Possible Points of Ulnar Nerve Entrapment in the Arm and Forearm: An Ultrasound, Anatomical, and Histological Study
Abstract
1. Introduction
2. Material and Methods
2.1. Ultrasound Study
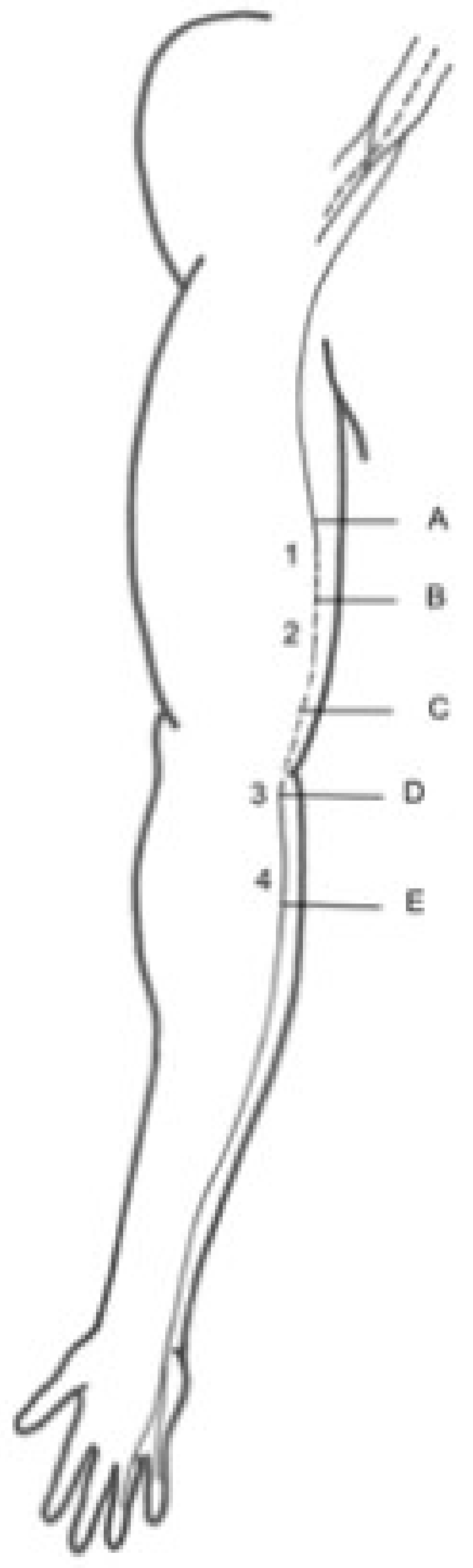
2.2. Anatomical Study
- (A)
- Two centimetres proximal to the IMS, the first anatomical landmark studied by US [1];
- (B)
- Two centimetres distal to the IMS, the first anatomical landmark studied by US [1];
- (C)
- Three centimetres proximal to the medial epicondyle (ME), the second anatomical landmark studied by US [2];
- (D)
- Before the traversing below the hiatus between the two heads of the FCU, the third anatomical landmark studied by US [3];
- (E)
- After the piercing of the intermuscular aponeurosis between the FCU and FDS, the fourth anatomical landmark studied by US [4].
