The Association of Circulating L-Carnitine, γ-Butyrobetaine and Trimethylamine N-Oxide Levels with Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Overall Design
2.2. Study Population
2.3. Measurement of Levels of L-Carnitine, GBB and TMAO by UPLC/MS/MS
2.4. Statistical Analysis
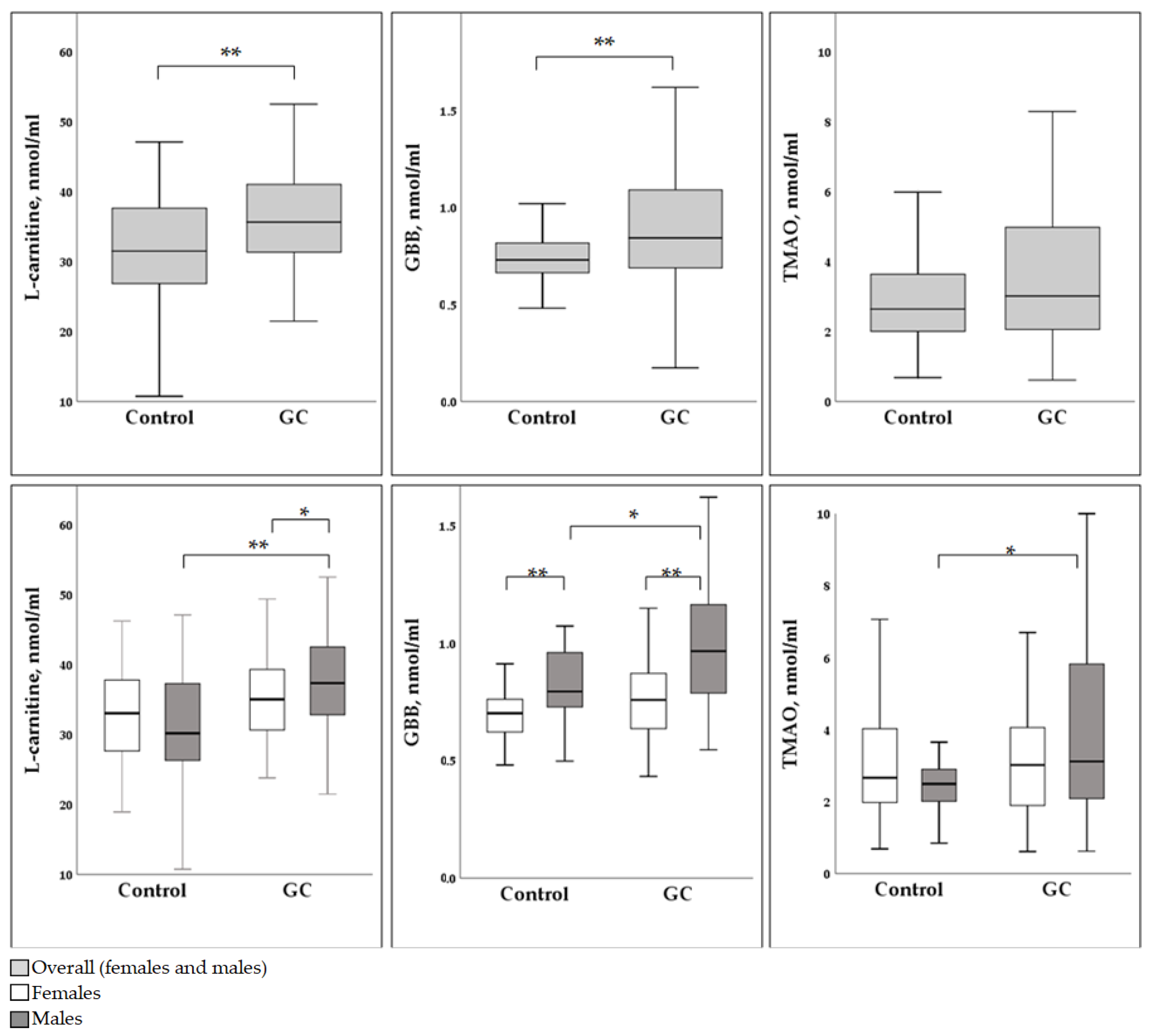
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Park, J.Y.; Murillo, R.; Liepniece-Karele, I.; Isajevs, S.; Kikuste, I.; Rudzite, D.; Krike, P.; Parshutin, S.; Polaka, I.; et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: The GISTAR study. BMJ Open 2017, 7, e016999. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Howes, N.; Stephens, N.; Pritchard, D.M. Review: Gastric malignancies–Clinical aspects & prevention. Microbiota Health Dis. 2022, 4, e715. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Hewetson, J.T. Report LXXXVIII: The Bacteriology of Certain Parts of the Human Alimentary Canal and of the Inflammatory Processes Arising Therefrom. Br. Med. J. 1904, 2, 1457–1460. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Tan, X.Y.; Li, Q.J.; Liao, G.C.; Fang, A.P.; Zhang, D.M.; Chen, P.Y.; Wang, X.Y.; Luo, Y.; Long, J.A.; et al. Trimethylamine N-oxide, a gut microbiota-dependent metabolite of choline, is positively associated with the risk of primary liver cancer: A case-control study. Nutr. Metab. 2018, 15, 81. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Xu, H.; Li, S.; Lau, H.C.; Chen, Q.; Zhang, B.; Zhao, L.; Chen, H.; Sung, J.J.; et al. Microbial Community Heterogeneity Within Colorectal Neoplasia and its Correlation With Colorectal Carcinogenesis. Gastroenterology 2021, 160, 2395–2408. [Google Scholar] [CrossRef]
- Jaensch, R.; Jonaitis, P.; Kupcinskas, J. Microbiota in colorectal cancer: Advances in 2022. Microb. Health Dis. 2022, 4, e778. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, T.; Perez Perez, G.I. Microbiota and gastric diseases in 2022. Microb. Health Dis. 2022, 4, e746. [Google Scholar] [CrossRef]
- Chen, C.C.; Liou, J.M.; Lee, Y.C.; Hong, T.C.; El-Omar, E.M.; Wu, M.S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021, 13, 1909459. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.I.; Pan, C.Y.; Kao, J.Y.; Tsay, F.W.; Peng, N.J.; Kao, S.S.; Wang, H.M.; Tsai, T.J.; Wu, D.C.; Chen, C.L.; et al. Helicobacter pylori eradication with bismuth quadruple therapy leads to dysbiosis of gut microbiota with an increased relative abundance of Proteobacteria and decreased relative abundances of Bacteroidetes and Actinobacteria. Helicobacter 2018, 23, e12498. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide With Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- van der Laan, T.; Kloots, T.; Beekman, M.; Kindt, A.; Dubbelman, A.C.; Harms, A.; van Duijn, C.M.; Slagboom, P.E.; Hankemeier, T. Fast LC-ESI-MS/MS analysis and influence of sampling conditions for gut metabolites in plasma and serum. Sci. Rep. 2019, 9, 12370. [Google Scholar] [CrossRef]
