Preliminary Findings of the Role of FAPi in Prostate Cancer Theranostics
Abstract
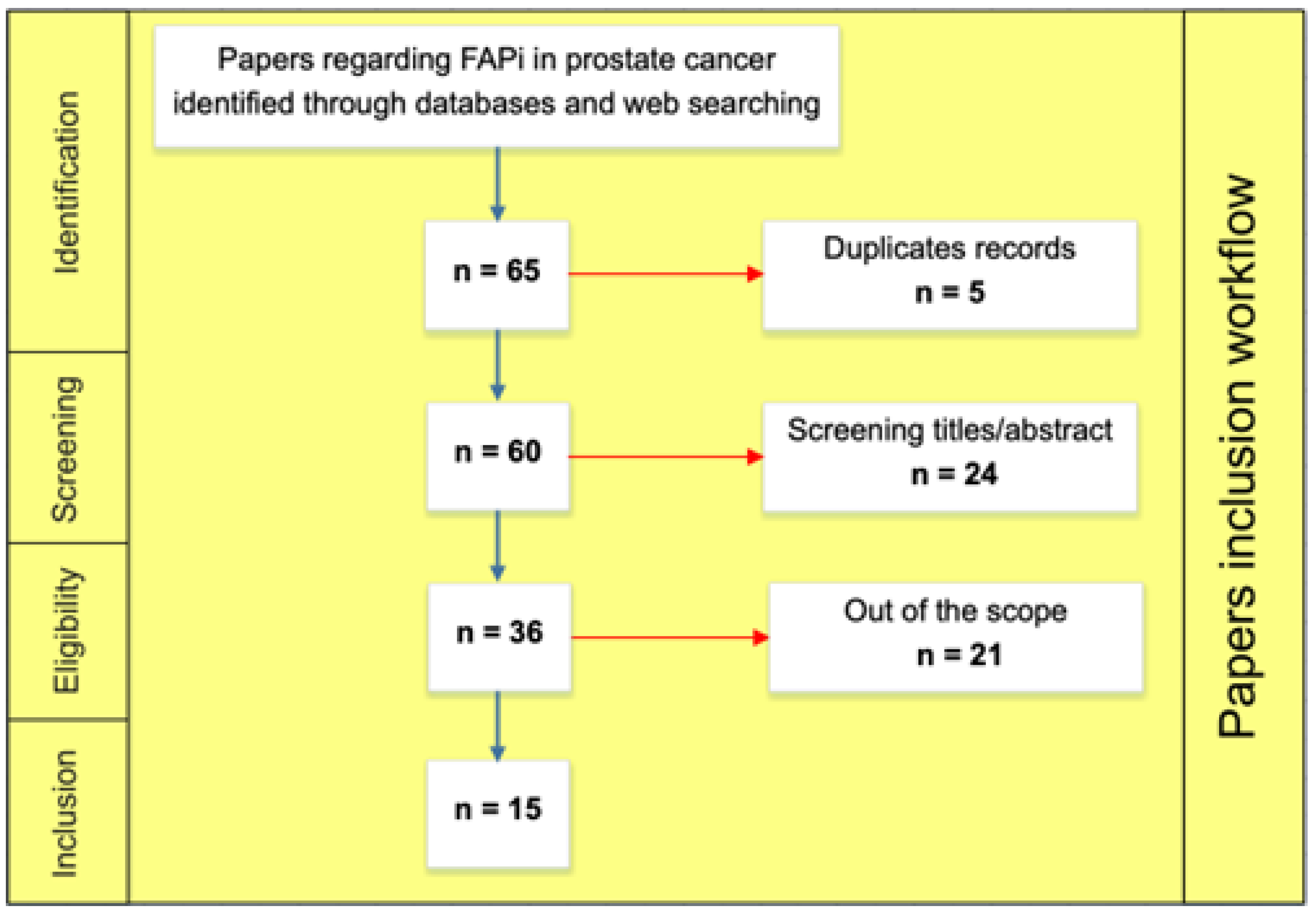
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. FAPi Immunohistochemistry (IHC) and Biodistribution Studies of PCa
4.2. Comparison of FAPi and PSMA PET for PCa
4.3. Comparison of FAPi and FDG PET for PCa
4.4. FAPi Theranostics Applications for PCa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | androgen deprivation therapy |
| BCR | biochemical recurrence |
| CAF | cancer-associated fibroblasts |
| FAP | fibroblast-activation-protein |
| FAPi | fibroblast-activation-protein inhibitor |
| FDG | fluorodeoxyglucose |
| IHC | immunohistochemistry |
| IMRT | intensity-modulated radiation therapy |
| ISUP | international society for urological pathology |
| mCRPCa | metastatic castration-resistant prostate cancer |
| MRI | magnetic resonance imaging |
| NA | not available |
| NEPCa | neuroendocrine differentiation PCa |
| OS | overall survival |
| PCa | prostate cancer |
| PET/CT | positron emission tomography/computer tomography |
| PRRT | peptide-receptor radiotherapy |
| PSA | prostate-specific antigen |
| PSMA | prostate-specific membrane antigen |
| RLT | radioligand therapy |
| RPE | radical prostatectomy |
| SUV | standardised uptake value |
| TBR | tumour-to-background ratios |
| TMA | tissue microarrays |
| TME | tumour microenvironment |
| TURP | transurethral prostate resection |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Lanzafame, H.; Farolfi, A.; Mapelli, P.; Picchio, M.; Burger, I.A.; Iagaru, A.; Minutoli, F.; Evangelista, L. PSMA and Choline PET for the Assessment of Response to Therapy and Survival Outcomes in Prostate Cancer Patients: A Systematic Review from the Literature. Cancers 2022, 14, 1770. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.A.; Laudicella, R.; Zeimpekis, K.; Mebert, I.; Müller, J.; Maurer, A.; Grünig, H.; Donati, O.; Sapienza, M.T.; Rueschoff, J.H.; et al. Hot needles can confirm accurate lesion sampling intraoperatively using [(18)F]PSMA-1007 PET/CT-guided biopsy in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1721–1730. [Google Scholar] [CrossRef]