2.3. Histological Study
2.4. Statistical Study
3. Results
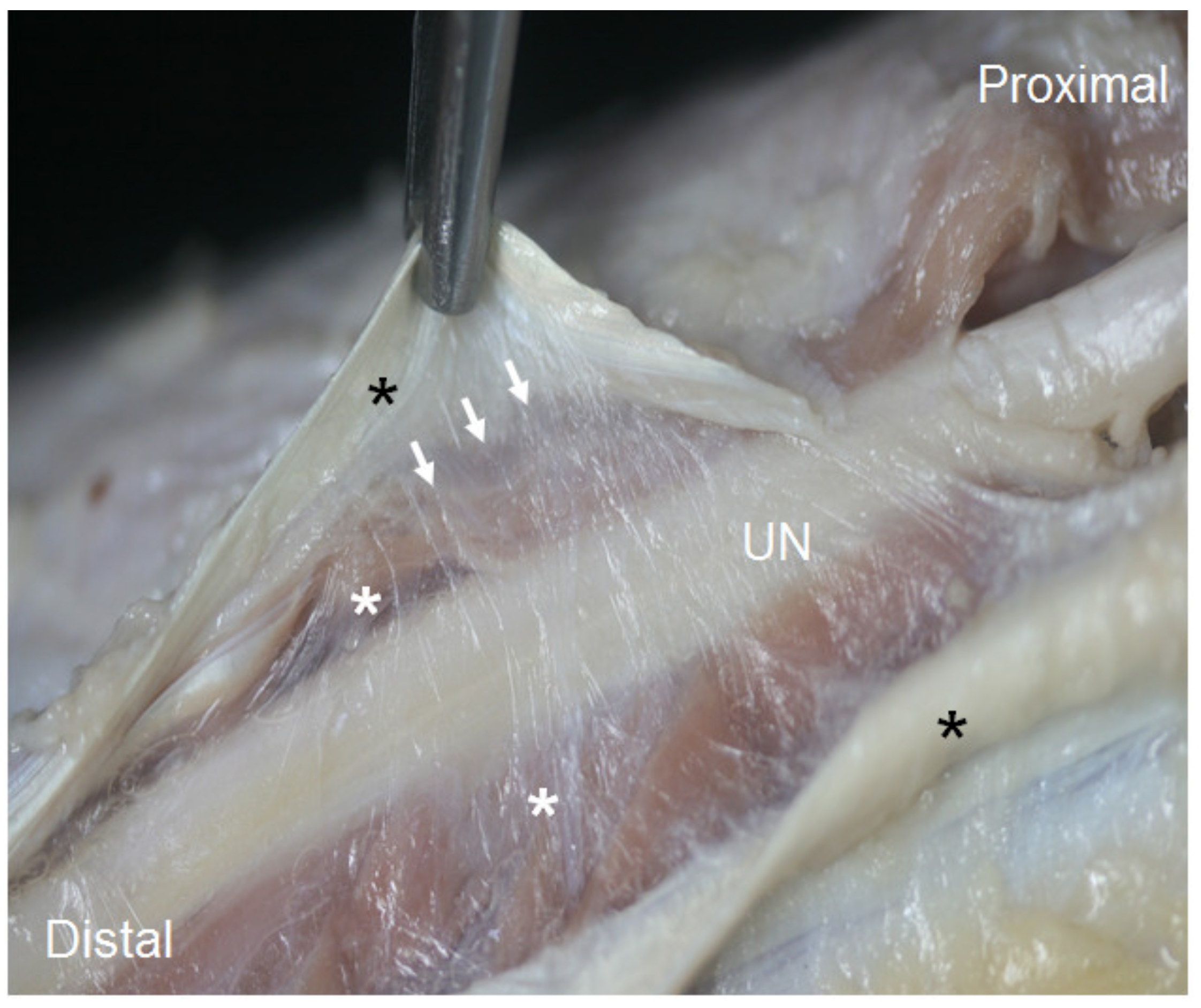
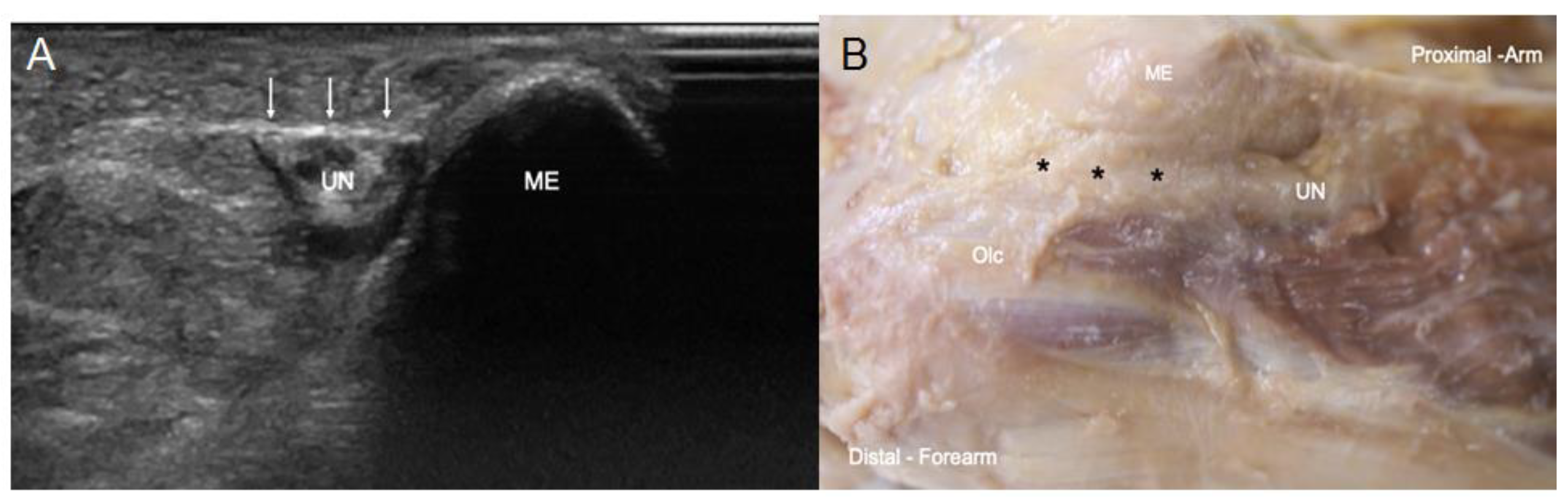
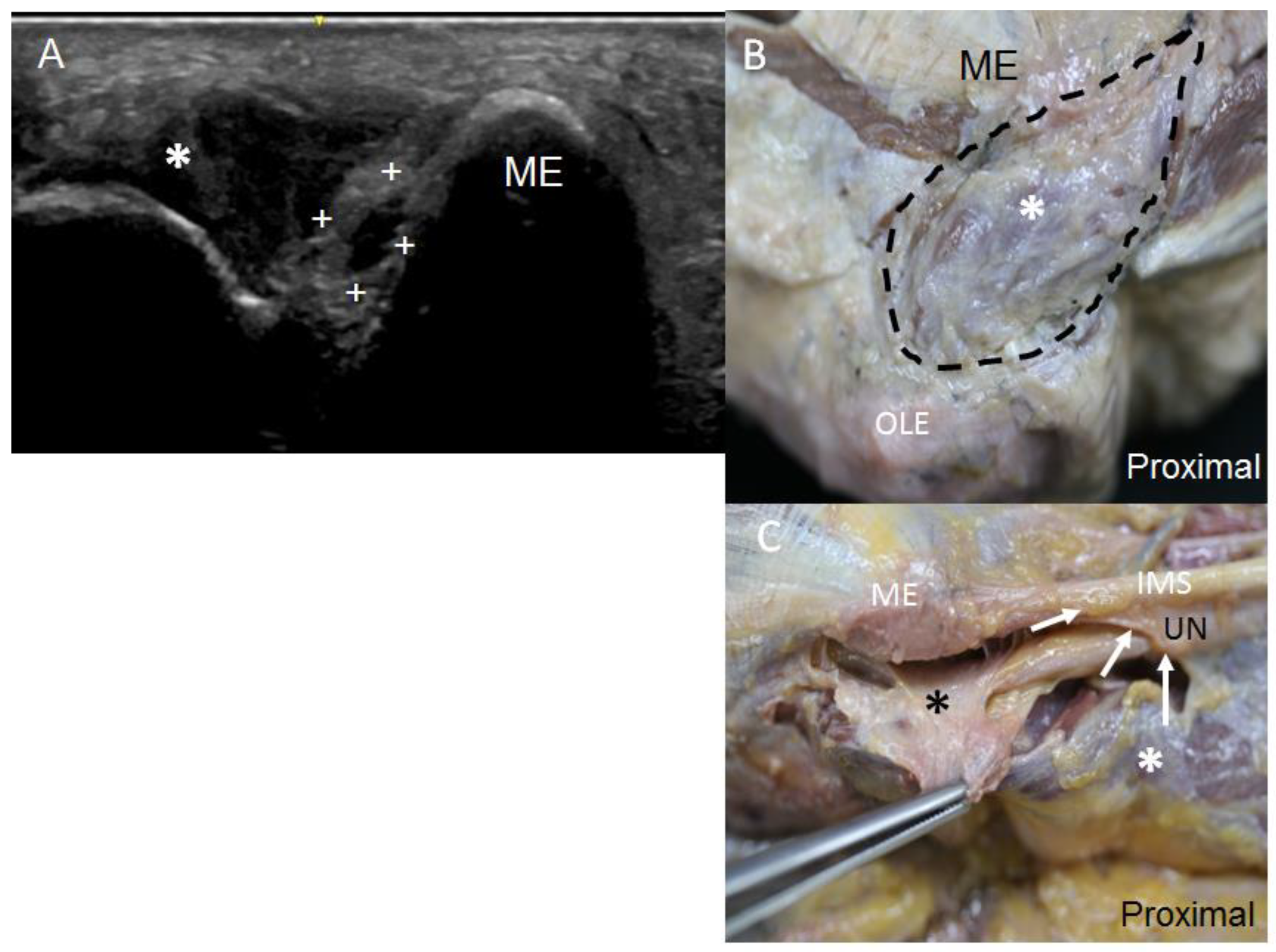
3.1. Ultrasound and Anatomical Studies
3.2. Histological Study
3.3. Statistical Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UN | Ulnar nerve |
| IMS | Intermuscular septum |
| FCU | Flexor carpi ulnaris |
| FDS | Flexor digitorum superficialis |
| FDP | Flexor digitorum profundus |
| ME | Medial epicondyle |
| Olc | Olecranon |
| US | Ultrasound |
References
- Bianchi, S.; Draghi, F.; Beggs, I. Ultrasound of the peripheral nerves. In Clinical Ultrasound, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 2, pp. 1158–1167. [Google Scholar] [CrossRef]
- Miller, T.T.; Reinus, W.R. Nerve entrapment syndromes of the elbow, forearm, and wrist. Am. J. Roentgenol. 2010, 195, 585–594. [Google Scholar] [CrossRef]
- Barrell, K.; Smith, A.G. Peripheral Neuropathy. Med. Clin. N. Am. 2019, 103, 383–397. [Google Scholar] [CrossRef]
- Martinoli, C.; Bianchi, S.; Pugliese, F.; Bacigalupo, L.; Gauglio, C.; Valle, M.; Derchi, L.E. Sonography of entrapment neuropathies in the upper limb (wrist excluded). J. Clin. Ultrasound 2004, 32, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Masih, S.; Patel, D.B.; Matcuk, G.R. MR anatomy and pathology of the ulnar nerve involving the cubital tunnel and Guyon’s canal. Clin. Imaging 2016, 40, 263–274. [Google Scholar] [CrossRef]
- Testut, L.; Latarjet, A. Tratado de Anatomia Humana, 9th ed.; Salvat Editores: Barcelona, Spain, 1990; pp. 1–1153. [Google Scholar]
- Chadwick, N.; Morag, Y.; Smith, B.W.; Yablon, C.; Kim, S.M.; Yang, L.J.S. Imaging appearance following surgical decompression of the ulnar nerve. Br. J. Radiol. 2019, 92, 20180757. [Google Scholar] [CrossRef]
