A Deep Learning Approach for Diagnosis Support in Breast Cancer Microwave Tomography
Abstract
1. Introduction
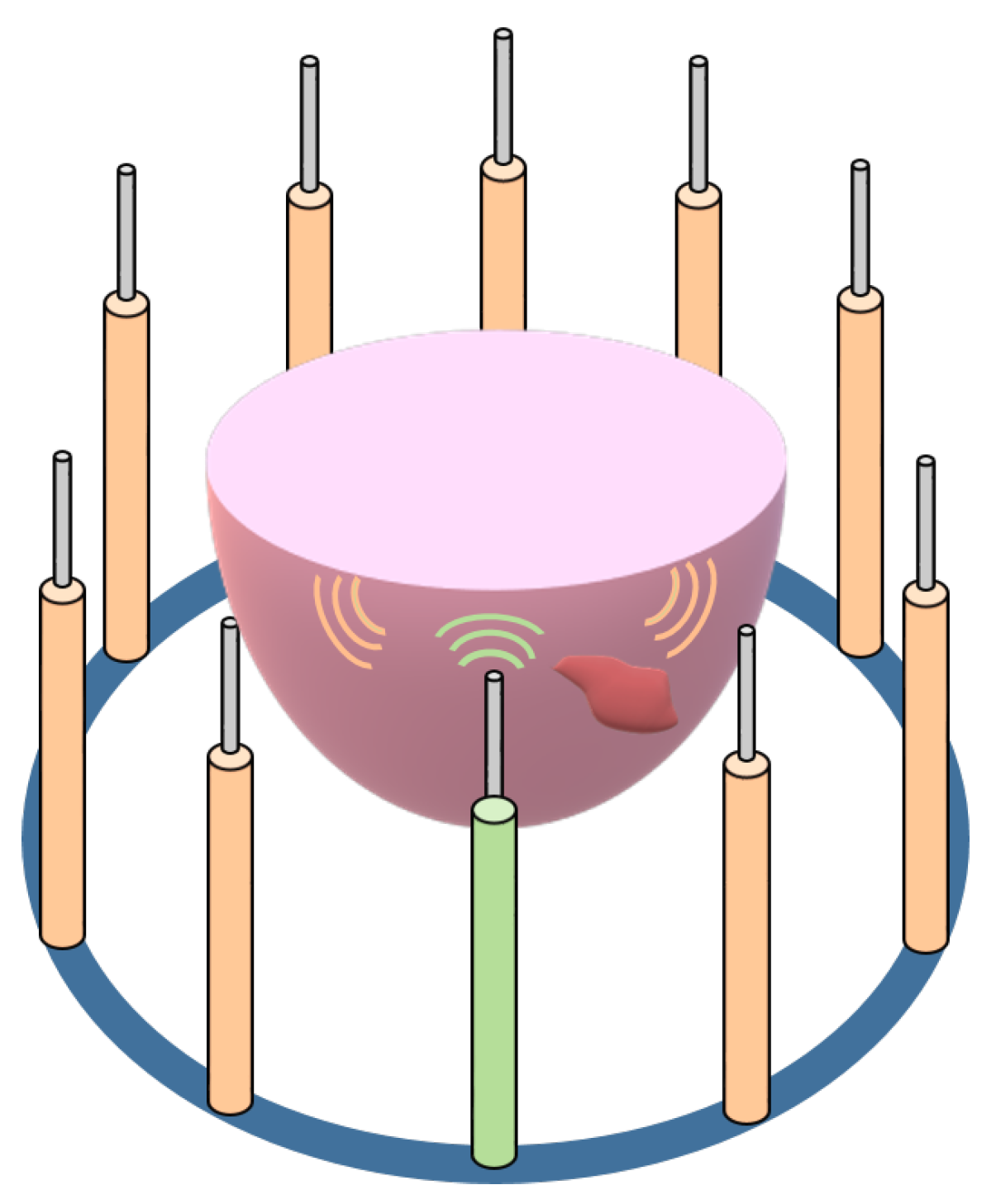
2. Methodology
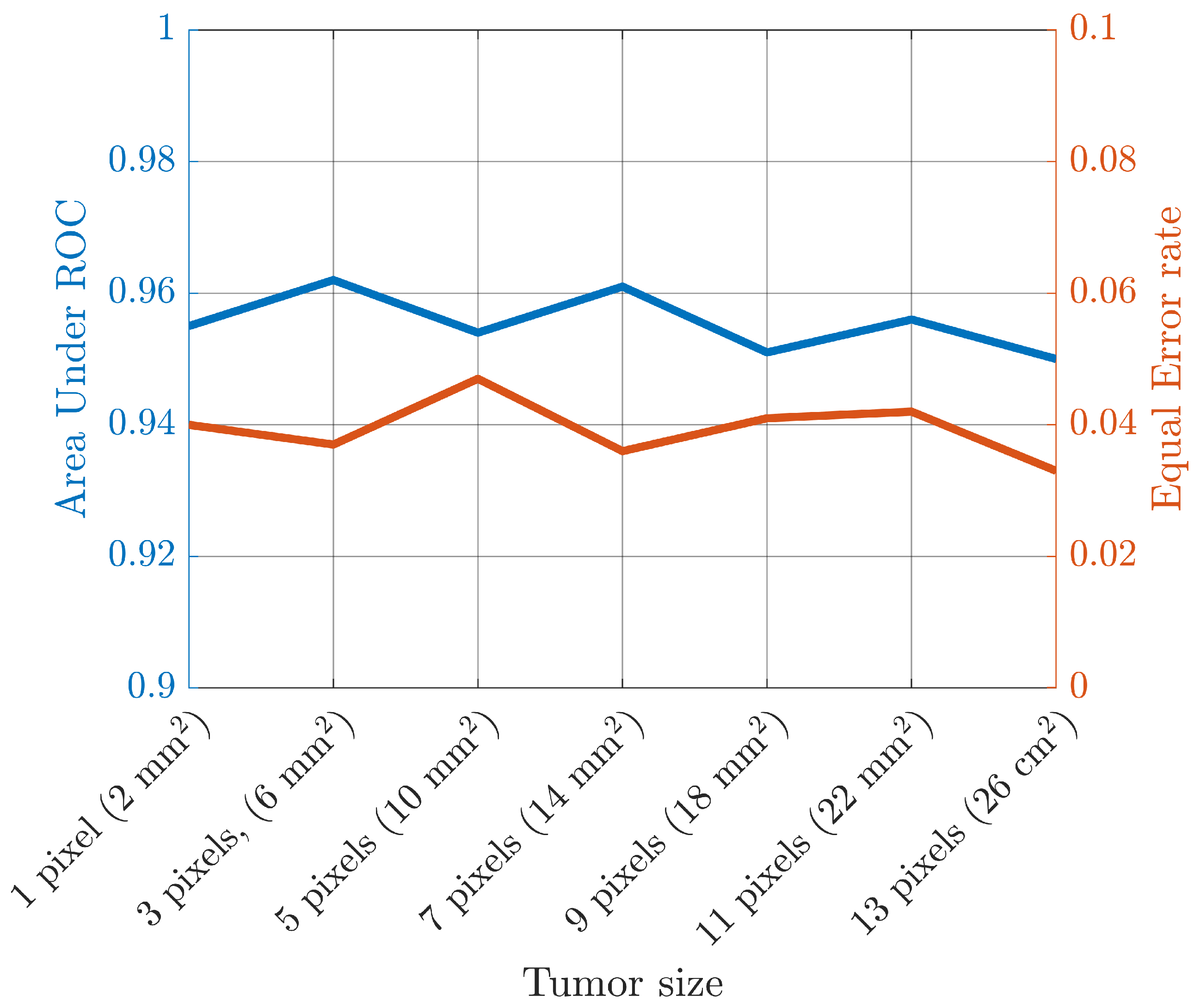
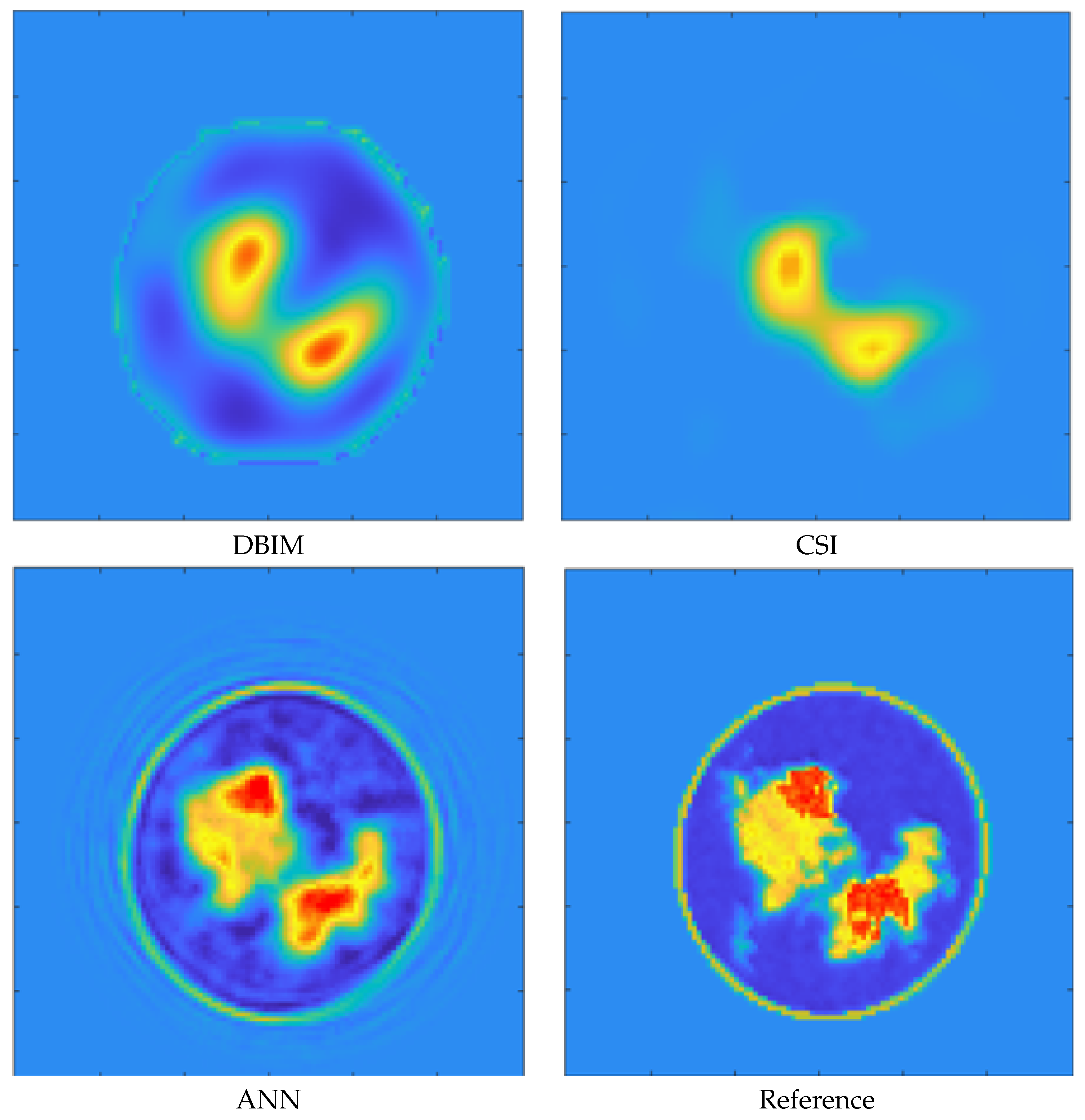
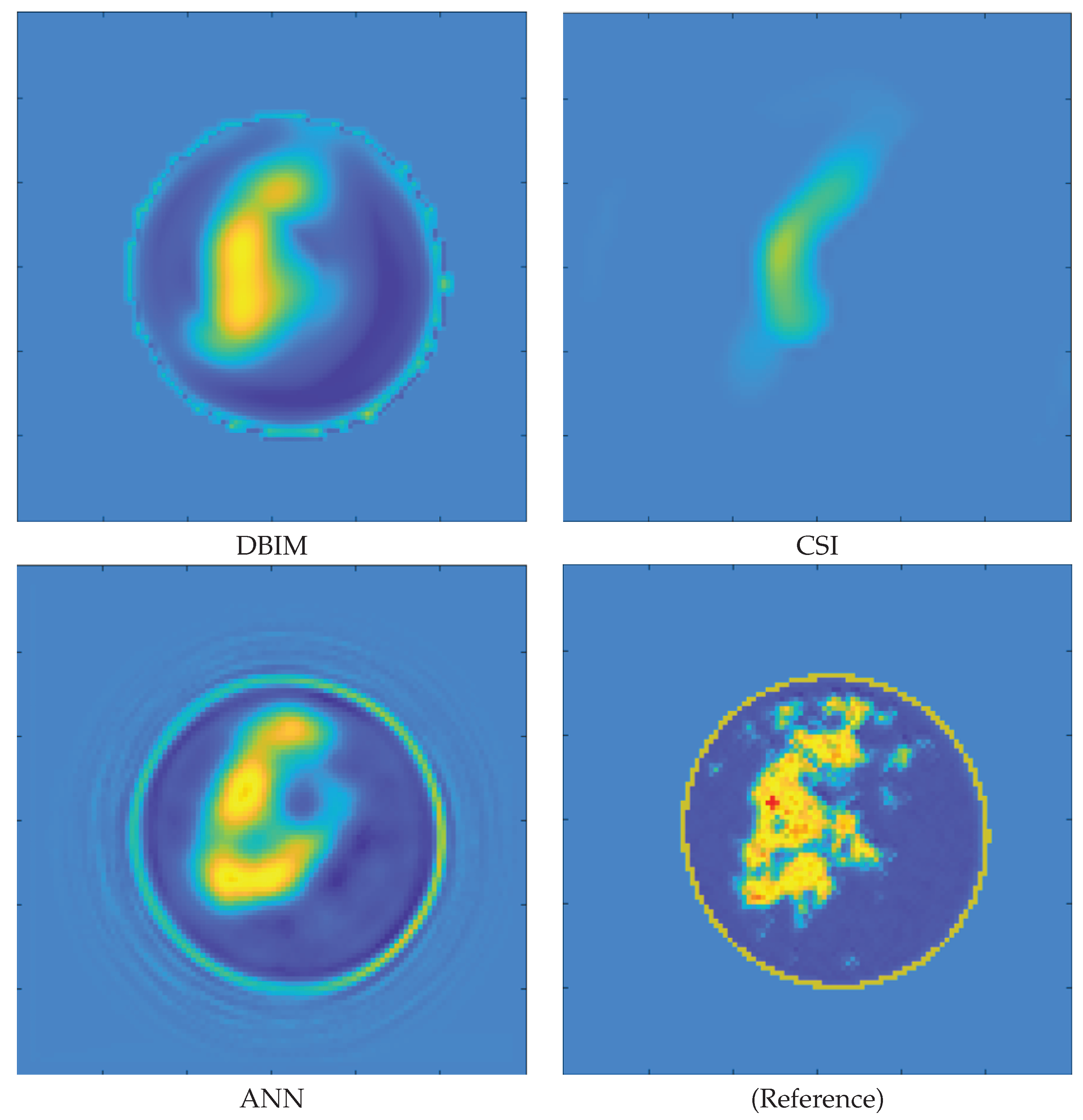
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| US | UltraSound |
| NN | Neural Network |
| CT | Computed Tomography |
| RF | Radio Frequency |
| SNR | Signal-to-Noise Ratio |
| EIS | Electromagnetic Inverse Scattering |
| CNN | Convolutional Neural Network |
| ANN | Artificial Neural Network |
| MoM | Method of Moments |
| FFT-CG | Fast Fourier Transform-Conjugate Gradient |
| TPR | True Positive Rate |
| FPR | False Positive Rate |
| TNR | True Negative Rate |
| FNR | False Negative Rate |
| ROC | Receiver Operative Curve |
| AUR | Area Under ROC |
| EER | Equal Error Rate |
| DBIM | Distorted Born Iterative Method |
| CSI | Contrast Source Inversion |
References
- Siegel, R.; Miller, K.D.; Wagle, N.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Webb, A. Introduction to Medical Imaging: Physics, Engineering and Clinical Applications; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- McNitt-Gray, M. AAPM/RSNA Physics Tutorial for Residents: Topics in CT. RadioGraphics 2002, 22, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Dasarathy, V. Medical image fusion: A survey of the state of the art. Inf. Fusion 2014, 19, 4–19. [Google Scholar] [CrossRef]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef]
- Zhurbenko, V.; Rubæk, T.; Krozer, V.; Meincke, P. Design and realisation of a microwave three-dimensional imaging system with application to breast-cancer detection. IET Microw. Antennas Propag. 2010, 4, 2200–2211. [Google Scholar] [CrossRef]
