Safety and Diagnostic Accuracy of the Transnasal Approach for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varela-Lema, L.; Fernandez-Villar, A.; Ruano-Ravina, A. Effectiveness and Safety of Endobronchial Ultrasound-Transbronchial Needle Aspiration: A Systematic Review. Eur. Respir. J. 2009, 33, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Sampsonas, F.; Kakoullis, L.; Lykouras, D.; Karkoulias, K.; Spiropoulos, K. EBUS: Faster, Cheaper and Most Effective in Lung Cancer Staging. Int. J. Clin. Pract. 2018, 72, e13053. [Google Scholar] [CrossRef]
- Vilmann, P.; Clementsen, P.F.; Colella, S.; Siemsen, M.; De Leyn, P.; Dumonceau, J.-M.; Herth, F.J.; Larghi, A.; Vazquez-Sequeiros, E.; Hassan, C.; et al. Combined Endobronchial and Oesophageal Endosonography for the Diagnosis and Staging of Lung Cancer. Eur. Respir. J. 2015, 46, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, D.A.; Crombag, L.M.; Cohen, J.F.; Spijker, R.; Bossuyt, P.M.; Annema, J.T. Added Value of Combined Endobronchial and Oesophageal Endosonography for Mediastinal Nodal Staging in Lung Cancer: A Systematic Review and Meta-Analysis. Lancet Respir. Med. 2016, 4, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Sawabata, N. Mediastinal Lymph Node Staging for Lung Cancer. Mediastinum 2019, 3, 33. [Google Scholar] [CrossRef]
- Chung, H.S.; Pak, K.; Lee, G.; Eom, J.S. Combined Procedure with Radial Probe and Convex Probe Endobronchial Ultrasound. Thorac. Cancer 2022, 13, 2837–2843. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, H.; Ning, Y.; Han, J.; Shen, Y.; Shi, H.; Wang, Q.; Bai, C.; Li, Q.; Michael, S.; et al. Radial Probe Endobronchial Ultrasound Assisted Conventional Transbronchial Needle Aspiration in the Diagnosis of Solitary Peribronchial Pulmonary Lesion Located in the Segmental Bronchi. J. Cancer 2019, 10, 634–642. [Google Scholar] [CrossRef]
- Ali, M.S.; Trick, W.; Mba, B.I.; Mohananey, D.; Sethi, J.; Musani, A.I. Radial Endobronchial Ultrasound for the Diagnosis of Peripheral Pulmonary Lesions: A Systematic Review and Meta-Analysis. Respirology 2017, 22, 443–453. [Google Scholar] [CrossRef]
- Sarkiss, M.; Kennedy, M.; Riedel, B.; Norman, P.; Morice, R.; Jimenez, C.; Eapen, G. Anesthesia Technique for Endobronchial Ultrasound-Guided Fine Needle Aspiration of Mediastinal Lymph Node. J. Cardiothorac. Vasc. Anesth. 2007, 21, 892–896. [Google Scholar] [CrossRef]
