.; Gonzalez-Hernandez, G. Clinical NER and Relation Extraction using Bi-Char-LSTMs and Random Forest Classifiers. In Proceedings of the PMLR 1st International Workshop on Medication and Adverse Drug Event Detection, Auckland, New Zealand, 4 May 2018; Liu, F., Jagannatha, A., Yu, H., Eds.; IEEE: Piscataway, NJ, USA, 2018; Volume 90, pp. 25–30. [Google Scholar]
- Kuling, G.; Curpen, B.; Martel, A.L. BI-RADS BERT and using section segmentation to understand radiology reports. J. Imaging 2022, 8, 131. [Google Scholar] [CrossRef]
- Zeng, Z.; Espino, S.; Roy, A.; Li, X.; Khan, S.A.; Clare, S.E.; Jiang, X.; Neapolitan, R.; Yuan, L. Using natural language processing and machine learning to identify breast cancer local recurrence. BMC Bioinform. 2018, 19, 65–74. [Google Scholar] [CrossRef]
- Ribelles, N.; Jerez, J.M.; Rodriguez-Brazzarola, P.; Jimenez, B.; Diaz-Redondo, T.; Mesa, H.; Marquez, A.; Sanchez-Muñoz, A.; Pajares, B.; Carabantes, F.; et al. Machine learning and natural language processing (NLP) approach to predict early progression to first-line treatment in real-world hormone receptor-positive (HR+)/HER2-negative advanced breast cancer patients. Eur. J. Cancer 2021, 144, 224–231. [Google Scholar] [CrossRef]
- Alkaitis, M.S.; Agrawal, M.N.; Riely, G.J.; Razavi, P.; Sontag, D. Automated NLP Extraction of Clinical Rationale for Treatment Discontinuation in Breast Cancer. JCO Clin. Cancer Inform. 2021, 5, 550–560. [Google Scholar] [CrossRef]
- Diamond, C.J.; Laurentiev, J.; Jie, Y.; Wint, A.; Harris, K.A.; Dang, T.H.; Mecker, A.; Carpenter, E.B.; Tosteson, A.N.; Wright, A.; et al. Natural Language Processing to Identify Abnormal Breast, Lung, and Cervical Cancer Screening Test Results from Unstructured Reports to Support Timely Follow-up. In MEDINFO 2021: One World, One Health—Global Partnership for Digital Innovation: Proceedings of the 18th World Congress on Medical and Health Informatics, Virtual Event, 2–4 October 2021; IOS Press: Amsterdam, The Netherland, 2022; pp. 433–437. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Phalnikar, R. Prognostic elements extraction from documents to detect prognostic stage. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 371–386. [Google Scholar] [CrossRef]
- Carrel, D.S.; Halgrim, S.; Tran, D.T.; Buist, D.S.M.; Chubak, J.; Chapman, W.W.; Savova, G. Using natural language processing to improve efficiency of manual chart abstraction in research: The case of breast cancer recurrence. Am. J. Epidemiol. 2014, 179, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Grann, V.R.; Friedman, C. Facilitating cancer research using natural language processing of pathology reports. Stud. Health Technol. Inform. 2004, 107, 565–572. [Google Scholar]
- Cai, T.; Giannopoulos, A.A.; Yu, S.; Kelil, T.; Ripley, B.; Kumamaru, K.K.; Rybicki, F.J.; Mitsouras, D. Natural Language Processing Technologies in Radiology Research and Clinical Applications. RadioGraphics 2016, 36, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Joulin, A.; Grave, E.; Bojanowski, P.; Mikolov, T. Bag of tricks for efficient text classification. arXiv 2016, arXiv:1607.01759. [Google Scholar]
- Goldberg, Y. A primer on neural network models for natural language processing. J. Artif. Intell. Res. 2016, 57, 345–420. [Google Scholar] [CrossRef]
- Otter, D.W.; Medina, J.R.; Kalita, J.K. A Survey of the Usages of Deep Learning for Natural Language Processing. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 604–624. [Google Scholar] [CrossRef]
- Jurafsky, D.; Martin, J.H. Speech and Language Processing, 3rd ed.; Pearson: London, UK, 2019. [Google Scholar]
- Xia, F.; Wang, S.; Sun, H.; Zhang, W.; Liu, Q. A machine learning approach to extract clinical entities and their assertions from radiology reports. BMC Med. Inform. Decis. Mak. 2011, 19, 601–606. [Google Scholar]
- Shin, B.; Chokshi, F.H.; Lee, T.; Choi, J.D. Classification of radiology reports using neural attention models. In Proceedings of the International Joint Conference on Neural Networks (IJCNN), Anchorage, AK, USA, 14–19 May 2017; pp. 4363–4370. [Google Scholar] [CrossRef]