- Michelin, P.; Leleup, G.; Ould-Slimane, M.; Merlet, M.C.; Dubourg, B.; Duparc, F. Ultrasound biomechanical anatomy of the soft structures in relation to the ulnar nerve in the cubital tunnel of the elbow. Surg. Radiol. Anat. 2017, 39, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.; Brigido, M.K.; Antic, M.; Lenchik, L.; Milants, A.; Vereecke, E.; Jager, T.; Shahabpour, M. Ultrasound of the elbow with emphasis on detailed assessment of ligaments, tendons, and nerves. Eur. J. Radiol. 2015, 84, 671–681. [Google Scholar] [CrossRef]
- Granger, A.; Sardi, J.P.; Iwanaga, J.; Wilson, T.J.; Yang, L.; Loukas, M.; Oskouian, R.J.; Tubbs, R.S. Osborne’s Ligament: A Review of its History, Anatomy, and Surgical Importance. Cureus 2017, 9, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martinoli, C.; Bianchi, S.; Gandolfo, N.; Valle, M.; Simonetti, S.; Derchi, L.E. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics 2000, 20, 199–213. [Google Scholar] [CrossRef]
- Mahan, M.A.; Gasco, J.; Mokhtee, D.B.; Brown, J.M. Anatomical considerations of fascial release in ulnar nerve transposition: A concept revisited. J. Neurosurg. 2015, 123, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Gregoli, B.; Bortolotto, C.; Draghi, F. Elbow nerves: Normal sonographic anatomy and identification of the structures potentially associated with nerve compression. A short pictorial-video article. J. Ultrasound 2013, 16, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Della Santa, D.; Glauser, T.; Beaulieu, J.Y.; Van Aaken, J. Sonography of masses of the wrist and hand. Am. J. Roentgenol. 2008, 191, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Joo, B.E.; Kim, K.H.; Park, B.K.; Cha, J.; Kim, D.H. Ultrasonographic and Electrophysiological Evaluation of Ulnar Nerve Instability and Snapping of the Triceps Medial Head in Healthy Subjects. Am. J. Phys. Med. Rehabil. 2017, 96, e141–e146. [Google Scholar] [CrossRef]