- Kennedy, M.P.; Shweihat, Y.; Sarkiss, M.; Eapen, G.A. Complete Mediastinal and Hilar Lymph Node Staging of Primary Lung Cancer by Endobronchial Ultrasound: Moderate Sedation or General Anesthesia? Chest 2008, 138, 1350–1351. [Google Scholar] [CrossRef]
- Alon, D.; Pertzov, B.; Gershman, E.; Frishman, M.; Rahman, N.A.; Rosengarten, D.; Kramer, M.R. The Safety of Laryngeal Mask Airway-Assisted Bronchoscopy versus Standard Nasal Bronchoscopy. Respiration 2017, 93, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Codazzi, D.; Paleari, M.C.; Previtali, P.; Delconte, G.; Fumagalli, L.; Manzi, R.; Faustini, M.; Persiani, L.; Rizzi, M.; et al. Endosonographic Evaluation of the Mediastinum through the I-Gel O2 Supraglottic Airway Device. Tumori J. 2021, 107, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Casal, R.F.; Lazarus, D.R.; Kuhl, K.; Nogueras-González, G.; Perusich, S.; Green, L.K.; Ost, D.E.; Sarkiss, M.; Jimenez, C.A.; Eapen, G.A.; et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration under General Anesthesia versus Moderate Sedation. Am. J. Respir. Crit. Care Med. 2015, 191, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Piro, R.; Casalini, E.; Fontana, M.; Galeone, C.; Ruggiero, P.; Taddei, S.; Ghidoni, G.; Patricelli, G.; Facciolongo, N. Efficacy and Safety of EBUS-TBNA under Conscious Sedation with Meperidine and Midazolam. Thorac. Cancer 2022, 13, 533–538. [Google Scholar] [CrossRef]
- Cornelissen, C.G.; Dapper, J.; Dreher, M.; Müller, T. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration under General Anesthesia versus Bronchoscopist-Directed Deep Sedation: A Retrospective Analysis. Endosc. Ultrasound 2019, 8, 204–208. [Google Scholar] [CrossRef]
- Steinfort, D.P.; Irving, L.B. Patient Satisfaction during Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Performed under Conscious Sedation. Respir. Care 2010, 55, 702–706. [Google Scholar] [PubMed]
- Skinner, T.R.; Churton, J.; Edwards, T.P.; Bashirzadeh, F.; Zappala, C.; Hundloe, J.T.; Tan, H.; Pattison, A.J.; Todman, M.; Hartel, G.F.; et al. A Randomised Study of Comfort during Bronchoscopy Comparing Conscious Sedation and Anaesthetist-Controlled General Anaesthesia, Including the Utility of Bispectral Index Monitoring. ERJ Open Res. 2021, 7, 00895–2020. [Google Scholar] [CrossRef]