- Doing-Harris, K.; Livnat, Y.; Meystre, S. Automated concept and relationship extraction for the semi-automated ontology management (SEAM) system. J. Biomed. Semant. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Popov, B.; Kiryakov, A.; Kirilov, A.; Manov, D.; Ognyanoff, D.; Goranov, M. KIM–semantic annotation platform. In Proceedings of the Second International Semantic Web Conference, Sanibel Island, FL, USA, 20–23 October 2003. [Google Scholar]
- Collobert, R.; Weston, J.; Bottou, L.; Karlen, M.; Kavukcuoglu, K.; Kuksa, P. Natural language processing (almost) from scratch. J. Mach. Learn. Res. 2011, 12, 2493–2537. [Google Scholar]
- Jurafsky, D.; Martin, J.H. Speech and Language Processing: An Introduction to Natural Language Processing, Computational Linguistics, and Speech Recognition, 1st ed.; Prentice Hall PTR: New York, NY, USA, 2000. [Google Scholar]
- Mikolov, T.; Chen, K.; Corrado, G.; Dean, J. Efficient estimation of word representations in vector space. arXiv 2013, arXiv:1301.3781. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. In Proceedings of the Advances in Neural Information Processing Systems 30 (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Mozayan, A.; Fabbri, A.R.; Maneevese, M.; Tocino, I.; Chheang, S. Practical Guide to Natural Language Processing for Radiology. RadioGraphics 2021, 41, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Savova, G.; Masanz, J.; Ogren, P.; Zheng, J.; Sohn, S.; Kipper-Schuler, K.; Chute, C. Mayo Clinic Clinical Text Analysis and Knowledge Extraction System (cTAKES): Architecture, component evaluation and applications. JAMIA 2010, 17, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Lacson, R.; Harris, K.; Brawarsky, P.; Tosteson, T.D.; Onega, T.; Tosteson, A.N.; Kaye, A.; Gonzalez, I.; Birdwell, R.; Haas, J.S. Evaluation of an automated information extraction tool for imaging data elements to populate a breast cancer screening registry. J. Digit. Imaging 2015, 28, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Lacson, R.; Wang, A.; Cochon, L.; Giess, C.; Desai, S.; Eappen, S.; Khorasani, R. Factors Associated With Optimal Follow-up in Women With BI-RADS 3 Breast Findings. J. Am. Coll. Radiol. 2020, 17, 3. [Google Scholar] [CrossRef]
- Short, R.G.; Bralich, J.; Bogaty, D.; Befera, N.T. Comprehensive Word-Level Classification of Screening Mammography Reports Using a Neural Network Sequence Labeling Approach. J. Digit. Imaging 2019, 32, 141. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, N.; Wang, L.; Liu, H.; Zhang, R. CancerBERT: A cancer domain-specific language model for extracting breast cancer phenotypes from electronic health records. J. Am. Med. Inform. Assoc. 2022, 29, 1208–1216. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, Q.; Ren, Y.; Qiu, T.; Ma, J.; Sun, Q. Extracting comprehensive clinical information for breast cancer using deep learning methods. Int. J. Med. Inform. 2019, 132, 103985. [Google Scholar] [CrossRef]
- Datta, S.; Bernstam, E.V.; Roberts, K. A frame semantic overview of NLP-based information extraction for cancer-related EHR notes. J. Biomed. Inform. 2019, 100, 103301. [Google Scholar] [CrossRef]
- Levine, M.N.; Alexander, G.; Sathiyapalan, A.; Agrawal, A.; Pond, G. Learning Health System for Breast Cancer: Pilot Project Experience. JCO Clin. Cancer Inform. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, L.; Zou, V.Z.; Hollander, Z.; Ng, R.T.; Isaac, K.V. Automated medical chart review for breast cancer outcomes research: A novel. BMC Med. Res. Methodol. 2022, 22, 136. [Google Scholar] [CrossRef]
- Hughes, K.S.; Zhou, J.; Bao, Y.; Singh, P.; Wang, J.; Yin, K. Natural language processing to facilitate breast cancer research and management. Breast J. 2020, 26, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Vey, B.L.; Bhimireddy, A.; Kim, T.; Santos, T.; Correa, R.; Dutt, R.; Mosunjac, M.; Oprea-Ilies, G.; Smith, G.; et al. The EMory BrEast imaging Dataset (EMBED): A Racially Diverse, Granular Dataset of 3.4 Million Screening and Diagnostic Mammographic Images. Radiol. Artif. Intell. 2023, 5, e220047. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yan, K.; Sandfort, V.; Summers, R.M.; Lu, Z. A self-attention based deep learning method for lesion attribute detection from CT reports. In Proceedings of the 2019 IEEE International Conference on Healthcare Informatics (ICHI), Xi’an, China, 10–13 June 2019; IEEE Computer Society: Los Alamitos, CA, USA, 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Banerjee, I.; Chen, M.C.; Lungren, M.P.; Rubin, D.L. Radiology report annotation using intelligent word embeddings: Applied to multi-institutional chest CT cohort. J. Biomed. Inform. 2018, 77, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Percha, B.; Zhang, Y.; Bozkurt, S.; Rubin, D.; Altman, R.B.; Langlotz, C.P. Expanding a radiology lexicon using contextual patterns in radiology reports. J. Am. Med. Inform. Assoc. 2018, 25, 679–685. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, D.; Van Genderen, M.E.; Smit, J.M.; Huiskens, J.; Visser, J.J.; Veen, R.E.R.; van Unen, E.; BA, O.H.; Gommers, D.; Bommel, J.v. Developing, implementing and governing artificial intelligence in medicine: A step-by-step approach to prevent an artificial intelligence winter. BMJ Health Care Inform. 2022, 29, 100495. [Google Scholar] [CrossRef]
- Smit, A.; Jain, S.; Rajpurkar, P.; Pareek, A.; Ng, A.; Lungren, M. Combining Automatic Labelers and Expert Annotations for Accurate Radiology Report Labeling Using BERT. In Proceedings of the 2020 Conference on Empirical Methods in Natural Language Processing (EMNLP), Online, 1 June 2020; pp. 1500–1519. [Google Scholar] [CrossRef]
- Grivas, A.; Alex, B.; Grover, C.; Tobin, R.; Whiteley, W. Not a cute stroke: Analysis of Rule- and Neural Network-based Information Extraction Systems for Brain Radiology Reports. In Proceedings of the 11th International Workshop on Health Text Mining and Information Analysis, Online, 20 November 2020; pp. 24–37. [Google Scholar] [CrossRef]
- Ettinger, A. What BERT Is Not: Lessons from a New Suite of Psycholinguistic Diagnostics for Language Models. Trans. Assoc. Comput. Linguist. 2020, 8, 34–48. [Google Scholar] [CrossRef]
- Medical Imaging Use Cases. Available online: https://www.acrdsi.org/DSI-Services/Define-AI (accessed on 21 February 2023).
- Yen, A.; Pfeffer, Y.; Blumenfeld, A.; Balcombe, J.N.; Berland, L.L.; Tanenbaum, L.; Kligerman, S.J. Use of a dual artificial intelligence platform to detect unreported lung nodules. J. Comput. Assist. Tomogr. 2021, 45, 318–322. [Google Scholar] [CrossRef]

| Metric | Description | Formula |
|---|---|---|
| Accuracy | Correctness on average | (TP + TN)/(TP + FP + TN + FN) |
| PPV | Positive Predictive Value, Precision | TP/(TP + FP) |
| Sensitivity | true positive rate, Recall | TP/(TP + FN) |
| Specificity | true negative rate | TN/(TN + FP) |
| F score | F1, harmonic mean of precision and recall | 2(Prec. × Recall)/(Prec. + Recall) |
| Task, [Papers] | Methods | Summary | Results |
|---|---|---|---|
| Staging [2,6,7] | Information extracted from pathology reports with machine learning and rule-based systems. | Pathology reports are processed with NLP to extract parameters for breast cancer staging, namely tumors, lymph nodes, and metastases. | Results have been promising with multiple NLP models achieving over 90% accuracy in identifying breast cancer staging. |
| Breast cancer recurrence [8,9,10,11] | NLP with BERT model, data from OncoShare Database | NLP has been used to detect patient-specific timing of metastatic recurrence, and calculate the probability and identify both distant and local recurrences. | Best NLP models were able to identify over 90% percent of recurrences and estimate diagnoses dates for most patients within 30 days. |
| Screening and Diagnoses [12,13,14,15,16] | Studies examined free-formed text reports and extracted features according to BI-RADS. | NLP has been used to identify index lesions in breast cancer patients and also extract information on them. Manual review was conducted to ensure the accuracy of NLP models. | Identification of index lesion has shown to be extremely accurate, almost 100% |
| Information Extraction [17,18,19] | Text classification, named entity recognition, sentiment analysis, and concept extraction. | Information extraction with NLP has been used for prognostic stage detection. Able to identify patterns and insights in a short amount of time, performance improves significantly with manual assistance. | NLP systems have been able to accurately extract information with over 90% sensitivity and precision. |
