Circulating Regulatory T Cell Subsets in Patients with Sarcoidosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Methods of the Study
2.3. Sample Collection
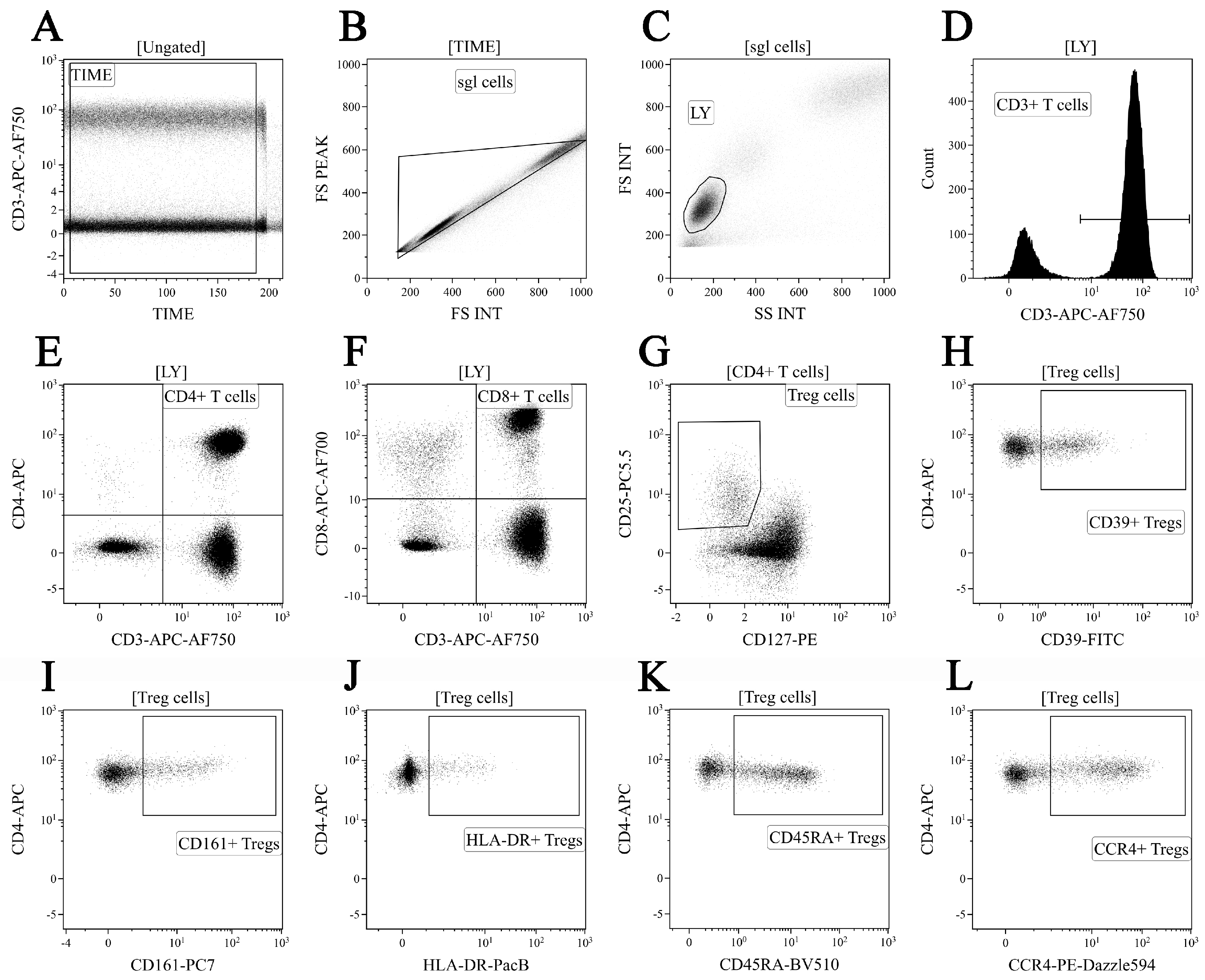
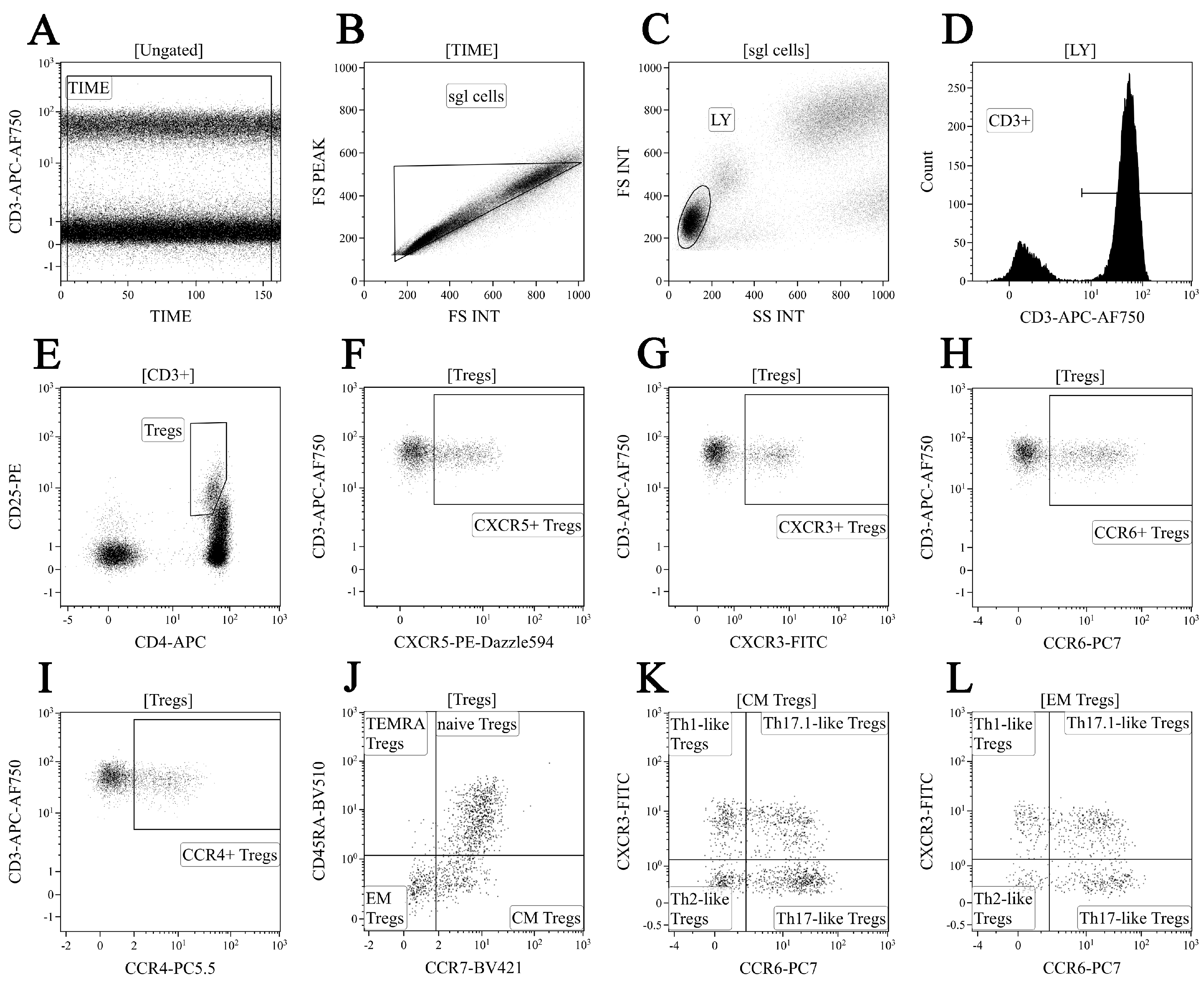
2.4. Regulatory T Cell Immunophenotyping
2.5. Statistical Analysis
3. Results
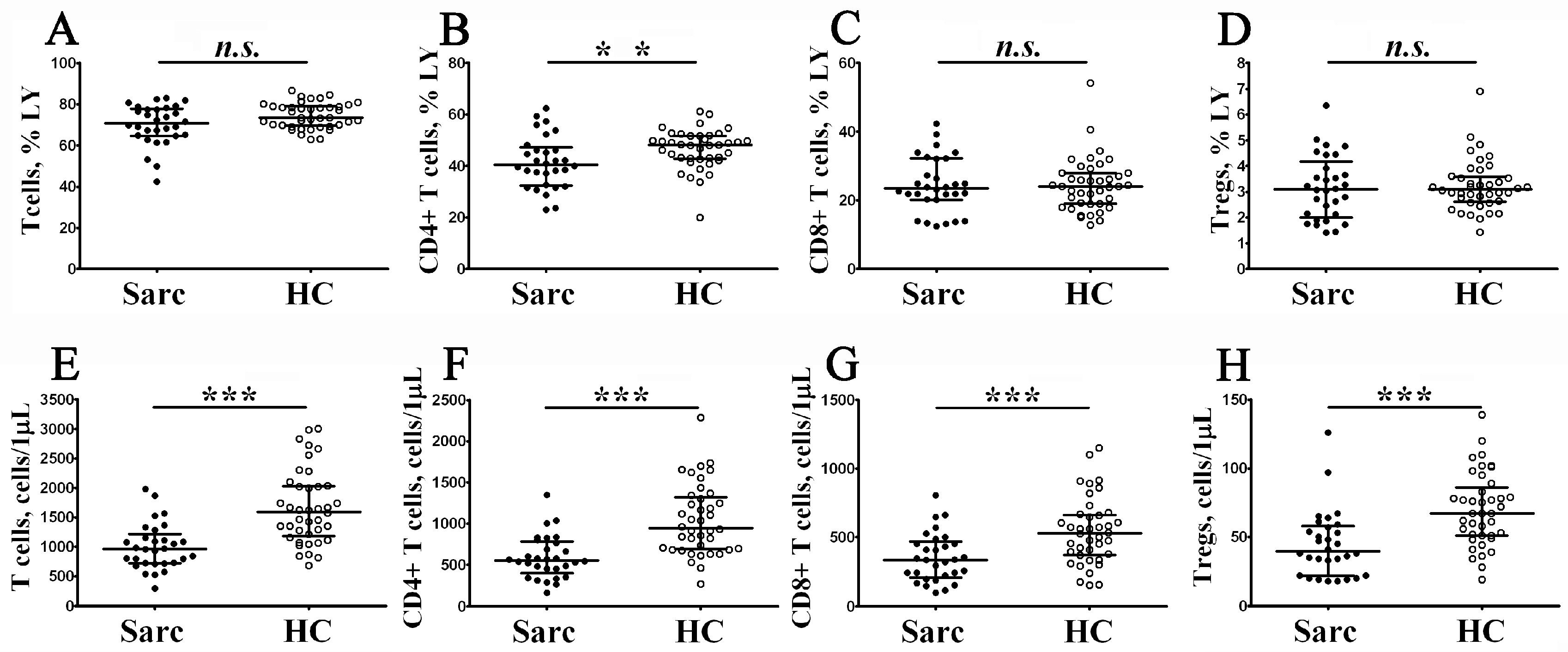
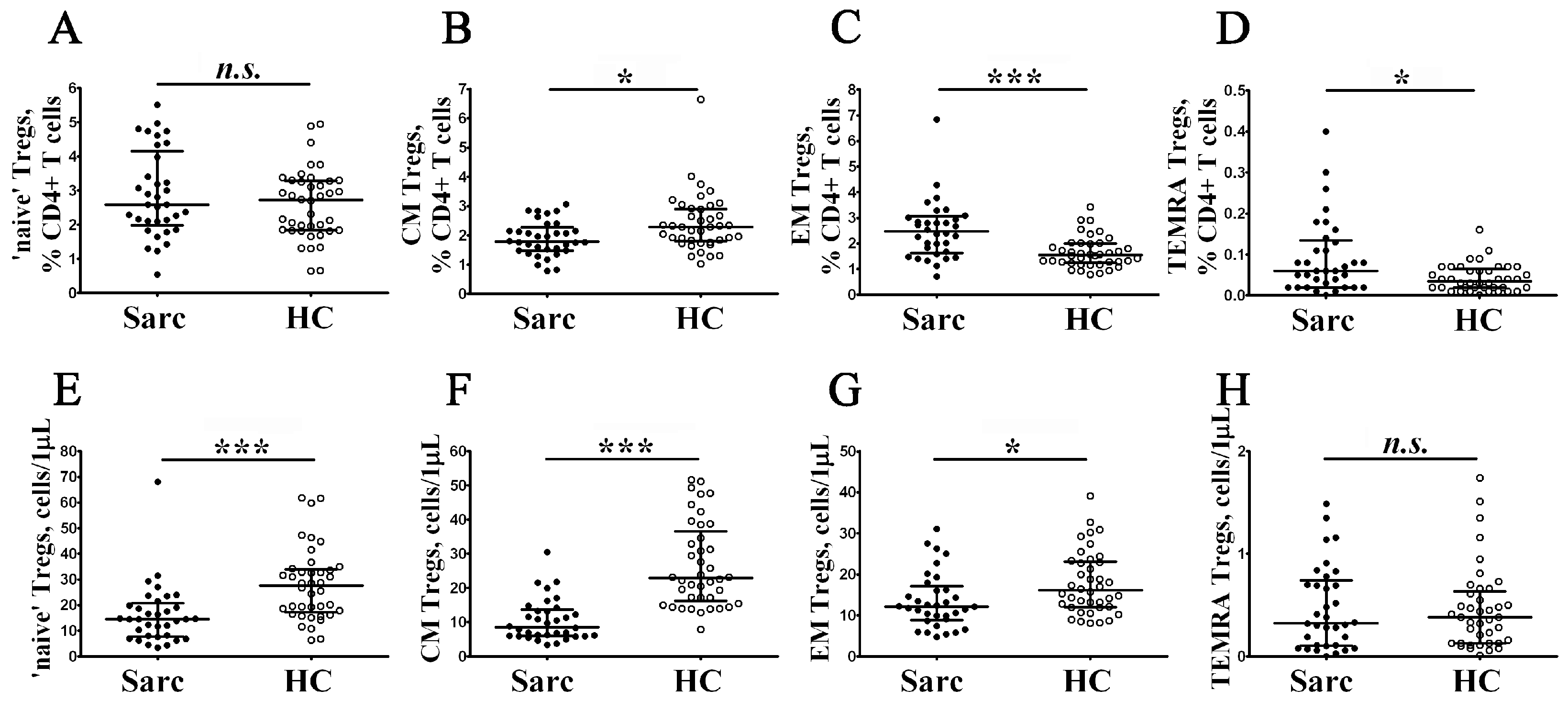
3.1. Alterations in Circulating T Cell Subsets in Patients with Sarcoidosis
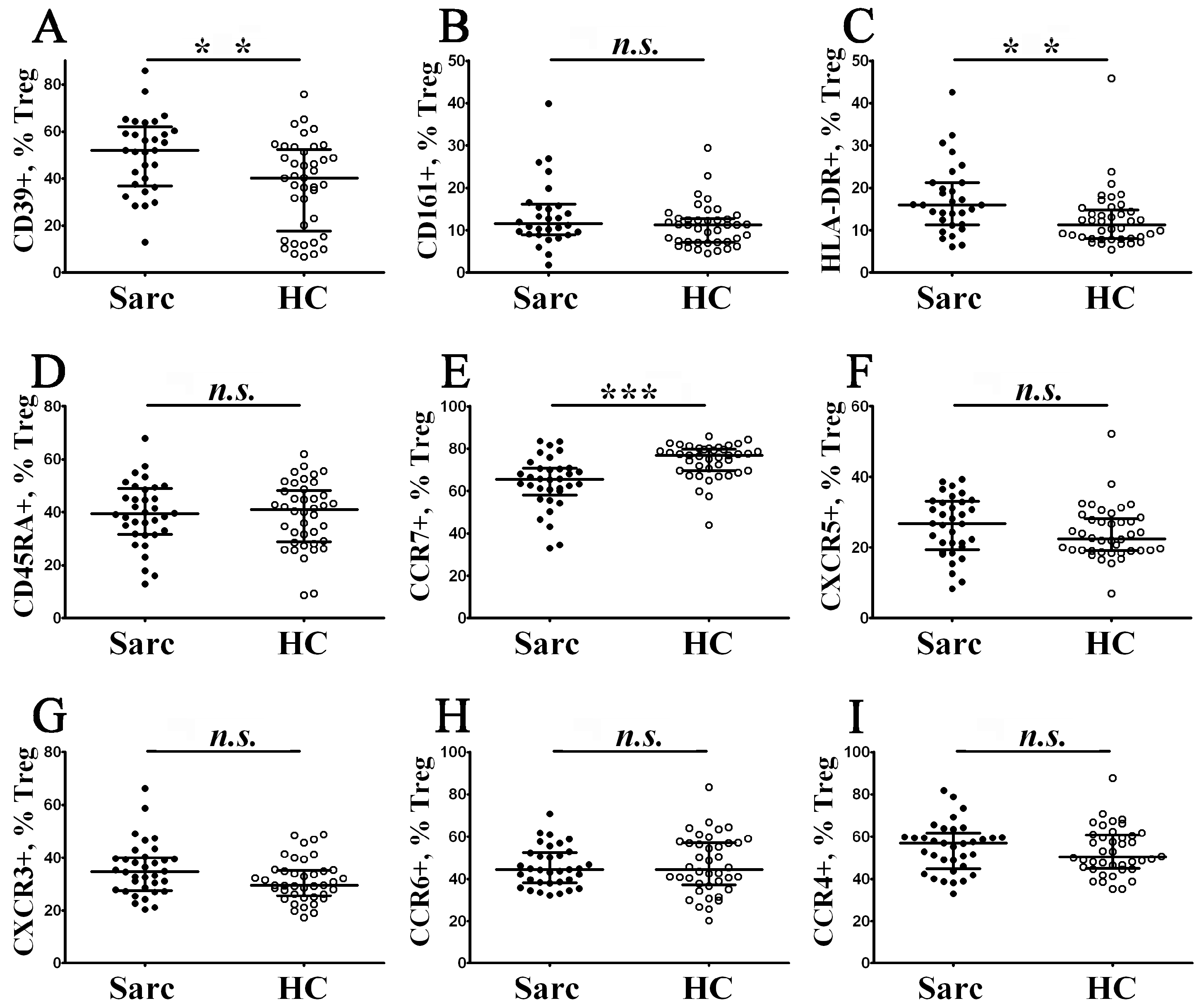
3.2. Altered Phenotype of Peripheral Blood Regulatory T Cells in Patients with Sarcoidosis
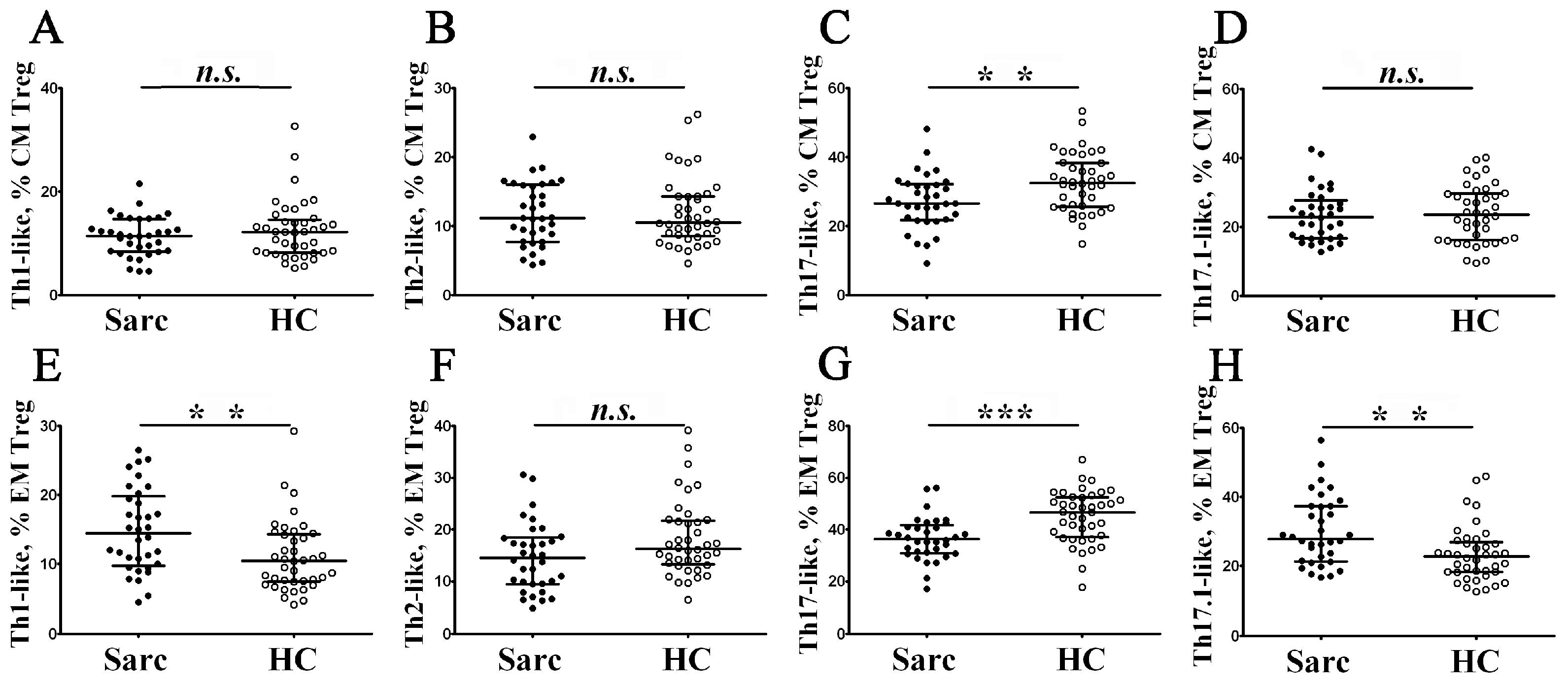
3.3. Identification of Phenotypically Distinct Maturation Subsets of Tregs in Sarcoidosis Patients
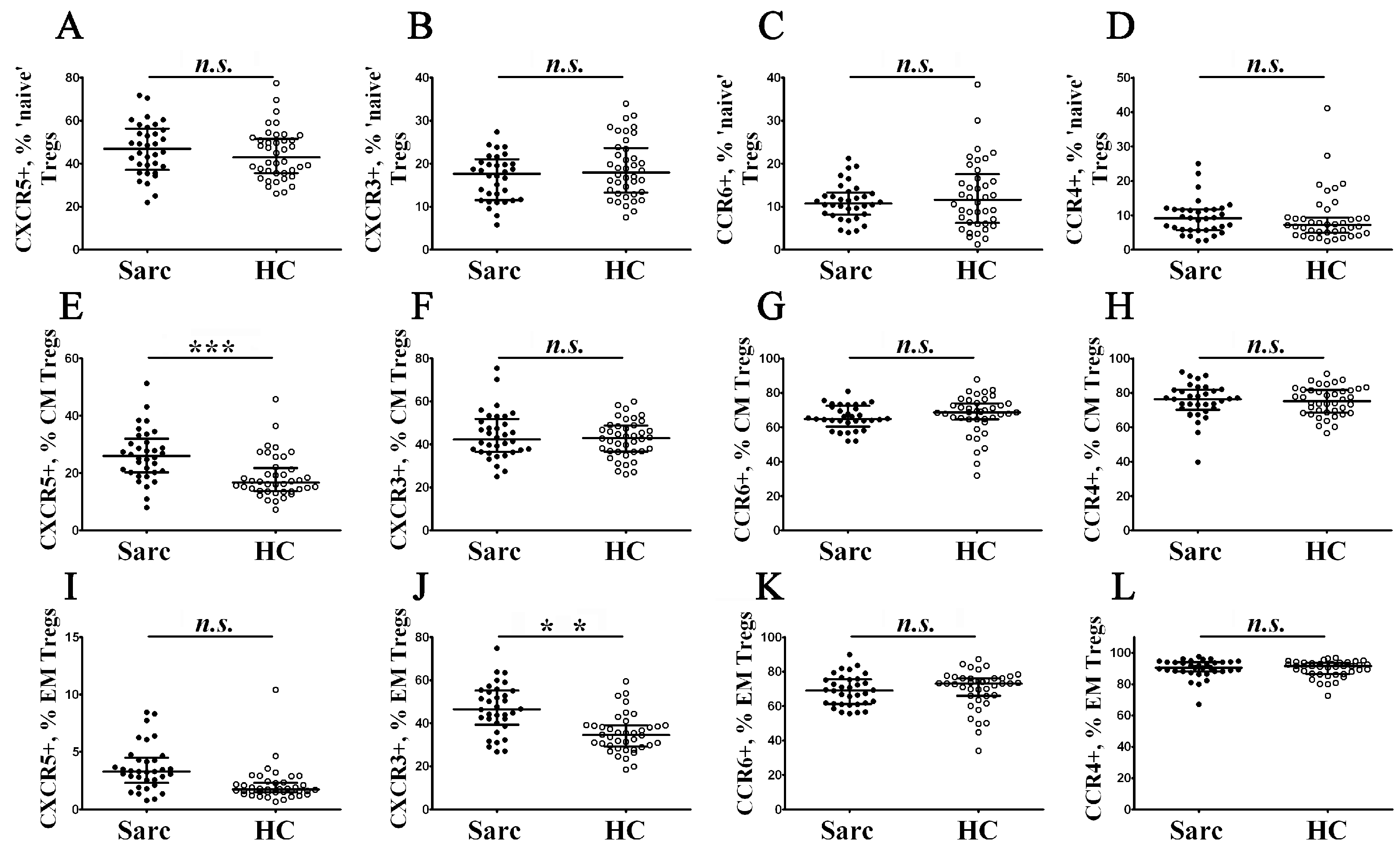
3.4. Alterations of Chemokine Receptor Expression on Treg Maturation Subsets from Patients with Sarcoidosis
3.5. Imbalance in Main Peripheral Blood Treg Cell Subsets during Sarcoidosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Starshinova, A.A.; Malkova, A.M.; Basantsova, N.Y.; Zinchenko, Y.S.; Kudryavtsev, I.V.; Ershov, G.A.; Soprun, L.A.; Mayevskaya, V.A.; Churilov, L.P.; Yablonskiy, P.K. Sarcoidosis as an Autoimmune Disease. Front. Immunol. 2020, 10, 2933. [Google Scholar] [CrossRef]
- Dua, A.; Manadan, A. Heerfordt’s Syndrome, or Uveoparotid Fever. N. Engl. J. Med. 2013, 369, 458. [Google Scholar] [CrossRef]
- Badar, F.; Azfar, S.F.; Ahmad, I.; Yasmeen, S.; Kirmani, S. Diagnostic difficulties in differentiating sarcoidosis from tuberculosis. Oman Med. J. 2011, 26, 210–211. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; Du Bois, R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. Statement on sarcoidosis. Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [Google Scholar] [CrossRef]
- Sellares, J.; Strambu, I.; Crouser, E.D.; Freudenberg, M.A.; Gulati, M.; Hart, S.; Herzog, E.; Kolb, M.; Weichhart, T.; Drake, W.P.; et al. New advances in the development of sarcoidosis models: A synopsis of a symposium sponsored by the Foundation for Sarcoidosis Research, Sarcoidosis. Vasc. Diffus. Lung Dis. 2018, 35, 2–4. [Google Scholar] [CrossRef]
- Moller, D.R.; Rybicki, B.A.; Hamzeh, N.Y.; Montgomery, C.G.; Chen, E.S.; Drake, W.; Fontenot, A. Genetic, immunologic, and environmental basis of sarcoidosis. Ann. Am. Thorac. Soc. 2017, 14, S429–S436. [Google Scholar] [CrossRef]
- Spagnolo, P.; Rossi, G.; Trisolini, R.; Sverzellati, N.; Baughman, R.P.; Wells, A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Rotsinger, J.E.; Celada, L.J.; Polosukhin, V.V.; Atkinson, J.B.; Drake, W.P. Molecular Analysis of Sarcoidosis Granulomas Reveals Antimicrobial Targets. Am. J. Respir. Cell. Mol. Biol. 2016, 55, 128–134. [Google Scholar] [CrossRef]
- Deigendesch, N.; Stenzel, W. Acute and chronic bacterial infections and sarcoidosis. Handb. Clin. Neurol. 2017, 145, 217–226. [Google Scholar] [CrossRef]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef]