- Treacy, E.P.; Akerman, B.R.; Chow, L.M.; Youil, R.; Bibeau, C.; Lin, J.; Bruce, A.G.; Knight, M.; Danks, D.M.; Cashman, J.R.; et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum. Mol. Genet. 1998, 7, 839–845. [Google Scholar] [CrossRef]
- Abbasi, J. TMAO and Heart Disease: The New Red Meat Risk? JAMA 2019, 321, 2149–2151. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Papandreou, C.; More, M.; Bellamine, A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health-Cause or Effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef]
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.Y.; et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Yuan, C.; Zhang, Y.; Wang, W.; Hu, S.; Liu, L.; Wang, Y. Preoperative serum TMAO level is a new prognostic marker for colorectal cancer. Biomark. Med. 2017, 11, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Guertin, K.A.; Li, X.S.; Graubard, B.I.; Albanes, D.; Weinstein, S.J.; Goedert, J.J.; Wang, Z.; Hazen, S.L.; Sinha, R. Serum Trimethylamine N-oxide, Carnitine, Choline, and Betaine in Relation to Colorectal Cancer Risk in the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Luu, H.N.; Butler, L.M.; Midttun, O.; Ulvik, A.; Wang, R.; Jin, A.; Gao, Y.T.; Tan, Y.; Ueland, P.M.; et al. A prospective evaluation of serum methionine-related metabolites in relation to pancreatic cancer risk in two prospective cohort studies. Int. J. Cancer 2020, 147, 1917–1927. [Google Scholar] [CrossRef]
- Brierley, J.D. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; p. 272. [Google Scholar]
- Rugge, M.; Meggio, A.; Pennelli, G.; Piscioli, F.; Giacomelli, L.; De Pretis, G.; Graham, D.Y. Gastritis staging in clinical practice: The OLGA staging system. Gut 2007, 56, 631–636. [Google Scholar] [CrossRef]
- Dambrova, M.; Skapare-Makarova, E.; Konrade, I.; Pugovics, O.; Grinberga, S.; Tirzite, D.; Petrovska, R.; Kalvins, I.; Liepins, E. Meldonium decreases the diet-increased plasma levels of trimethylamine N-oxide, a metabolite associated with atherosclerosis. J. Clin. Pharmacol. 2013, 53, 1095–1098. [Google Scholar] [CrossRef]
- Liepinsh, E.; Konrade, I.; Skapare, E.; Pugovics, O.; Grinberga, S.; Kuka, J.; Kalvinsh, I.; Dambrova, M. Mildronate treatment alters gamma-butyrobetaine and l-carnitine concentrations in healthy volunteers. J. Pharm. Pharmacol. 2011, 63, 1195–1201. [Google Scholar] [CrossRef]
- Grinberga, S.; Dambrova, M.; Latkovskis, G.; Strele, I.; Konrade, I.; Hartmane, D.; Sevostjanovs, E.; Liepinsh, E.; Pugovics, O. Determination of trimethylamine-N-oxide in combination with L-carnitine and gamma-butyrobetaine in human plasma by UPLC/MS/MS. Biomed. Chromatogr. BMC 2015, 29, 1670–1674. [Google Scholar] [CrossRef]