- Rao, M.; Somayaji, S.; Raghu, J.; Pamidi, N.; Swamy, R. Bilateral additional slips of triceps brachii forming osseo-musculo-fibrous tunnels for ulnar nerves. Ann. Med. Health Sci. Res. 2013, 3, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Shuttlewood, K.; Beazley, J.; Smith, C.D. Distal triceps injuries (including snapping triceps): A systematic review of the literature. World J. Orthop. 2017, 8, 507–513. [Google Scholar] [CrossRef]
- Fabrizio, P.A.; Clemente, F.R. Variation in the triceps brachii muscle: A fourth muscular head. Clin. Anat. 1997, 10, 259–263. [Google Scholar] [CrossRef]
- Beekman, R.; Visser, L.H. High-resolution sonography of the peripheral nervous system—A review of the literature. Eur. J. Neurol. 2004, 11, 305–314. [Google Scholar] [CrossRef]
- Draghi, F.; Bortolotto, C. Importance of the ultrasound in cubital tunnel syndrome. Surg. Radiol. Anat. 2016, 38, 265–268. [Google Scholar] [CrossRef]
- Caetano, E.B.; Sabongi Neto, J.J.; Vieira, L.A.; Caetano, M.F. The arcade of Struthers: An anatomical study and clinical implications. Rev. Bras. Ortop. 2017, 52, 331–336. [Google Scholar] [CrossRef]
- Siqueira, M.G.; Martins, R.S. The controversial arcade of Struthers. Surg. Neurol. 2005, 64, 21–24. [Google Scholar] [CrossRef]
- Von Schroeder, H.P.; Scheker, L.R. Redefining the “Arcade of Struthers”. J. Hand Surg. Am. 2003, 28, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, L.; Oberlin, C. The internal brachial ligament versus the arcade of Struthers: An anatomical study. Plast. Reconstr. Surg. 2005, 115, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Gray, H. Anatomy of the Human Body, 20th ed.; Lewis, W.H., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1918. [Google Scholar]
- Athlani, L.; Delgove, A.; Dautel, G.; Casoli, V. Anatomy of the ulnar nerve in the posterior compartment of the upper arm: Relationships with the triceps brachii muscle. Morphologie 2020, 104, 85–90. [Google Scholar] [CrossRef]