- Salah, S.; Abad, S.; Monnet, D.; Brézin, A.P. Sarcoidosis. J. Fr. Ophtalmol. 2018, 41, e451–e467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.R.; Arce, S. Key Players and Biomarkers of the Adaptive Immune System in the Pathogenesis of Sarcoidosis. Int. J. Mol. Sci. 2020, 21, 7398. [Google Scholar] [CrossRef] [PubMed]
- Broos, C.E.; van Nimwegen, M.; Hoogsteden, H.C.; Hendriks, R.W.; Kool, M.; Van den Blink, B. Granuloma formation in pulmonary sarcoidosis. Front. Immunol. 2013, 4, 437. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-cells in autoimmune diseases: Challenges, controversies and—Yet—unanswered questions. Autoimmun. Rev. 2015, 14, 105–116. [Google Scholar] [CrossRef]
- Hatzioannou, A.; Boumpas, A.; Papadopoulou, M.; Papafragkos, I.; Varveri, A.; Alissafi, T.; Verginis, P. Regulatory T Cells in Autoimmunity and Cancer: A Duplicitous Lifestyle. Front. Immunol. 2021, 12, 731947. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Alape, D.; de Lima, A.; Ascanio, J.; Majid, A.; Gangadharan, S.P. Regulatory T Cells in Respiratory Health and Diseases. Pulm. Med. 2019, 2019, 1907807. [Google Scholar] [CrossRef]
- Zhang, H.; Costabel, U.; Dai, H. The Role of Diverse Immune Cells in Sarcoidosis. Front. Immunol. 2021, 12, 788502. [Google Scholar] [CrossRef]
- Miyara, M.; Amoura, Z.; Parizot, C.; Badoual, C.; Dorgham, K.; Trad, S.; Kambouchner, M.; Valeyre, D.; Chapelon-Abric, C.; Debré, P.; et al. The immune paradox of sarcoidosis and regulatory T cells. J. Exp. Med. 2006, 203, 359–370. [Google Scholar] [CrossRef]
- Kachamakova-Trojanowska, N.; Jazwa-Kusior, A.; Szade, K.; Kasper, L.; Soja, J.; Andrychiewicz, A.; Jakiela, B.; Plutecka, H.; Sanak, M.; Jozkowicz, A.; et al. Molecular profiling of regulatory T cells in pulmonary sarcoidosis. J. Autoimmun. 2018, 94, 56–69. [Google Scholar] [CrossRef]
- Idali, F.; Wahlström, J.; Müller-Suur, C.; Eklund, A.; Grunewald, J. Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin. Exp. Immunol. 2008, 152, 127–137. [Google Scholar] [CrossRef]
- Huang, H.; Lu, Z.; Jiang, C.; Liu, J.; Wang, Y.; Xu, Z. Imbalance between Th17 and regulatory T-Cells in sarcoidosis. Int. J. Mol. Sci. 2013, 14, 21463–21473. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Lozanski, G.; Fox, C.C.; Hauswirth, D.W.; Raveendran, R.; Julian, M.W. The CD4+ lymphopenic sarcoidosis phenotype is highly responsive to anti-tumor necrosis factor-{alpha} therapy. Chest 2010, 137, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015, 36, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Dhaeze, T.; Stinissen, P.; Liston, A.; Hellings, N. Humoral autoimmunity: A failure of regulatory T cells? Autoimmun. Rev. 2015, 14, 735–741. [Google Scholar] [CrossRef]
- Shan, Y.; Qi, C.; Zhao, J.; Liu, Y.; Gao, H.; Zhao, D.; Ding, F.; Wang, J.; Jiang, Y. Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clin. Exp. Pharmacol. Physiol. 2015, 42, 154–161. [Google Scholar] [CrossRef]
- Pandya, J.M.; Lundell, A.; Hallström, M.; Andersson, K.; Nordström, I.; Rudin, A. Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J. Leukoc. Biol. 2015, 100, 823–833. [Google Scholar] [CrossRef]