- Fedeli, A.; Maffongelli, M.; Monleone, R.; Pagnamenta, C.; Pastorino, M.; Poretti, S.; Randazzo, A.; Salvadè, A. A tomograph prototype for quantitative microwave imaging: Preliminary experimental results. J. Imaging 2018, 4, 139. [Google Scholar] [CrossRef]
- Tobon Vasquez, J.A.; Scapaticci, R.; Turvani, G.; Bellizzi, G.; Joachimowicz, N.; Duchêne, B.; Tedeschi, E.; Casu, M.R.; Crocco, L.; Vipiana, F. Design and experimental assessment of a 2d microwave imaging system for brain stroke monitoring. Int. J. Antennas Propag. 2019, 2019, 8065036. [Google Scholar] [CrossRef]
- Pagliari, D.J.; Pulimeno, A.; Vacca, M.; Tobon, J.A.; Vipiana, F.; Casu, M.R.; Solimene, R.; Carloni, L.P. A low-cost, fast, and accurate microwave imaging system for breast cancer detection. In Proceedings of the 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS), Atlanta, GA, USA, 22–24 October 2015; pp. 1–4. [Google Scholar]
- Fear, E.C.; Bourqui, J.; Curtis, C.; Mew, D.; Docktor, B.; Romano, C. Microwave breast imaging with a monostatic radar-based system: A study of application to patients. IEEE Trans. Microw. Theory Tech. 2013, 61, 2119–2128. [Google Scholar] [CrossRef]
- Porter, E.; Kirshin, E.; Santorelli, A.; Coates, M.; Popović, M. Time-domain multistatic radar system for microwave breast Screening. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 229–232. [Google Scholar] [CrossRef]
- Santorelli, A.; Porter, E.; Kang, E.; Piske, T.; Popović, M.; Schwartz, J.D. A time-domain microwave system for breast cancer detection using a flexible circuit board. IEEE Trans. Instrum. Meas. 2015, 64, 2986–2994. [Google Scholar] [CrossRef]
- Meaney, P.; Hartov, A.; Bulumulla, S.; Raynolds, T.; Davis, C.; Schoenberger, F.; Richter, S.; Paulsen, K. A 4-channel, vector network analyzer microwave imaging prototype based on software defined radio technology. Rev. Sci. Instrum. 2019, 90, 044708. [Google Scholar] [CrossRef] [PubMed]