- Wahidi, M.M.; Herth, F.; Yasufuku, K.; Shepherd, R.W.; Yarmus, L.; Chawla, M.; Lamb, C.; Casey, K.R.; Patel, S.; Silvestri, G.A.; et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration CHEST Guideline and Expert Panel Report. Chest 2016, 149, 816–835. [Google Scholar] [CrossRef]
- Facciolongo, N.; Piro, R.; Menzella, F.; Lusuardi, M.; Salio, M.; Agli, L.L.; Patelli, M. Training and Practice in Bronchoscopy: A National Survey in Italy. Monaldi Arch. Chest Dis. 2013, 79, 128–133. [Google Scholar] [CrossRef]
- Beaudoin, S.; Ferland, N.; Martel, S.; Delage, A. Feasibility of Using the Nasal Route for Linear Endobronchial Ultrasound. Lung 2014, 192, 921–926. [Google Scholar] [CrossRef]
- Beaudoin, S.; Martel, S.; Pelletier, S.; Lampron, N.; Simon, M.; Laberge, F.; Delage, A. Randomized Trial Comparing Patient Comfort between the Oral and Nasal Insertion Routes for Linear Endobronchial Ultrasound. J. Bronchol. Interv. Pulmonol. 2016, 23, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Madan, K.; Mohan, A.; Hadda, V. Nasal Route for Endoscopic Ultrasound-Guided Fine-Needle Aspiration Using Echobronchoscope: The Last Resort. Lung India 2020, 37, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society Guideline for Diagnostic Flexible Bronchoscopy in Adults: Accredited by NICE. Thorax 2013, 68, i1–i44. [Google Scholar] [CrossRef] [PubMed]
- Abuqayyas, S.; Raju, S.; Bartholomew, J.R.; Hweij, R.A.; Mehta, A.C. Management of Antithrombotic Agents in Patients Undergoing Flexible Bronchoscopy. Eur. Respir. Rev. 2017, 26, 170001. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Spyropoulos, A.C.; Murad, M.H.; Arcelus, J.I.; Dager, W.E.; Dunn, A.S.; Fargo, R.A.; Levy, J.H.; Samama, C.M.; Shah, S.H.; et al. Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest 2022, 162, e207–e243. [Google Scholar] [CrossRef] [PubMed]
- Jeffus, S.K.; Joiner, A.K.; Siegel, E.R.; Massoll, N.A.; Meena, N.; Chen, C.; Post, S.R.; Bartter, T. Rapid On-Site Evaluation of EBUS-TBNA Specimens of Lymph Nodes: Comparative Analysis and Recommendations for Standardization. Cancer Cytopathol. 2015, 123, 362–372. [Google Scholar] [CrossRef]
- Harrell, J.H. Transnasal Approach for Fiberoptic Bronchoscopy. Chest 1978, 73, 704–706. [Google Scholar] [CrossRef]
- Mehta, A.C.; Dweik, R.A. Nasal versus Oral Insertion of the Flexible Bronchoscope: Pro-Nasal Insertion. J. Bronchol. Interv. Pulmonol. 1996, 3, 224–228. [Google Scholar] [CrossRef]
- Borchers, S.D.; Beamis, J.F.J. Flexible Bronchoscopy. Chest Surg. Clin. N. Am. 1996, 6, 169–192. [Google Scholar]