- Dhaeze, T.; Peelen, E.; Hombrouck, A.; Peeters, L.; Van Wijmeersch, B.; Lemkens, N.; Lemkens, P.; Somers, V.; Lucas, S.; Broux, B.; et al. Circulating follicular regulatory T cells are defective in multiple sclerosis. J. Immunol. 2015, 195, 832–840. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Tu, B.; Hong, W.G.; Zhang, Z.; Chen, W.W.; Zhao, M. Alterations of the frequency and functions of follicular regulatory T cells and related mechanisms in HIV infection. J. Infect. 2020, 81, 776–784. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, R.; Freyn, A.W.; Wang, J.; Strohmeier, S.; Lederer, K.; Locci, M.; Zhao, H.; Angeletti, D.; O’Connor, K.C.; et al. CD4+ follicular regulatory T cells optimize the influenza virus-specific B cell response. J. Exp. Med. 2021, 218, e20200547. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; Bois, R.D.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffus. Lung Dis. 1999, 16, 149–173. [Google Scholar]
- Kudryavtsev, I.; Serebriakova, M.; Starshinova, A.; Zinchenko, Y.; Basantsova, N.; Malkova, A.; Soprun, L.; Churilov, L.P.; Toubi, E.; Yablonskiy, P.; et al. Imbalance in B cell and T Follicular Helper Cell Subsets in Pulmonary Sarcoidosis. Sci. Rep. 2020, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Sweiss, N.J.; Salloum, R.; Gandhi, S.; Alegre, M.L.; Sawaqed, R.; Badaracco, M.; Pursell, K.; Pitrak, D.; Baughman, R.P.; Moller, D.R.; et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS ONE 2010, 5, e9088. [Google Scholar] [CrossRef]
- Vagts, C.; Ascoli, C.; Fraidenburg, D.R.; Baughman, R.P.; Huang, Y.; Edafetanure-Ibeh, R.; Ahmed, S.; Levin, B.; Lu, Y.; Perkins, D.L.; et al. Unsupervised Clustering Reveals Sarcoidosis Phenotypes Marked by a Reduction in Lymphocytes Relate to Increased Inflammatory Activity on 18FDG-PET/CT. Front. Med. 2021, 8, 595077. [Google Scholar] [CrossRef] [PubMed]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef]
- Pesenacker, A.M.; Bending, D.; Ursu, S.; Wu, Q.; Nistala, K.; Wedderburn, L.R. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 2013, 121, 2647–2658. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Wolf, E.; Hafler, D.A. MHC class II expression identifies functionally distinct human regulatory T cells. J. Immunol. 2006, 176, 4622–4631. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Lord, G.M.; Rao, R.M.; Choe, H.; Sullivan, B.M.; Lichtman, A.H.; Luscinskas, F.W.; Glimcher, L.H. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood 2005, 106, 3432–3439. [Google Scholar] [CrossRef]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Yoshitomi, H.; Hashimoto, M.; Maeda, S.; Teradaira, S.; Sugimoto, N.; Yamaguchi, T.; Nomura, T.; Ito, H.; Nakamura, T.; et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007, 204, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Akesson, K.; Tompa, A.; Rydén, A.; Faresjö, M. Low expression of CD39(+) /CD45RA(+) on regulatory T cells (Treg) cells in type 1 diabetic children in contrast to high expression of CD101(+) /CD129(+) on Treg cells in children with coeliac disease. Clin. Exp. Immunol. 2015, 180, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.Y.; Kong, H.; Zhao, Y.L.; Peng, L.S.; Chen, W.; Zhang, J.Y.; Cheng, P.; Wang, T.T.; Lv, Y.P.; Teng, Y.S.; et al. Increased tumor-infiltrating CD45RA-CCR7- regulatory T-cell subset with immunosuppressive properties foster gastric cancer progress. Cell Death Dis. 2017, 8, e3002. [Google Scholar] [CrossRef] [PubMed]