| Treatment [20,21,22] | Trained and tested on electronic health records of real-world breast-cancer patients. | NLP to develop early predictive models for patient response. | Best predictive models with NLP achieved area under the curve (AUC) of 0.758. |
| Task | Pro’s | Con’s |
|---|---|---|
| Cancer Screening and Diagnoses | Can standardize and streamline the diagnosis process, reducing human error and improving consistency in interpretation of data. Very accurate in diagnosis and identification of index lesions. | Needs large amounts of labeled data to train the model. Still has the potential for error and can therefore lead to false positives and negatives. Potential to increase the workload for medical professionals who will need to verify the results of the NLP model. |
| Cancer Staging | NLP Can take into account all of the available data when dealing with breast cancer staging to improve accuracy. | NLP may be unable to fully replicate a clinical examination. |
| Recurrence | NLP Has proven to be very accurate in predicting breast cancer recurrence. | Key differences from patient to patient can pose challenges for NLP models to adapt to. |
| Information Extraction | Can save a lot of time by quickly analyzing and interpreting large amounts of clinical data. | Difficulties dealing with different medical terminologies. |
| Treatment | Can improve the quality and completeness of patient information that is available to physicians and researchers. Can be used to identify patterns and insights from large amounts of data that would have gone unnoticed. Can also be used to monitor online platforms for early detection. | Difficulties in interpreting nuances and context of human language can lead to mistakes and inaccuracies with treatment. Privacy and security concerns. |
| Task | Methods | Accuracy | AUC | Recall | Precision | Sample Size |
|---|---|---|---|---|---|---|
| Staging | Extracting parameters from pathology reports [2] | 72 | - | 82 | 73 | 150 |
| Prognostic stage detection in rural/urban regions [23] | - | 93/83 | - | - | 465 | |
| Screening and Diagnosis [12] | Identification of Index Lesions | - | - | 100 | 99.6 | 478 |
| Identification of BI-RADS Categories | - | - | 96.6 | 94.8 | 478 | |
| Extracting Imaging Features | - | - | 91 | 92.6 | 478 | |
| Recurrence | Identifying recurrences [24] | 92 | - | - | - | 1472 |
| Identifying patients who experienced recurrence (BERT-base) [10] | - | 0.9883 | - | - | 112,285 | |
| Predicting timing of metastatic recurrence [9] | - | - | - | - | 894 | |
| Information Extraction | IE to Determine Recruit Eligibility for Studies [25] | - | - | - | 91.6 | - |
| Clinical NER/RE using Bi-char-LSTMs and random forest classifiers [17] | - | - | 0.82/0.94 | 0.80/0.82 | 800 | |
| BI-RADS BERT perform section segmentation and extract information (density, previous cancer) [18] | 95.9 | - | - | - | 155,000 | |
| Treatment | Identifying toxicity events in early stage patients [21] | - | 0.857 | - | - | 6115 |
| NLP free-text to predict early/long progression to first-line treatment [20] | - | 0.758/0.752 | - | - | 610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, K.M.; Deng, J.; Wu, Y.; Yesha, Y.; Collado-Mesa, F.; Nguyen, P. Natural Language Processing for Breast Imaging: A Systematic Review. Diagnostics 2023, 13, 1420. https://doi.org/10.3390/diagnostics13081420
Diab KM, Deng J, Wu Y, Yesha Y, Collado-Mesa F, Nguyen P. Natural Language Processing for Breast Imaging: A Systematic Review. Diagnostics. 2023; 13(8):1420. https://doi.org/10.3390/diagnostics13081420
Chicago/Turabian StyleDiab, Kareem Mahmoud, Jamie Deng, Yusen Wu, Yelena Yesha, Fernando Collado-Mesa, and Phuong Nguyen. 2023. "Natural Language Processing for Breast Imaging: A Systematic Review" Diagnostics 13, no. 8: 1420. https://doi.org/10.3390/diagnostics13081420
APA StyleDiab, K. M., Deng, J., Wu, Y., Yesha, Y., Collado-Mesa, F., & Nguyen, P. (2023). Natural Language Processing for Breast Imaging: A Systematic Review. Diagnostics, 13(8), 1420. https://doi.org/10.3390/diagnostics13081420