- de Boer, G.M.; Türk, Y.; Meuleman-van Waning, V.H.; Braunstahl, G.-J. Bronchoscopy: Oral or Nasal Insertion? J. Bronchol. Interv. Pulmonol. 2017, 24, 125–130. [Google Scholar] [CrossRef]
- Choi, C.M.; Yoon, H.I.; Lee, S.M.; Yoo, C.-G.; Kim, Y.W.; Han, S.K.; Shim, Y.-S.; Yim, J.-J. Oral Insertion of a Flexible Bronchoscope Is Associated with Less Discomfort than Nasal Insertion for Korean Patients. Int. J. Tuberc. Lung Dis. 2005, 9, 344–348. [Google Scholar] [PubMed]

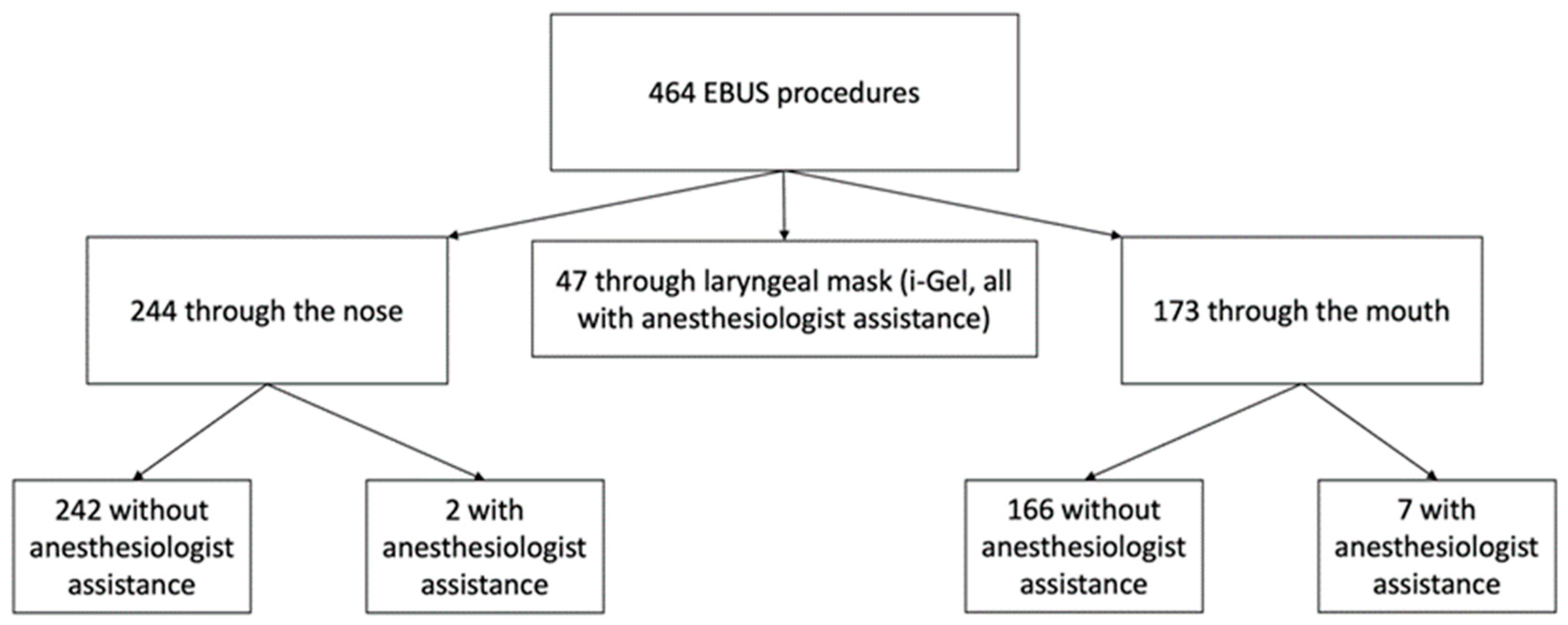
Inclusion criteria:
|
Exclusion criteria:
|
| Overall Population (464) | |
|---|---|
| Age (years, mean ± SD) | 66.7 (±11.4) |
| Sex (males, %) | 296 (63.8) |
| Death (%) | 236 (50.9) |
| Weight (kg, mean ± SD) | 73.5 (±16.3) |
| Height (cm, mean ± SD) | 167 (±9.9) |
| BMI (kg/m2, mean ± SD) | 26.2 (±4.9) |
| Previous biopsies (%) | 81 (17.5) |
| Mediastinal staging (%) | 136 (29.3) |
| Mediastinal adenopathies (%) | 444 (95.7) |
| Adequate visualization (%) | 455 (98.1) |
| Sample acquisition (%) | 458 (98.7) |
| No. of stations sampled (mean ± SD) | 1.7 (±1) |
| 2R (%) | 9 (1.9) |
| 2L (%) | 3 (0.6) |
| 3 (%) | 3 (0.6) |
| 4R (%) | 173 (37.3) |
| 4L (%) | 64 (13.8) |
| 7 (%) | 273 (58.8) |
| 8 (%) | 3 (0.6) |
| 10R (%) | 23 (4.9) |
| 10L (%) | 14 (3) |
| 11R (%) | 108 (23.3) |
| 11L (%) | 81 (17.5) |
| 12R (%) | 0 (0) |
| 12L (%) | 2 (0.4) |
| T (%) | 30 (6.5) |
| No. of total needle passes (mean ± SD) | 5.9 (±2.8) |
| Anesthesiologist assistance (%) | 56 (12.1) |
| Diagnostic sample (%) | 381 (82) |
| Chronic inflammation (%) | 10 (2.2) |
| Anthracosis (%) | 85 (18.3) |
| NSCLC (%) | 177 (38.1) |
| SCLC (%) | 40 (8.6) |
| Neuroendocrine large cell tumors (%) | 1 (0.2) |
| Metastasis of other solid tumors (%) | 19 (4.1) |
| Benign neoplasms (%) | 1 (0.2) |
| Sarcoidosis (%) | 37 (8) |
| Lymphoma (%) | 10 (2.2) |
| Lymphocytes (%) | 234 (50.4) |
| Suspicious for malignancy (%) | 3 (0.6) |
| Inadequate sample (%) | 24 (5.2) |
| False negative (%) | 2 (0.4) |
| Nose (244) | Mouth (173) | p Value | |
|---|---|---|---|
| Age (years, mean ± SD) | 69.1 (±11.5) | 68.3 (±11.4) | 0.49 |
| Sex (males, %) | 169 (69.3) | 96 (55.5) | 0.005 |
| Death (%) | 123 (50.4) | 94 (54.3) | 0.49 |
| Height (cm, mean ± SD) | 168.1 (±10.3) | 165.4 (±9.2) | 0.005 |
| Weight (kg, mean ± SD) | 74.8 (±16.4) | 71.7 (±16.8) | 0.03 |