- Duhen, T.; Duhen, R.; Lanzavecchia, A.; Sallusto, F.; Campbell, D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012, 119, 4430–4440, Erratum in Blood 2012, 120, 4447. [Google Scholar] [CrossRef] [PubMed]
- Halim, L.; Romano, M.; McGregor, R.; Correa, I.; Pavlidis, P.; Grageda, N.; Hoong, S.J.; Yuksel, M.; Jassem, W.; Hannen, R.F.; et al. An Atlas of Human Regulatory T Helper-like Cells Reveals Features of Th2-like Tregs that Support a Tumorigenic Environment. Cell. Rep. 2017, 20, 757–770. [Google Scholar] [CrossRef]
- Oliver, S.J.; Kikuchi, T.; Krueger, J.G.; Kaplan, G. Thalidomide induces granuloma differentiation in sarcoid skin lesions associated with disease improvement. Clin. Immunol. 2002, 102, 225–236. [Google Scholar] [CrossRef]
- Carvajal Alegria, G.; Gazeau, P.; Hillion, S.; Daïen, C.I.; Cornec, D.Y. Could Lymphocyte Profiling be Useful to Diagnose Systemic Autoimmune Diseases? Clin. Rev. Allergy Immunol. 2017, 53, 219–236. [Google Scholar] [CrossRef]
- Nakken, B.; Munthe, L.A.; Konttinen, Y.T.; Sandberg, A.K.; Szekanecz, Z.; Alex, P.; Szodoray, P. B-cells and their targeting in rheumatoid arthritis—Current concepts and future perspectives. Autoimmun. Rev. 2011, 11, 28–34. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Kurata, I.; Matsumoto, I.; Sumida, T. T follicular helper cell subsets: A potential key player in autoimmunity. Immunol. Med. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Tangye, S.G.; Ma, C.S.; Brink, R.; Deenick, E.K. The good, the bad and the ugly-T FH cells in human health and disease. Nat. Rev. Immunol. 2013, 13, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.L.; Weiss, S.A.; Pauken, K.E.; Sen, D.R.; Sharpe, A.H. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021, 22, 809–819. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, S.; Khan, S.; Surendra Lele, S.; Prabhakar, B.S. Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cell. Immunol. 2019, 339, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tøndell, A.; Moen, T.; Børset, M.; Salvesen, Ø.; Rø, A.D.; Sue-Chu, M. Bronchoalveolar lavage fluid IFN-γ+ Th17 cells and regulatory T cells in pulmonary sarcoidosis. Mediat. Inflamm. 2014, 2014, 438070. [Google Scholar] [CrossRef]
- Jia, X.; Zhai, T.; Wang, B.; Yao, Q.; Li, Q.; Mu, K.; Zhang, J.A. Decreased number and impaired function of type 1 regulatory T cells in autoimmune diseases. J. Cell. Physiol. 2019, 234, 12442–12450. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, L.; Wang, Y.; Aimurola, H.; Zhao, Y.; Li, S.; Xu, Z. The Circulating Treg/Th17 Cell Ratio Is Correlated with Relapse and Treatment Response in Pulmonary Sarcoidosis Patients after Corticosteroid Withdrawal. PLoS ONE 2016, 11, e0148207. [Google Scholar] [CrossRef]
