National Knowledge-Driven Management of Obstructive Sleep Apnea—The Swedish Approach
Abstract
1. Introduction
Aims
2. Materials and Methods
2.1. The National System for Knowledge-Driven Management within Swedish Healthcare
2.1.1. The Swedish Working Group for OSA in Adults
2.1.2. Evidence Extraction
2.1.3. Delphi Round for National Consensus
2.2. The Swedish Sleep Apnea Registry (SESAR)
3. Results
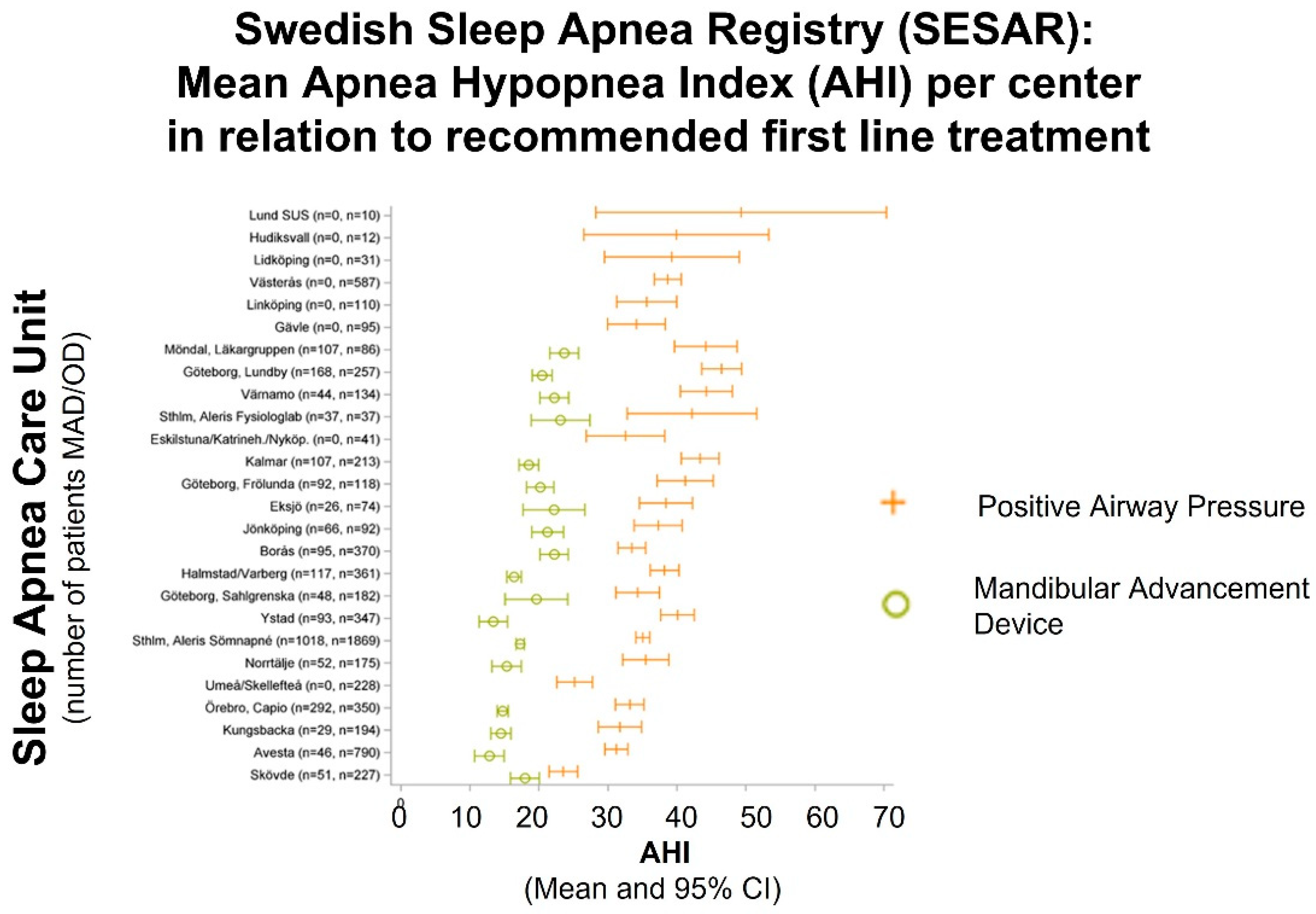
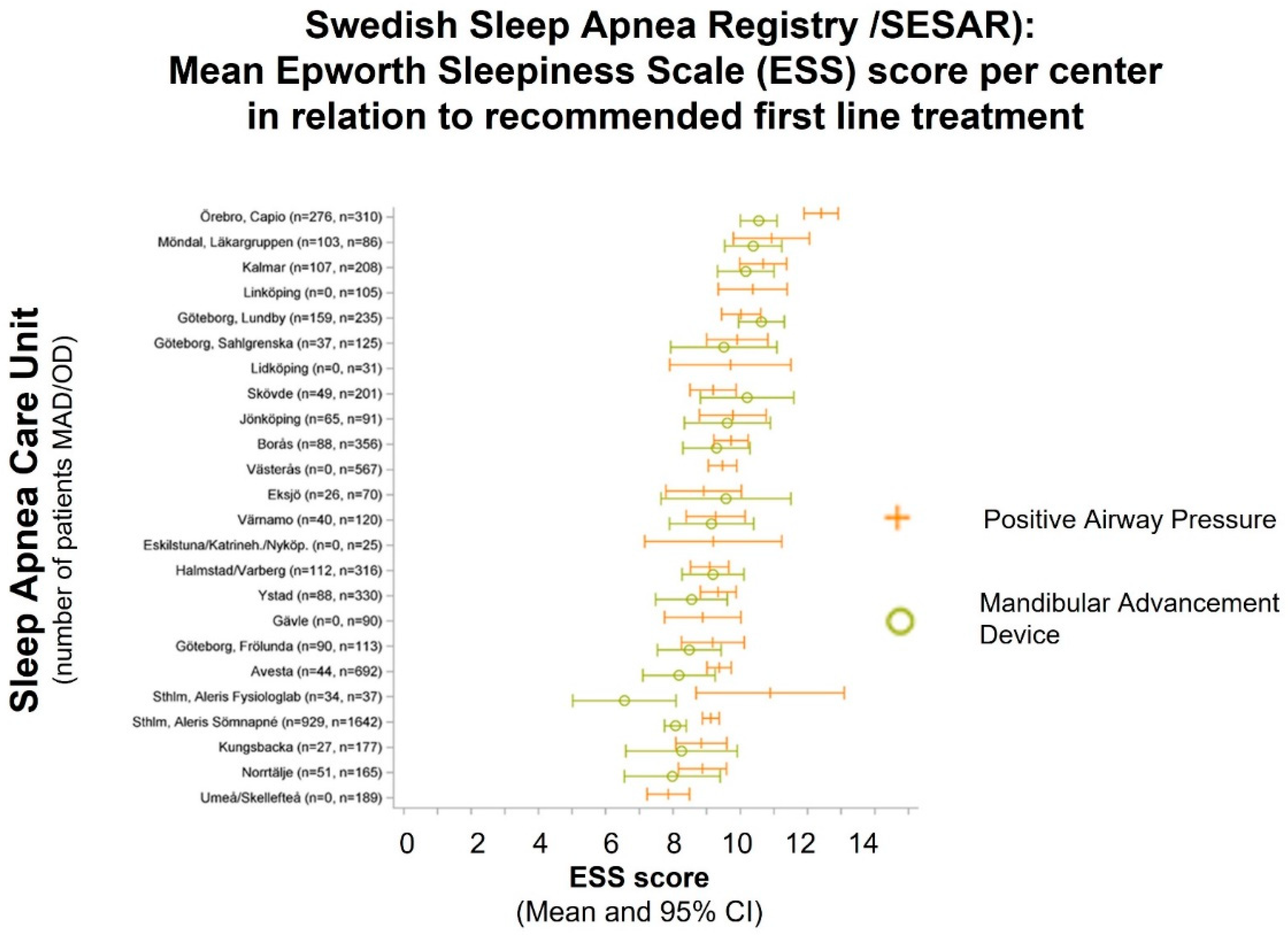
3.1. Current Status on Treatment Prescription in Sweden
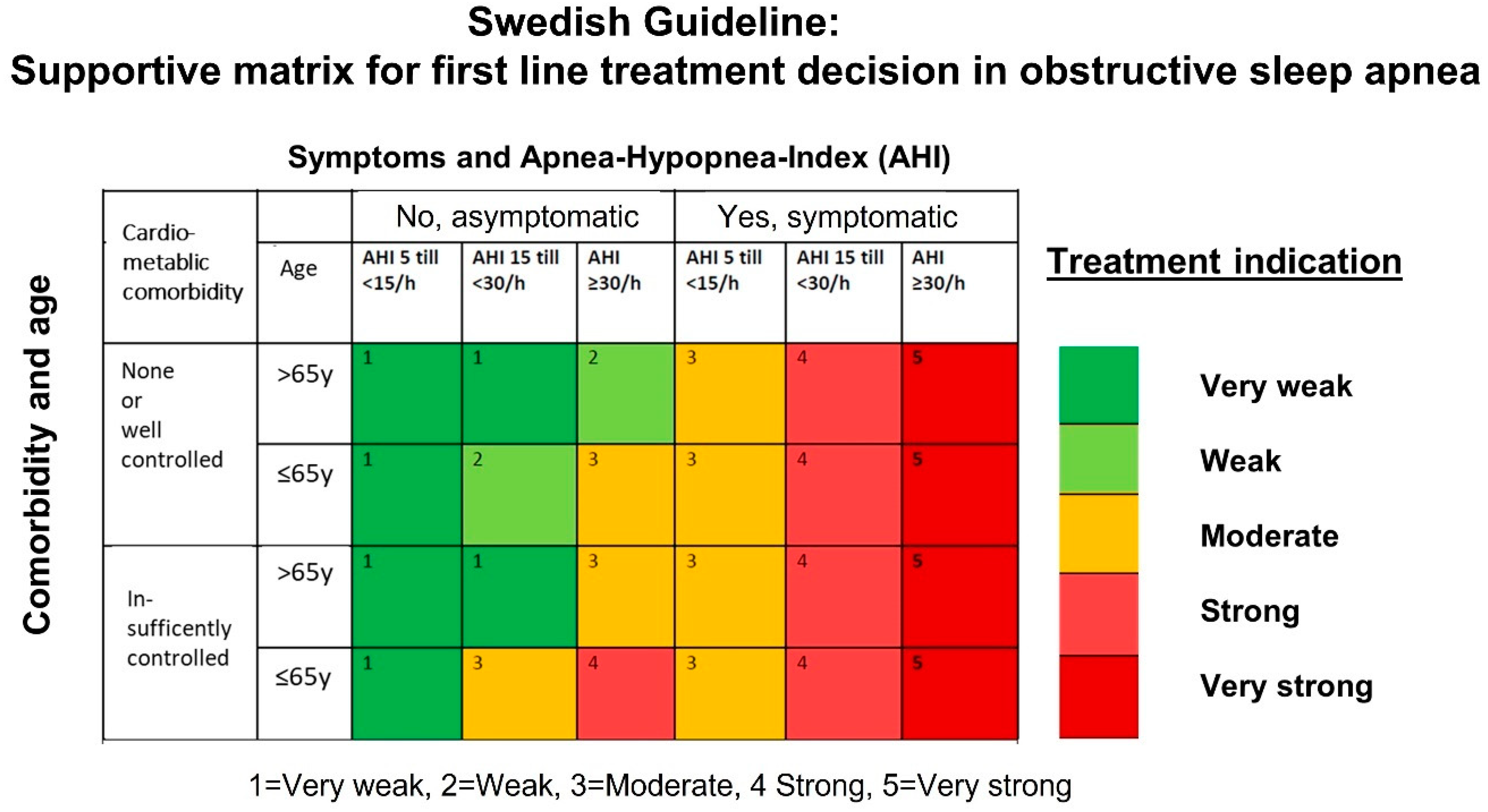
3.2. Nationwide Knowledge Management and Shared Decision Making for Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Prim. 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Randerath, W.; Vignatelli, L.; Ferini-Strambi, L.; Brill, A.K.; Bonsignore, M.R.; Grote, L.; Jennum, P.; Leys, D.; Minnerup, J.; et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur. Respir. J. 2020, 55, 1901104. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Hedner, J.; Häbel, H.; Nerman, O.; Grote, L. Sleep apnea-related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish Traffic Accident Registry data. Sleep 2015, 38, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the rise and fall of the apnea-hypopnea index: A historical review and critical appraisal. J. Sleep Res. 2020, 29, e13066. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Sánchez-de-la-Torre, M.; White, D.P.; Azarbarzin, A. Hypoxic Burden in Obstructive Sleep Apnea: Present and Future. Arch. Bronconeumol. 2023, 59, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, S.; Töyräs, J.; Oksenberg, A.; Korkalainen, H.; Sefa, S.; Kulkas, A.; Leppänen, T. Severity of Desaturations Reflects OSA-Related Daytime Sleepiness Better than AHI. J. Clin. Sleep Med. 2019, 15, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Grote, L.; Sommermeyer, D.; Zou, D.; Eder, D.N.; Hedner, J. Oximeter-based autonomic state indicator algorithm for cardiovascular risk assessment. Chest 2011, 139, 253–259. [Google Scholar] [CrossRef]