| BMI (kg/m2, mean ± SD) | 26.3 (±4.6) | 26 (±5.4) | 0.25 |
| Previous biopsies (%) | 37 (15.2) | 29 (16.8) | 0.68 |
| Mediastinal staging (%) | 74 (30.3) | 37 (21.4) | 0.04 |
| Mediastinal adenopathies (%) | 231 (94.7) | 168 (97.1) | 0.33 |
| Adequate visualization (%) | 238 (98.3) | 170 (97.5) | 0.74 |
| Sample acquisition (%) | 240 (98.4) | 171 (98.8) | >0.9 |
| No. of stations sampled (mean ± SD) | 1.7 (±1.1) | 1.6 (±0.9) | 0.47 |
| No. of total needle passes (mean ± SD) | 5.9 (±2.9) | 5.5 (±2.3) | 0.1 |
| Anesthesiologist assistance (%) | 2 (0.8) | 7 (4) | 0.037 |
| Sedative drugs | |||
| Meperidine (mg, mean ± SD) | 56.5 (±36.2) | 50.1 (±36.3) | 0.08 |
| Midazolam (mg, mean ± SD) | 5.9 (±2.7) | 5.5 (±2.6) | 0.14 |
| Fentanyl (mg, mean ± SD) | 0.044 (±0.059) | 0.047 (±0.055) | 0.58 |
| Remifentanil (mg, mean ± SD) * | 0 (±0) | 0.36 (±0.75) | >0.9 |
| Propofol (mg, mean ± SD) * | 250 (±0) | 454.3 (±296.6) | 0.12 |
| Adverse events (%) | 25 (10.2) | 17 (9.8) | >0.9 |
| Epistaxis (%) | 5 (0) | 0 (0) | 0.08 |
| Mild airway bleeding (%) | 11 (4.5) | 1 (0.6) | 0.017 |
| Desaturation (%) | 1 (0.4) | 6 (3.5) | 0.022 |
| Hypertension (%) | 1 (0.4) | 1 (0.6) | >0.9 |
| Use of sedative antagonists (%) | 1 (0.4) | 0 (0) | >0.9 |
| Cough (%) | 2 (0.8) | 4 (2.3) | 0.24 |
| Agitation (%) | 2 (0.8) | 6 (3.4) | 0.07 |
| Stridor or bronchospasm (%) | 3 (1.2) | 4 (2.3) | 0.45 |
| Diagnostic sample (%) | 205 (84) | 142 (82) | 0.59 |
| Inadequate sample (%) | 12 (4.9) | 9 (5.2) | >0.9 |
| False negative (%) | 2 (0.8) | 0 (0) | 0.51 |
| Nose (244) | Mouth (173) | Laryngeal Mask (47) | p Value | |
|---|---|---|---|---|
| Previous biopsies (%) | 37 (15.2) | 29 (16.8) | 15 (31.9) | 0.02 |
| Mediastinal staging (%) | 74 (30.3) | 37 (21.4) | 25 (53.2) | 0.0001 |
| Mediastinal adenopathies (%) | 231 (94.7) | 168 (97.1) | 45 (95.7) | 0.5 |
| Adequate visualization (%) | 238 (98.3) | 170 (97.5) | 47 (100) | 0.5 |
| Sample acquisition (%) | 240 (98.4) | 171 (98.8) | 47 (100) | 0.7 |
| No. of stations sampled (mean ± SD) | 1.7 (±1.1) | 1.6 (±0.9) | 2.1 (±1.3) | 0.02 |
| No. of total needle passes (mean ± SD) | 5.9 (±2.9) | 5.5 (±2.3) | 7.4 (±3.2) | 0.0003 |
| Anesthesiologist assistance (%) | 2 (0.8) | 7 (4) | 47 (100) | <0.0001 |
| Sedative drugs | ||||
| Meperidine (mg, mean ± SD) | 56.5 (±36.2) | 50.1 (±36.3) | 0 (±0) | <0.0001 |
| Midazolam (mg, mean ± SD) | 5.9 (±2.7) | 5.5 (±2.6) | 1.3 (±2.1) | <0.0001 |