- Cederblad, G. Plasma carnitine and body composition. Clin. Chim. Acta 1976, 67, 207–212. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Wang, X.; Wei, Y.; Li, S.; Song, Y.; Chen, P.; Liu, L.; Wang, B.; Shi, H. The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension. Nutrients 2022, 14, 4999. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Zhang, C.; Zhu, C.; Tao, G.; Zhao, L.; Tang, S.; Shu, Z.; Cai, J.; Dai, S.; et al. Red and processed meat intake is associated with higher gastric cancer risk: A meta-analysis of epidemiological observational studies. PLoS ONE 2013, 8, e70955. [Google Scholar] [CrossRef]
- Wu, W.K.; Chen, C.C.; Liu, P.Y.; Panyod, S.; Liao, B.Y.; Chen, P.C.; Kao, H.L.; Kuo, H.C.; Kuo, C.H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef]
- Lin, T.J.; Tang, S.C.; Liao, P.Y.; Dongoran, R.A.; Yang, J.H.; Liu, C.H. A comparison of L-carnitine and several cardiovascular-related biomarkers between healthy vegetarians and omnivores. Nutrition 2019, 66, 29–37. [Google Scholar] [CrossRef]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Console, L.; Scalise, M.; Mazza, T.; Pochini, L.; Galluccio, M.; Giangregorio, N.; Tonazzi, A.; Indiveri, C. Carnitine Traffic in Cells. Link With Cancer. Front. Cell Dev. Biol. 2020, 8, 583850. [Google Scholar] [CrossRef]
- Chen, T.; Wu, G.; Hu, H.; Wu, C. Enhanced fatty acid oxidation mediated by CPT1C promotes gastric cancer progression. J. Gastrointest. Oncol. 2020, 11, 695–707. [Google Scholar] [CrossRef]
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018, 9, 228. [Google Scholar] [CrossRef]
- Pal, S.; Sharma, A.; Mathew, S.P.; Jaganathan, B.G. Targeting cancer-specific metabolic pathways for developing novel cancer therapeutics. Front. Immunol. 2022, 13, 955476. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Liao, X.; Hu, H.; Chen, L.; Meng, L.; Gao, W.; Li, Q. Carnitine Palmitoyltransferase System: A New Target for Anti-Inflammatory and Anticancer Therapy? Front. Pharmacol. 2021, 12, 760581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, G.; Zhu, H.; Yang, F.; Yang, S.; Vuong, A.M.; Li, J.; Zhu, D.; Sun, Y.; Tao, W. Circulating Carnitine Levels and Breast Cancer: A Matched Retrospective Case-Control Study. Front. Oncol. 2022, 12, 891619. [Google Scholar] [CrossRef] [PubMed]
- Rabito, E.I.; Leme, I.A.; Demenice, R.; Portari, G.V.; Jordao, A.A., Jr.; dos Santos, J.S.; Marchini, J.S. Lower carnitine plasma values from malnutrition cancer patients. J. Gastrointest. Cancer 2013, 44, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Hawke, P.; Tokuda, S.; Toda, T.; Higashizono, K.; Nagai, E.; Watanabe, M.; Nakatani, E.; Kanemoto, H.; Oba, N. Serum carnitine as a biomarker of sarcopenia and nutritional status in preoperative gastrointestinal cancer patients. J. Cachexia Sarcopenia Muscle 2022, 13, 287–295. [Google Scholar] [CrossRef]