- Sani, L.; Ghavami, N.; Vispa, A.; Paoli, M.; Raspa, G.; Ghavami, M.; Sacchetti, F.; Vannini, E.; Ercolani, S.; Saracini, A.; et al. Novel microwave apparatus for breast lesions detection: Preliminary clinical results. Biomed. Signal Process. Control 2019, 52, 257–263. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Svenson, R.H.; Bulyshev, A.E.; Souvorov, A.E.; Nazarov, A.G.; Sizov, Y.E.; Pavlovsky, A.V.; Borisov, V.Y.; Voinov, B.A.; Simonova, G.I.; et al. Three-dimensional microwave tomography: Experimental prototype of the system and vector Born reconstruction method. IEEE Trans. Biomed. Eng. 1999, 46, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, T.M.; Meaney, P.M.; Kaufman, P.A.; Paulsen, K.D. Fast 3-D tomographic microwave imaging for breast cancer detection. IEEE Trans. Med. Imag. 2012, 31, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Simonov, N.; Kim, H.J.; Lee, J.M.; Jeon, S.I. Preclinical prototype development of a microwave tomography system for breast cancer detection. ETRI J. 2010, 32, 901–910. [Google Scholar] [CrossRef]
- Benny, R.; Anjit, T.A.; Mythili, P. An overview of microwave imaging for breast tumor detection. Prog. Electromagn. Res. 2020, 87, 61–91. [Google Scholar] [CrossRef]
- Ambrosanio, M.; Kosmasy, P.; Pascazio, V. An adaptive multi-threshold iterative shrinkage algorithm for microwave imaging applications. In Proceedings of the 2016 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–15 April 2016; pp. 1–3. [Google Scholar]
- Meaney, P.M.; Fanning, M.W.; Li, D.; Poplack, S.P.; Paulsen, K.D. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar]