- Strassberger, C.; Zou, D.; Penzel, T.; Fietze, I.; Hedner, J.; Ficker, J.H.; Randerath, W.; Sanner, B.; Sommermeyer, D.; Grote, L. Beyond the AHI-pulse wave analysis during sleep for recognition of cardiovascular risk in sleep apnea patients. J. Sleep Res. 2021, 30, e13364. [Google Scholar] [CrossRef]
- Kim, J.T.K.; Seal, K.; Almeida, F.; Ross Gl Messier, M.; Tsoi, B.; Garland, S.; Rader, T.; Duthie, K.; Bond, K.; Mann, J.; et al. Interventions for the Treatment of Obstructive Sleep Apnea in Adults: A Health Technology Assessment; CADTH: Ottawa, ON, Canada, 2017. [Google Scholar]
- Randerath, W.; Bassetti, C.L.; Bonsignore, M.R.; Farre, R.; Ferini-Strambi, L.; Grote, L.; Hedner, J.; Kohler, M.; Martinez-Garcia, M.A.; Mihaicuta, S.; et al. Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur. Respir. J. 2018, 52, 1702616. [Google Scholar] [CrossRef]
- Randerath, W.J.; Herkenrath, S.; Treml, M.; Grote, L.; Hedner, J.; Bonsignore, M.R.; Pépin, J.L.; Ryan, S.; Schiza, S.; Verbraecken, J.; et al. Evaluation of a multicomponent grading system for obstructive sleep apnoea: The Baveno classification. ERJ Open Res. 2021, 7, 00928–02020. [Google Scholar] [CrossRef] [PubMed]
- Hedner, J.; Grote, L.; Friberg, D.; Ejnell, H.; Harlid, R.; Isacsson, G.; Lindberg, E.; Midgren, B.; Puzio, J.; Tegelberg, Å.; et al. National Guidelines for the Evaluation of Suspected Sleep Apnea in Adults. Nationell Kvalitetsregister SESAR/Registercentrum Väst, 2018; pp. 1–39. Available online: https://www.sesar.se/riktlinjer (accessed on 10 January 2023). (In Swedish).