- Kawai, A.; Matsumoto, H.; Endou, Y.; Honda, Y.; Kubota, H.; Higashida, M.; Hirai, T. Repeated Combined Chemotherapy with Cisplatin Lowers Carnitine Levels in Gastric Cancer Patients. Ann. Nutr. Metab. 2017, 71, 261–265. [Google Scholar] [CrossRef]
- Mirji, G.; Worth, A.; Bhat, S.A.; El Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.Y.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, J.; Lian, J.; Yang, X.; Zhou, J. Gut Metabolite Trimethylamine-N-Oxide in Atherosclerosis: From Mechanism to Therapy. Front. Cardiovasc. Med. 2021, 8, 723886. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Q.; Li, L. A genome-wide systems analysis reveals strong link between colorectal cancer and trimethylamine N-oxide (TMAO), a gut microbial metabolite of dietary meat and fat. BMC Genom. 2015, 16 (Suppl. S7), S4. [Google Scholar] [CrossRef]
- Miller, C.A.; Corbin, K.D.; da Costa, K.A.; Zhang, S.; Zhao, X.; Galanko, J.A.; Blevins, T.; Bennett, B.J.; O’Connor, A.; Zeisel, S.H. Effect of egg ingestion on trimethylamine-N-oxide production in humans: A randomized, controlled, dose-response study. Am. J. Clin. Nutr. 2014, 100, 778–786. [Google Scholar] [CrossRef]
- Canyelles, M.; Plaza, M.; Rotllan, N.; Llobet, D.; Julve, J.; Mojal, S.; Diaz-Ricart, M.; Soria, J.M.; Escola-Gil, J.C.; Tondo, M.; et al. TMAO and Gut Microbial-Derived Metabolites TML and gammaBB Are Not Associated with Thrombotic Risk in Patients with Venous Thromboembolism. J. Clin. Med. 2022, 11, 1425. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedo, L.; Farras, M.; Escola-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Khodabakhshi, A.; Monfared, V.; Arabpour, Z.; Vahid, F.; Hasani, M. Association between Levels of Trimethylamine N-Oxide and Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 75, 402–414. [Google Scholar] [CrossRef]
- Haro, C.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- Chan, C.W.H.; Law, B.M.H.; Waye, M.M.Y.; Chan, J.Y.W.; So, W.K.W.; Chow, K.M. Trimethylamine-N-oxide as One Hypothetical Link for the Relationship between Intestinal Microbiota and Cancer—Where We Are and Where Shall We Go? J. Cancer 2019, 10, 5874–5882. [Google Scholar] [CrossRef]
- Ufnal, M.; Pham, K. The gut-blood barrier permeability—A new marker in cardiovascular and metabolic diseases? Med. Hypotheses 2017, 98, 35–37. [Google Scholar] [CrossRef]
- Drapala, A.; Szudzik, M.; Chabowski, D.; Mogilnicka, I.; Jaworska, K.; Kraszewska, K.; Samborowska, E.; Ufnal, M. Heart Failure Disturbs Gut-Blood Barrier and Increases Plasma Trimethylamine, a Toxic Bacterial Metabolite. Int. J. Mol. Sci. 2020, 21, 6161. [Google Scholar] [CrossRef]