- Porter, E.; Coates, M.; Popović, M. An early clinical study of time-domain microwave radar for breast health monitoring. IEEE Trans. Biomed. Eng. 2015, 63, 530–539. [Google Scholar] [CrossRef]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. Maria m4: Clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef]
- Bertero, M.; Boccacci, P. Introduction to Inverse Problems in Imaging; CRC press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Isernia, T.; Pascazio, V.; Pierri, R. A nonlinear estimation method in tomographic imaging. IEEE Trans. Geosci. Remote Sens. 1997, 35, 910–923. [Google Scholar] [CrossRef]
- Bucci, O.M.; Isernia, T. Electromagnetic inverse scattering: Retrievable information and measurement strategies. Radio Sci. 1997, 32, 2123–2137. [Google Scholar] [CrossRef]
- Cui, T.J.; Qin, Y.; Wang, G.L.; Chew, W.C. Low-frequency detection of two-dimensional buried objects using high-order extended born approximations. Inverse Probl. 2004, 20, S41. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference On Computer Vision Furthermore, Pattern Recognition (CVPR), Las Vegas, NV, USA, 26 June–1 July 2016; pp. 770–778. [Google Scholar]
- Franceschini, S.; Ambrosanio, M.; Pascazio, V.; Baselice, F. Hand Gesture Signatures Acquisition and Processing by Means of a Novel Ultrasound System. Bioengineering 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Ambrosanio, M.; Vitale, S.; Baselice, F.; Gifuni, A.; Grassini, G.; Pascazio, V. Hand gesture recognition via radar sensors and convolutional neural networks. In Proceedings of the 2020 IEEE Radar Conference (RadarConf20), Florence, Italy, 21–25 September 2020; pp. 1–5. [Google Scholar]
- Vitale, S.; Ferraioli, G.; Pascazio, V. Analysis on the Building of Training Dataset for Deep Learning SAR Despeckling. IEEE Geosci. Remote Sens. Lett. 2021, 19, 4015005. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.; Setio, A.; Ciompi, F.; Ghafoorian, M.; Van der Laak, J.; Van Ginneken, B.; Sánchez, C. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Salucci, M.; Arrebola, M.; Shan, T.; Li, M. Artificial Intelligence: New Frontiers in Real-Time Inverse Scattering and Electromagnetic Imaging. IEEE Trans. Antennas Propag. 2022, 70, 6349–6364. [Google Scholar] [CrossRef]
- Rekanos, I.T. Neural-network-based inverse-scattering technique for online microwave medical imaging. IEEE Trans. Magn. 2002, 38, 1061–1064. [Google Scholar] [CrossRef]
- Ashtari, A.; Noghanian, S.; Sabouni, A.; Aronsson, J.; Thomas, G.; Pistorius, S. Using a priori information for regularization in breast microwave image reconstruction. IEEE Trans. Biomed. Eng. 2010, 57, 2197–2208. [Google Scholar] [CrossRef]
- Khoshdel, V.; Asefi, M.; Ashraf, A.; LoVetri, J. Full 3D microwave breast imaging using a deep-learning technique. J. Imaging 2020, 6, 80. [Google Scholar] [CrossRef]
- Shah, P.; Moghaddam, M. Super resolution for microwave imaging: A deep learning approach. In Proceedings of the International Symposium on Antennas and Propagation & USNC/URSI National Radio Science Meeting, San Diego, CA, USA, 9–14 July 2017; pp. 849–850. [Google Scholar]
- Li, L.; Wang, L.G.; Teixeira, F.L.; Liu, C.; Nehorai, A.; Cui, T.J. Deepnis: Deep neural network for nonlinear electromagnetic inverse scattering. IEEE Trans. Antennas Propag. 2018, 67, 1819–1825. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, X. Deep-learning schemes for full-wave nonlinear inverse scattering problems. IEEE Trans. Geosci. Remote Sens. 2018, 57, 1849–1860. [Google Scholar] [CrossRef]
- Wu, H.; Ren, X.; Guo, L.; Li, Z. A Non-Iterative Method Combined with Neural Network Embedded in Physical Model to Solve the Imaging of Electromagnetic Inverse Scattering Problem. Electronics 2021, 10, 3104. [Google Scholar] [CrossRef]