- Mroz, R.M.; Korniluk, M.; Stasiak-Barmuta, A.; Ossolinska, M.; Chyczewska, E. Increased levels of Treg cells in bronchoalveolar lavage fluid and induced sputum of patients Garman with active pulmonary sarcoidosis. Eur. J. Med. Res. 2009, 14 (Suppl. 4), 165–169. [Google Scholar] [CrossRef]
- Oswald-Richter, K.A.; Richmond, B.W.; Braun, N.A.; Isom, J.; Abraham, S.; Taylor, T.R.; Drake, J.M.; Culver, D.A.; Wilkes, D.S.; Drake, W.P. Reversal of global CD4+ subset dysfunction is associated with spontaneous clinical resolution of pulmonary sarcoidosis. J. Immunol. 2013, 190, 5446–5453. [Google Scholar] [CrossRef]
- Broos, C.E.; van Nimwegen, M.; Kleinjan, A.; ten Berge, B.; Muskens, F.; in’t Veen, J.C.; Annema, J.T.; Lambrecht, B.N.; Hoogsteden, H.C.; Hendriks, R.W.; et al. Impaired survival of regulatory T cells in pulmonary sarcoidosis. Respir. Res. 2015, 16, 108. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell. Rep. 2020, 30, 1039–1051.e5. [Google Scholar] [CrossRef] [PubMed]
- Kataria, Y.P.; LoBuglio, A.F.; Bromberg, P.A.; Hurtubise, P.E. Sarcoid lymphocytes: B- and T-cell quantitation. Ann. N. Y. Acad. Sci. 1976, 278, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Li, X.; Song, W.; Li, D.; Yu, D.; Zeng, X.; Li, M.; Leng, X.; Li, X. CD4(+)CD25 (+)CD127 (low/-) T cells: A more specific Treg population in human peripheral blood. Inflammation 2012, 35, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Esendagli, D.; Koksal, D.; Emri, S. Recovery of pulmonary and skin lesions of sarcoidosis after thymectomy. Acta Clin. Belg. 2016, 71, 441–443. [Google Scholar] [CrossRef]
- Garman, L.; Pelikan, R.C.; Rasmussen, A.; Lareau, C.A.; Savoy, K.A.; Deshmukh, U.S.; Bagavant, H.; Levin, A.M.; Daouk, S.; Drake, W.P.; et al. Single Cell Transcriptomics Implicate Novel Monocyte and T Cell Immune Dysregulation in Sarcoidosis. Front. Immunol. 2020, 11, 567342. [Google Scholar] [CrossRef]
- Weeratunga, P.; Moller, D.R.; Ho, L.P. Immune mechanisms in fibrotic pulmonary sarcoidosis. Eur. Respir. Rev. 2022, 31, 220178. [Google Scholar] [CrossRef]
- Zhao, M.; Tian, C.; Cong, S.; Di, X.; Wang, K. From COVID-19 to Sarcoidosis: How Similar Are These Two Diseases? Front. Immunol. 2022, 13, 877303. [Google Scholar] [CrossRef]
- Ozdemir, O.K.; Celik, G.; Dalva, K.; Ulger, F.; Elhan, A.; Beksac, M. High CD95 expression of BAL lymphocytes predicts chronic course in patients with sarcoidosis. Respirology 2007, 12, 869–873. [Google Scholar] [CrossRef]
- Hartigan-O’Connor, D.J.; Poon, C.; Sinclair, E.; McCune, J.M. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J. Immunol. Methods 2007, 319, 41–52. [Google Scholar] [CrossRef]
- Nishioka, Y.; Manabe, K.; Kishi, J.; Wang, W.; Inayama, M.; Azuma, M.; Sone, S. CXCL9 and 11 in patients with pulmonary sarcoidosis: A role of alveolar macrophages. Clin. Exp. Immunol. 2007, 149, 317–326. [Google Scholar] [CrossRef]