| Fentanyl (mg, mean ± SD) | 0.044 (±0.059) | 0.047 (±0.055) | 0.118 (±0.078) | <0.0001 |
| Remifentanil (mg, mean ± SD) * | 0 (±0) | 0.36 (±0.75) | 0.32 (±1) | 0.64 |
| Propofol (mg, mean ± SD) * | 250 (±0) | 454.3 (±296.6) | 550.6 (±325.8) | 0.13 |
| Adverse events (%) | 25 (10.2) | 17 (9.8) | 5 (10.6) | 0.98 |
| Epistaxis (%) | 5 (0) | 0 (0) | 0 (0) | 0.1 |
| Mild airway bleeding (%) | 11 (4.5) | 1 (0.6) | 2 (4.2) | 0.06 |
| Desaturation (%) | 1 (0.4) | 6 (3.5) | 1 (2.1) | 0.06 |
| Hypertension (%) | 1 (0.4) | 1 (0.6) | 0 (0) | 0.9 |
| Use of sedative antagonists (%) | 1 (0.4) | 0 (0) | 0 (0) | 0,6 |
| Cough (%) | 2 (0.8) | 4 (2.3) | 0 (0) | 0.3 |
| Agitation (%) | 2 (0.8) | 6 (3.4) | 2 (4.2) | 0.1 |
| Stridor or bronchospasm (%) | 3 (1.2) | 4 (2.3) | 0 (0) | 0.4 |
| Diagnostic sample (%) | 205 (84) | 142 (82) | 34 (72) | 0.16 |
| Inadequate sample (%) | 12 (4.9) | 9 (5.2) | 3 (6.4) | 0.9 |
| False negative (%) | 2 (0.8) | 0 (0) | 0 (0) | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piro, R.; Fontana, M.; Casalini, E.; Rossi, L.; Simeone, M.S.; Ghinassi, F.; Ruggiero, P.; Pollorsi, C.; Taddei, S.; Beghe’, B.; et al. Safety and Diagnostic Accuracy of the Transnasal Approach for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA). Diagnostics 2023, 13, 1405. https://doi.org/10.3390/diagnostics13081405
Piro R, Fontana M, Casalini E, Rossi L, Simeone MS, Ghinassi F, Ruggiero P, Pollorsi C, Taddei S, Beghe’ B, et al. Safety and Diagnostic Accuracy of the Transnasal Approach for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA). Diagnostics. 2023; 13(8):1405. https://doi.org/10.3390/diagnostics13081405
Chicago/Turabian StylePiro, Roberto, Matteo Fontana, Eleonora Casalini, Laura Rossi, Maria Serena Simeone, Federica Ghinassi, Patrizia Ruggiero, Chiara Pollorsi, Sofia Taddei, Bianca Beghe’, and et al. 2023. "Safety and Diagnostic Accuracy of the Transnasal Approach for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA)" Diagnostics 13, no. 8: 1405. https://doi.org/10.3390/diagnostics13081405
APA StylePiro, R., Fontana, M., Casalini, E., Rossi, L., Simeone, M. S., Ghinassi, F., Ruggiero, P., Pollorsi, C., Taddei, S., Beghe’, B., & Facciolongo, N. C. (2023). Safety and Diagnostic Accuracy of the Transnasal Approach for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA). Diagnostics, 13(8), 1405. https://doi.org/10.3390/diagnostics13081405