- Ambrosanio, M.; Franceschini, S.; Pascazio, V.; Baselice, F. An End-to-End Deep Learning Approach for Quantitative Microwave Breast Imaging in Real-Time Applications. Bioengineering 2022, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Ambrosanio, M.; Baselice, F.; Pascazio, V. Neural Networks for Inverse Problems: The Microwave Imaging Case. In Proceedings of the 2021 15th European Conference on Antennas and Propagation (EuCAP), Düsseldorf, Germany, 22–26 March 2021. [Google Scholar]
- Ambrosanio, M.; Franceschini, S.; Baselice, F.; Pascazio, V. Artificial neural networks for quantitative microwave breast imaging. In Proceedings of the 7th International Conference on Bioimaging, Proceedings (BIOIMAGING), Valletta, Malta, 24–26 February 2020. [Google Scholar]
- Colton, D.; Kress, R. Inverse Acoustic and Electromagnetic Scattering Theory; Springer Nature: Berlin/Heidelberg, Germany, 2019; Volume 93. [Google Scholar]
- Schertzer, D.; Lovejoy, S. Nonlinear variability in geophysics: Multifractal simulations and analysis. In Fractals’ Physical Origin and Properties; Springer: Berlin/Heidelberg, Germany, 1989; pp. 49–79. [Google Scholar]
- Burfeindt, M.J.; Colgan, T.J.; Mays, R.O.; Shea, J.D.; Behdad, N.; Van Veen, B.D.; Hagness, S.C. MRI-derived 3-D-printed breast phantom for microwave breast imaging validation. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1610–1613. [Google Scholar] [CrossRef]
- Jain, A.K.; Ross, A.; Prabhakar, S. An introduction to biometric recognition. IEEE Trans. Circuits Syst. Video Technol. 2004, 14, 4–20. [Google Scholar] [CrossRef]
- Chew, W.C.; Wang, Y.-M. Reconstruction of two-dimensional permittivity distribution using the distorted born iterative method. IEEE Trans. Med. Imag. 1990, 9, 218–225. [Google Scholar] [CrossRef]