- Arger, N.K.; Ho, M.; Woodruff, P.G.; Koth, L.L. Serum CXCL11 correlates with pulmonary outcomes and disease burden in sarcoidosis. Respir. Med. 2019, 152, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, N.M.; Baranova, O.P.; Kudryavtsev, I.V.; Arsentieva, N.A.; Liubimova, N.E.; Ses’, T.P.; Ilkovich, M.M.; Totolian, A.A. Features of cytokine profile in patients with sarcoidosis. Med. Immunol. 2020, 22, 993–1002. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Gangi, S.; Cavallaro, D.; Bergantini, L.; Mezzasalma, F.; Cattelan, S.; Baglioni, S.; Abbritti, M.; Cameli, P.; Bargagli, E. CD103 Expression on Regulatory and Follicular T Cells in Lymph Nodes, Bronchoalveolar Lavage Fluid and Peripheral Blood of Sarcoidosis Patients. Life 2022, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Höllbacher, B.; Duhen, T.; Motley, S.; Klicznik, M.M.; Gratz, I.K.; Campbell, D.J. Transcriptomic Profiling of Human Effector and Regulatory T Cell Subsets Identifies Predictive Population Signatures. Immunohorizons 2020, 4, 585–596. [Google Scholar] [CrossRef]
- Mohr, A.; Atif, M.; Balderas, R.; Gorochov, G.; Miyara, M. The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases. Clin. Exp. Immunol. 2019, 197, 24–35. [Google Scholar] [CrossRef]
- Prior, C.; Haslam, P.L. Increased levels of serum interferon-gamma in pulmonary sarcoidosis and relationship with response to corticosteroid therapy. Am. Rev. Respir. Dis. 1991, 143, 53–60. [Google Scholar] [CrossRef]
- Möllers, M.; Aries, S.P.; Drömann, D.; Mascher, B.; Braun, J.; Dalhoff, K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax 2001, 56, 487–493. [Google Scholar] [CrossRef]
- Koth, L.L.; Solberg, O.D.; Peng, J.C.; Bhakta, N.R.; Nguyen, C.P.; Woodruff, P.G. Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1153–1163. [Google Scholar] [CrossRef]
- Ramstein, J.; Broos, C.E.; Simpson, L.J.; Ansel, K.M.; Sun, S.A.; Ho, M.E.; Woodruff, P.G.; Bhakta, N.R.; Christian, L.; Nguyen, C.P.; et al. IFN-γ-Producing T-Helper 17.1 Cells Are Increased in Sarcoidosis and Are More Prevalent than T-Helper Type 1 Cells. Am. J. Respir. Crit. Care Med. 2016, 193, 1281–1291. [Google Scholar] [CrossRef]
- Kaiser, Y.; Lepzien, R.; Kullberg, S.; Eklund, A.; Smed-Sörensen, A.; Grunewald, J. Expanded lung T-bet+RORγT+ CD4+ T-cells in sarcoidosis patients with a favorable disease phenotype. Eur. Respir. J. 2016, 48, 484–494. [Google Scholar] [CrossRef]
- McClymont, S.A.; Putnam, A.L.; Lee, M.R.; Esensten, J.H.; Liu, W.; Hulme, M.A.; Hoffmüller, U.; Baron, U.; Olek, S.; Bluestone, J.A.; et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 2011, 186, 3918–3926. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, H.; Åkerman, L.; Pihl, M.; Ludvigsson, J.; Casas, R. Mass Cytometry Identifies Distinct Subsets of Regulatory T Cells and Natural Killer Cells Associated with High Risk for Type 1 Diabetes. Front. Immunol. 2019, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.D.; Lam, A.D.; Chiu, C.; Tran, G.T.; Hall, B.M.; Hodgkinson, S.J. Multiple sclerosis patients have reduced resting and increased activated CD4+CD25+FOXP3+T regulatory cells. Sci. Rep. 2021, 11, 10476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, H.; Xue, M.; Lin, L.; Wang, J.; Cai, G.; Shen, Q. Reprograming of peripheral Foxp3+ regulatory T cell towards Th17-like cell in patients with active systemic lupus erythematosus. Clin. Immunol. 2019, 209, 108267. [Google Scholar] [CrossRef]