- Michael, A.E.; Young, P. Is triceps hypertrophy associated with ulnar nerve luxation? Muscle Nerve 2018, 58, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Pripotnev, S.; White, C. Ulnar Nerve Neuropathy Secondary to “Snapping Triceps Syndrome”. Plast. Surg. Case Stud. 2017, 3, 2513826X17716452. [Google Scholar] [CrossRef]
- Won, H.S.; Liu, H.F.; Kim, J.H.; Kwak, D.S.; Chung, I.H.; Kim, I.B. Intermuscular aponeuroses between the flexor muscles of the forearm and their relationships with the ulnar nerve. Surg. Radiol. Anat. 2016, 38, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Miguel, S.; Miguel-Pérez, M.; Navarro, J.; Möller, I.; Pérez-Bellmunt, A.; Agullo, J.L.; Ortiz-Sagristà, J.; Blasi, J.; Martinoli, C. Compartments of the antebrachial fascia of the forearm: Clinically relevant ultrasound, anatomical and histological findings. Surg. Radiol. Anat. 2021, 43, 1569–1579. [Google Scholar] [CrossRef]







| Means | ICC | 95% CI | F Test (p) |
|---|---|---|---|
| Perimeter | 0.950 | 0.898–0.983 | 19.588 (0.000) |
| Diameter | 0.642 | 0.250–0.885 | 2.795 (0.003) |
| Anatomical Landmarks | Perimeter | Diameter | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | IC 95% | Median (IQR) | Shapiro–Wilk (p) | Mean (DE) | IC 95% | Median (IQR) | Shapiro–Wilk (p) | |
| A | 15.54 (1.08) | 14.85–16.22 | 15.65 (1.93) | 0.254 | 4.25 (0.33) | 4.03–4.48 | 4.33 (0.426) | 0.055 |
| B | 14.83 (0.94) | 14.23–15.43 | 15.01 (1.55) | 0.873 | 3.94 (0.66) | 3.50–4.38 | 3.66 (0.96) | 0.473 |
| C | 16.94 (1.42) | 16.04–17.84 | 16.95 (1.79) | 0.481 | 5.26 (1.14) | 4.5–6.02 | 4.93 (1.272) | 0.097 |
| D | 16.73 (0.93) | 16.14–17.32 | 16.73 (0.97) | 0.641 | 5.46 (0.75) | 4.96–5.97 | 5.58 (0.948) | 0.636 |
| E | 13.52 (1) | 12.88–15.16 | 13.27 (1.84) | 0.257 | 3.83 (0.35) | 3.6–4.06 | 3.75 (0.306) | 0.535 |
| Friedman Test | N Total | F | p |
|---|---|---|---|
| Perimeter | 12 | 37.867 | 0.000 |