| Control (N = 83) | GC Cases (N = 105) | |||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Number, N (%) | 52 (62.6) | 31 (37.4) | 45 (42.9) | 60 (57.1) |
| Age, mean ± SD, years | 66.83 ± 9.89 | 61.23 ± 13.81 | 64.13 ± 11.12 | 64.3 ± 10.30 |
| BMI, mean ± SD, kg/m2 | 30.37 ± 5.35 | 26.11 ± 4.94 | 26.09 ± 5.50 | 27.02 ± 4.66 |
| T stage of GC | ||||
| T3, N (%) | N/A | N/A | 13 (28.9) | 21 (35.0) |
| T4, N (%) | N/A | N/A | 32 (71.1) | 39 (65.0) |
| Grade of gastric atrophy | ||||
| OLGA 0 (no atrophy), N (%) | 6 (11.5) | 11 (35.5) | N/A | N/A |
| OLGA 1 (mild atrophy), N (%) | 46 (88.5) | 20 (64.5) | N/A | N/A |
| Females | |||||||
| Controls | GC | ||||||
| R | L-carnitine | GBB | TMAO | R | L-carnitine | GBB | TMAO |
| L-carnitine | 1 | 0.29 * | −0.03 | L-carnitine | 1 | 0.53 ** | 0.36 * |
| GBB | 0.29 * | 1 | 0.13 | GBB | 0.53 ** | 1 | 0.34 * |
| TMAO | −0.03 | 0.13 | 1 | TMAO | 0.36 * | 0.34 * | 1 |
| Males | |||||||
| Controls | GC | ||||||
| R | L-carnitine | GBB | TMAO | R | L-carnitine | GBB | TMAO |
| L-carnitine | 1 | 0.05 | −0.03 | L-carnitine | 1 | 0.47 ** | 0.28 * |
| GBB | 0.05 | 1 | 0.20 | GBB | 0.47 ** | 1 | 0.27 * |
| TMAO | −0.03 | 0.20 | 1 | TMAO | 0.28 * | 0.27 * | 1 |
 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stonāns, I.; Kuzmina, J.; Poļaka, I.; Grīnberga, S.; Sevostjanovs, E.; Liepiņš, E.; Aleksandraviča, I.; Šantare, D.; Kiršners, A.; Škapars, R.; et al. The Association of Circulating L-Carnitine, γ-Butyrobetaine and Trimethylamine N-Oxide Levels with Gastric Cancer. Diagnostics 2023, 13, 1341. https://doi.org/10.3390/diagnostics13071341
Stonāns I, Kuzmina J, Poļaka I, Grīnberga S, Sevostjanovs E, Liepiņš E, Aleksandraviča I, Šantare D, Kiršners A, Škapars R, et al. The Association of Circulating L-Carnitine, γ-Butyrobetaine and Trimethylamine N-Oxide Levels with Gastric Cancer. Diagnostics. 2023; 13(7):1341. https://doi.org/10.3390/diagnostics13071341
Chicago/Turabian StyleStonāns, Ilmārs, Jelizaveta Kuzmina, Inese Poļaka, Solveiga Grīnberga, Eduards Sevostjanovs, Edgars Liepiņš, Ilona Aleksandraviča, Daiga Šantare, Arnis Kiršners, Roberts Škapars, and et al. 2023. "The Association of Circulating L-Carnitine, γ-Butyrobetaine and Trimethylamine N-Oxide Levels with Gastric Cancer" Diagnostics 13, no. 7: 1341. https://doi.org/10.3390/diagnostics13071341
APA StyleStonāns, I., Kuzmina, J., Poļaka, I., Grīnberga, S., Sevostjanovs, E., Liepiņš, E., Aleksandraviča, I., Šantare, D., Kiršners, A., Škapars, R., Pčolkins, A., Tolmanis, I., Sīviņš, A., Leja, M., & Dambrova, M. (2023). The Association of Circulating L-Carnitine, γ-Butyrobetaine and Trimethylamine N-Oxide Levels with Gastric Cancer. Diagnostics, 13(7), 1341. https://doi.org/10.3390/diagnostics13071341