- Chen, J.; Ye, H.; Xiao, W.; Mao, Y.; Ai, S.; Chen, R.; Lian, X.; Shi, L.; Wang, X.; Bi, S.; et al. Increased Dysfunctional and Plastic Regulatory T Cells in Idiopathic Orbital Inflammation. Front. Immunol. 2021, 12, 634847. [Google Scholar] [CrossRef]
- Wollenberg, I.; Agua-Doce, A.; Hernández, A.; Almeida, C.; Oliveira, V.G.; Faro, J.; Graca, L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 2011, 187, 4553–4560. [Google Scholar] [CrossRef]
- Maceiras, A.R.; Almeida, S.C.P.; Mariotti-Ferrandiz, E.; Chaara, W.; Jebbawi, F.; Six, A.; Hori, S.; Klatzmann, D.; Faro, J. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat. Commun. 2017, 8, 15067. [Google Scholar] [CrossRef]
- Aloulou, M.; Carr, E.J.; Gador, M.; Bignon, A.; Liblau, R.S.; Fazilleau, N.; Linterman, M.A. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat. Commun. 2016, 7, 10579. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Bergantini, L.; Cameli, P.; Mezzasalma, F.; Refini, R.M.; Pieroni, M.; Sestini, P.; Bargagli, E. Adaptive immune system in pulmonary sarcoidosis-Comparison of peripheral and alveolar biomarkers. Clin. Exp. Immunol. 2021, 205, 406–416. [Google Scholar] [CrossRef]
- Ly, N.T.M.; Ueda-Hayakawa, I.; Nguyen, C.T.H.; Okamoto, H. Exploring the imbalance of circulating follicular helper CD4+ T cells in sarcoidosis patients. J. Dermatol. Sci. 2020, 97, 216–224. [Google Scholar] [CrossRef]
- Liu, C.; Wang, D.; Song, Y.; Lu, S.; Zhao, J.; Wang, H. Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int. Immunopharmacol. 2018, 56, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.R.; Agua-Doce, A.; Maceiras, A.R.; Pierson, W.; Ribeiro, F.; Romão, V.C.; Pires, A.R.; da Silva, S.L.; Fonseca, J.E.; Sousa, A.E.; et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci. Immunol. 2017, 11, pii:eaan1487. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.R.; Romão, V.C.; Agua-Doce, A.; Santos, M.; López-Presa, D.; Ferreira, A.C.; Fonseca, J.E.; Graca, L. The Ratio of Blood T Follicular Regulatory Cells to T Follicular Helper Cells Marks Ectopic Lymphoid Structure Formation While Activated Follicular Helper T Cells Indicate Disease Activity in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2018, 70, 774–784. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, B.; Lu, J.; Zhang, J.; Yang, H.; Li, J. Imbalance of circulating CD4+CXCR5+FOXP3+ Tfr-like cells and CD4+CXCR5+FOXP3− Tfh-like cells in myasthenia gravis. Neurosci. Lett. 2016, 630, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Seddiki, N.; Santner-Nanan, B.; Martinson, J.; Zaunders, J.; Sasson, S.; Landay, A.; Solomon, M.; Selby, W.; Alexander, S.I.; Nanan, R.; et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006, 203, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.F.; Duggan, P.J.; Ponchel, F.; Matarese, G.; Lombardi, G.; Edwards, A.D.; Isaacs, J.D.; Lechler, R.I. Human CD4(+)CD25(+) cells: A naturally occurring population of regulatory T cells. Blood 2001, 98, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Brown, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25 high regulatory cells in human peripheral blood. J. Immunol. 2001, 167, 1245–1253. [Google Scholar] [CrossRef]