- Grote, L.; Hedner, J. Swedish Sleep Apnea Registry (SESAR) Annual Report 2021. Registercentrum Väst: Göteborg. Available online: https://www.sesar.se/om_sesar/årsrapporter (accessed on 10 January 2023). (In Swedish).
- Grote, L.; Friberg, D.; Tegelberg, Å.; Isaksson, G.; Theorell Haglöw, J.; Grundström, G.; Spaak, J.; Stillberg, G.; Söderberg, K.; Anderberg, C.P.; et al. National Guideline for the Treatment of Obstructive Sleep Apnea in Adults. Nationell Kunskapsstyrning, December 2021. Available online: https://www.sesar.se/riktlinjer (accessed on 10 January 2023). (In Swedish).
- Ulander, M.; Hedner, J.; Stillberg, G.; Sunnergren, O.; Grote, L. Correlates of excessive daytime sleepiness in obstructive sleep apnea: Results from the nationwide SESAR cohort including 34,684 patients. J. Sleep Res. 2022, 31, e13690. [Google Scholar] [CrossRef] [PubMed]
- Grote, L.; Theorell-Haglöw, J.; Ulander, M.; Hedner, J. Prolonged Effects of the COVID-19 Pandemic on Sleep Medicine Services-Longitudinal Data from the Swedish Sleep Apnea Registry. Sleep Med. Clin. 2021, 16, 409–416. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed.; (ICSD-3); American Academy of Sleep Medicine: Darien, IL, USA, 2014; ISBN 978-0991543410. [Google Scholar]
- Ferber, R.; Millman, R.; Coppola, M.; Fleetham, J.; Murray, C.F.; Iber, C.; McCall, W.V.; Nino-Murcia, G.; Pressman, M.; Sanders, M. Portable recording in the assessment of obstructive sleep apnea. ASDA Stand. Pract. Sleep 1994, 17, 378–392. [Google Scholar] [CrossRef]
- Collop, N.A.; Tracy, S.L.; Kapur, V.; Mehra, R.; Kuhlmann, D.; Fleishman, S.A.; Ojile, J.M. Obstructive sleep apnea devices for out-of-center (OOC) testing: Technology evaluation. J. Clin. Sleep. Med. 2011, 7, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Bargiotas, P.; Basoglu, O.K.; Dogas, Z.; Drummond, M.; Fanfulla, F.; Gislason, T.; Gouveris, H.; Grote, L.; et al. Management of obstructive sleep apnea in Europe—A 10-year follow-up. Sleep Med. 2022, 97, 64–72. [Google Scholar] [CrossRef]