| Diameter | 11 | 25.673 | 0.000 |
| A | B | C | D | E | Mean (DS) | Difference | IC 95% Difference | t-Statistic (p) | |
|---|---|---|---|---|---|---|---|---|---|
| Perimeter | 15.65 | 15.01 | 16.95 | 16.73 | 13.27 | 15.522 (1.68) | −1.54 | (3.73, 0.64) | −1.627 (0.142) |
| Circumference | 13.60 | 11.50 | 15.49 | 17.53 | 11.78 | 13.98 (1.29) | |||
| Diameter | 4.33 | 3.66 | 4.93 | 5.58 | 3.75 | 4.45 (0.41) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferre-Martinez, A.; Miguel-Pérez, M.; Möller, I.; Ortiz-Miguel, S.; Pérez-Bellmunt, A.; Ruiz, N.; Sanjuan, X.; Agullo, J.; Ortiz-Sagristà, J.; Martinoli, C. Possible Points of Ulnar Nerve Entrapment in the Arm and Forearm: An Ultrasound, Anatomical, and Histological Study. Diagnostics 2023, 13, 1332. https://doi.org/10.3390/diagnostics13071332
Ferre-Martinez A, Miguel-Pérez M, Möller I, Ortiz-Miguel S, Pérez-Bellmunt A, Ruiz N, Sanjuan X, Agullo J, Ortiz-Sagristà J, Martinoli C. Possible Points of Ulnar Nerve Entrapment in the Arm and Forearm: An Ultrasound, Anatomical, and Histological Study. Diagnostics. 2023; 13(7):1332. https://doi.org/10.3390/diagnostics13071332
Chicago/Turabian StyleFerre-Martinez, Andrea, Maribel Miguel-Pérez, Ingrid Möller, Sara Ortiz-Miguel, Albert Pérez-Bellmunt, Núria Ruiz, Xavier Sanjuan, Jose Agullo, Juan Ortiz-Sagristà, and Carlo Martinoli. 2023. "Possible Points of Ulnar Nerve Entrapment in the Arm and Forearm: An Ultrasound, Anatomical, and Histological Study" Diagnostics 13, no. 7: 1332. https://doi.org/10.3390/diagnostics13071332
APA StyleFerre-Martinez, A., Miguel-Pérez, M., Möller, I., Ortiz-Miguel, S., Pérez-Bellmunt, A., Ruiz, N., Sanjuan, X., Agullo, J., Ortiz-Sagristà, J., & Martinoli, C. (2023). Possible Points of Ulnar Nerve Entrapment in the Arm and Forearm: An Ultrasound, Anatomical, and Histological Study. Diagnostics, 13(7), 1332. https://doi.org/10.3390/diagnostics13071332