- Roncador, G.; Brown, P.J.; Maestre, L.; Hue, S.; Martínez-Torrecuadrada, J.L.; Ling, K.L.; Pratap, S.; Toms, C.; Fox, B.C.; Cerundolo, V.; et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 2005, 35, 1681–1691. [Google Scholar] [CrossRef]
- Garcia Santana, C.A.; Tung, J.W.; Gulnik, S. Human treg cells are characterized by low/negative CD6 expression. Cytom. A 2014, 85, 901–908. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Kretz, C.C.; Krammer, P.H.; Kuhn, A. CD127(low/-) and FoxP3(+) expression levels characterize different regulatory T-cell populations in human peripheral blood. J. Investig. Dermatol. 2010, 130, 492–499. [Google Scholar] [CrossRef] [PubMed]
| Characteristics: | Pulmonary Sarcoidosis, n (%), (n = 34) |
|---|---|
| Complaints: | |
| Clinical manifestations | 28 (82.3) |
| Weakness | 20 (58.8) |
| Cough | 18 (52.9) |
| Dyspnea | 10 (29.4) |
| Fever (37–37.9 °C) | 10 (29.4) |
| Chest pain | 3 (8.8) |
| Erithema nodosum | 6 (17.6) |
| Arthralgia | 7 (20.5) |
| Weight loss | 5 (14.7) |
| X-ray findings: | |
| Enlarged lymph nodes | 34 (100.0) |
| Foci in the lung tissue | 31 (91.1) |
| Infiltation | 3 (8.8) |
| Fibrosis | 1 (2.9) |
| Ground-glass opacity | 6 (17.6) |
| Medical history: | |
| Smoking | 14 (41.1) |
| Family history of autoimmune diseases | 4 (11.7) |
| Allergy | 13 (38.2) |
| Results of TB testing: | |
| TB.T-SPOT test (positive) | 0 |
| Mantoux test with 2 TE (positive > 5 mm) | 4 (11.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtsev, I.; Zinchenko, Y.; Starshinova, A.; Serebriakova, M.; Malkova, A.; Akisheva, T.; Kudlay, D.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Circulating Regulatory T Cell Subsets in Patients with Sarcoidosis. Diagnostics 2023, 13, 1378. https://doi.org/10.3390/diagnostics13081378
Kudryavtsev I, Zinchenko Y, Starshinova A, Serebriakova M, Malkova A, Akisheva T, Kudlay D, Glushkova A, Yablonskiy P, Shoenfeld Y. Circulating Regulatory T Cell Subsets in Patients with Sarcoidosis. Diagnostics. 2023; 13(8):1378. https://doi.org/10.3390/diagnostics13081378
Chicago/Turabian StyleKudryavtsev, Igor, Yulia Zinchenko, Anna Starshinova, Maria Serebriakova, Anna Malkova, Tatiana Akisheva, Dmitriy Kudlay, Anzhela Glushkova, Piotr Yablonskiy, and Yehuda Shoenfeld. 2023. "Circulating Regulatory T Cell Subsets in Patients with Sarcoidosis" Diagnostics 13, no. 8: 1378. https://doi.org/10.3390/diagnostics13081378
APA StyleKudryavtsev, I., Zinchenko, Y., Starshinova, A., Serebriakova, M., Malkova, A., Akisheva, T., Kudlay, D., Glushkova, A., Yablonskiy, P., & Shoenfeld, Y. (2023). Circulating Regulatory T Cell Subsets in Patients with Sarcoidosis. Diagnostics, 13(8), 1378. https://doi.org/10.3390/diagnostics13081378