- Fietze, I.; Penzel, T.; Alonderis, A.; Barbe, F.; Bonsignore, M.R.; Calverly, P.; De Backer, W.; Diefenbach, K.; Donic, V.; Eijsvogel, M.M.; et al. COST Action B26 Group. Management of obstructive sleep apnea in Europe. Sleep Med. 2011, 12, 190–197. [Google Scholar] [CrossRef]
- Riha, R.L.; Celmina, M.; Cooper, B.; Hamutcu-Ersu, R.; Kaditis, A.; Morley, A.; Pataka, A.; Penzel, T.; Roberti, L.; Ruehland, W.; et al. ERS technical standards for using type III devices (limited channel studies) in the diagnosis of sleep disordered breathing in adults and children. Eur. Respir. J. 2023, 61, 2200422. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef]
- Grote, L.; Pevernagie, D.; Bruni, O.; Deboer, T.; Garcia-Borreguero, D.; Hill, E.A.; Penzel, T.; Puertas, F.J.; Wiechmann, A.; Verspaandonk, M.; et al. 10-year anniversary of the European Somnologist examination—A historic overview and critical appraisal. J. Sleep Res. 2022, 31, e13667. [Google Scholar] [CrossRef]
- Ruehland, W.R.; Rochford, P.D.; O’Donoghue, F.J.; Pierce, R.J.; Singh, P.; Thornton, A.T. The new AASM criteria for scoring hypopneas: Impact on the apnea hypopnea index. Sleep 2009, 32, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.; Bailly, S.; Soccal, P.M.; Janssens, J.P.; Sapène, M.; Grillet, Y.; Stach, B.; Tamisier, R.; Pépin, J.L. Symptomatic response to CPAP in obstructive sleep apnea versus COPD- obstructive sleep apnea overlap syndrome: Insights from a large national registry. PLoS ONE 2021, 16, e0256230. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.; Ågren, K.; Grote, L.; Ljunggren, M.; Midgren, B.; Sundh, J.; Theorell-Haglöw, J.; Ekström, M. Course of DISease In patients reported to the Swedish CPAP Oxygen and VEntilator RegistrY (DISCOVERY) with population-based controls. BMJ Open 2020, 10, e040396. [Google Scholar] [CrossRef] [PubMed]