- Van den Berg, P.M.; Abubakar, A. Contrast source inversion method: State of art. Prog. Electromagn. Res. 2001, 34, 189–218. [Google Scholar] [CrossRef]
- Sun, S.; Kooij, B.J.; Jin, T.; Yarovoy, A.G. Cross-correlated contrast source inversion. IEEE Trans. Antennas Propag. 2017, 65, 2592–2603. [Google Scholar] [CrossRef]



| Tissue Type | Relative Permittivity | Conductivity [S/m] |
|---|---|---|
| Adipose | 1–8 | 0–0.12 |
| Transitional | 7–38 | 0.11–0.58 |
| Fibroglandular | 37–58 | 0.56–1.22 |
| Noise-Free | 30 dB SNR | 20 dB SNR | 10 dB SNR | |
|---|---|---|---|---|
| Area under ROC | 1.00 | 0.992 | 0.661 | 0.550 |
| Equal Error Rate | 0.001 | 0.041 | 0.367 | 0.430 |
| Accuracy | 0.995 | 0.960 | 0.625 | 0.567 |
| Sensitivity | 0.989 | 0.950 | 0.738 | 0.626 |
| Specificity | 0.999 | 0.950 | 0.502 | 0.495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschini, S.; Autorino, M.M.; Ambrosanio, M.; Pascazio, V.; Baselice, F. A Deep Learning Approach for Diagnosis Support in Breast Cancer Microwave Tomography. Diagnostics 2023, 13, 1693. https://doi.org/10.3390/diagnostics13101693
Franceschini S, Autorino MM, Ambrosanio M, Pascazio V, Baselice F. A Deep Learning Approach for Diagnosis Support in Breast Cancer Microwave Tomography. Diagnostics. 2023; 13(10):1693. https://doi.org/10.3390/diagnostics13101693
Chicago/Turabian StyleFranceschini, Stefano, Maria Maddalena Autorino, Michele Ambrosanio, Vito Pascazio, and Fabio Baselice. 2023. "A Deep Learning Approach for Diagnosis Support in Breast Cancer Microwave Tomography" Diagnostics 13, no. 10: 1693. https://doi.org/10.3390/diagnostics13101693
APA StyleFranceschini, S., Autorino, M. M., Ambrosanio, M., Pascazio, V., & Baselice, F. (2023). A Deep Learning Approach for Diagnosis Support in Breast Cancer Microwave Tomography. Diagnostics, 13(10), 1693. https://doi.org/10.3390/diagnostics13101693