| Daytime Symptoms | Sleep Related Symptoms | Comorbidities of Interest |
|---|---|---|
| Daytime sleepiness | Snoring, witnessed apneas | Systemic hypertension |
| Non-restorative sleep | Nocturia | Atrial fibrillation |
| Morning headache | Nocturnal dyspnea | Ischemic heart disease |
| Drowsy driving | Sweating | Heart Failure |
| Reduced work performance | Dry mouth | Stroke |
| Insomnia | Diabetes | |
| Nocturnal awakening | Obesity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grote, L.; Anderberg, C.-P.; Friberg, D.; Grundström, G.; Hinz, K.; Isaksson, G.; Murto, T.; Nilsson, Z.; Spaak, J.; Stillberg, G.; et al. National Knowledge-Driven Management of Obstructive Sleep Apnea—The Swedish Approach. Diagnostics 2023, 13, 1179. https://doi.org/10.3390/diagnostics13061179
Grote L, Anderberg C-P, Friberg D, Grundström G, Hinz K, Isaksson G, Murto T, Nilsson Z, Spaak J, Stillberg G, et al. National Knowledge-Driven Management of Obstructive Sleep Apnea—The Swedish Approach. Diagnostics. 2023; 13(6):1179. https://doi.org/10.3390/diagnostics13061179
Chicago/Turabian StyleGrote, Ludger, Carl-Peter Anderberg, Danielle Friberg, Gert Grundström, Kerstin Hinz, Göran Isaksson, Tarmo Murto, Zarita Nilsson, Jonas Spaak, Göran Stillberg, and et al. 2023. "National Knowledge-Driven Management of Obstructive Sleep Apnea—The Swedish Approach" Diagnostics 13, no. 6: 1179. https://doi.org/10.3390/diagnostics13061179
APA StyleGrote, L., Anderberg, C.-P., Friberg, D., Grundström, G., Hinz, K., Isaksson, G., Murto, T., Nilsson, Z., Spaak, J., Stillberg, G., Söderberg, K., Tegelberg, Å., Theorell-Haglöw, J., Ulander, M., & Hedner, J. (2023). National Knowledge-Driven Management of Obstructive Sleep Apnea—The Swedish Approach. Diagnostics, 13(6), 1179. https://doi.org/10.3390/diagnostics13061179