- Laudicella, R.; Skawran, S.; Ferraro, D.A.; Mühlematter, U.J.; Maurer, A.; Grünig, H.; Rüschoff, H.J.; Rupp, N.; Donati, O.; Eberli, D.; et al. Quantitative imaging parameters to predict the local staging of prostate cancer in intermediate- to high-risk patients. Insights Imaging 2022, 13, 75. [Google Scholar] [CrossRef]
- Fassbind, S.; Ferraro, D.A.; Stelmes, J.-J.; Fankhauser, C.D.; Guckenberger, M.; Kaufmann, P.A.; Eberli, D.; Burger, I.A.; Kranzbühler, B. (68)Ga-PSMA-11 PET imaging in patients with ongoing androgen deprivation therapy for advanced prostate cancer. Ann. Nucl. Med. 2021, 35, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Rüschoff, J.H.; Ferraro, D.A.; Muehlematter, U.J.; Laudicella, R.; Hermanns, T.; Rodewald, A.-K.; Moch, H.; Eberli, D.; Burger, I.A.; Rupp, N.J. What’s behind Ga-PSMA-11 uptake in primary prostate cancer PET? Investigation of histopathological parameters and immunohistochemical PSMA expression patterns. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4042–4053. [Google Scholar] [CrossRef]
- Laudicella, R.; Rüschoff, J.H.; Ferraro, D.A.; Brada, M.D.; Hausmann, D.; Mebert, I.; Maurer, A.; Hermanns, T.; Eberli, D.; Rupp, N.J.; et al. Infiltrative growth pattern of prostate cancer is associated with lower uptake on PSMA PET and reduced diffusion restriction on mpMRI. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3917–3928. [Google Scholar] [CrossRef] [PubMed]
- Laudicella, R.; La Torre, F.; Davì, V.; Crocè, L.; Aricò, D.; Leonardi, G.; Russo, S.; Minutoli, F.; Burger, I.A.; Baldari, S. Prostate Cancer Biochemical Recurrence Resulted Negative on [68Ga]Ga-PSMA-11 but Positive on [18F]Fluoromethylcholine PET/CT. Tomography 2022, 8, 205. [Google Scholar] [CrossRef]
- Kesch, C.; Yirga, L.; Dendl, K.; Handke, A.; Darr, C.; Krafft, U.; Radtke, J.P.; Tschirdewahn, S.; Szarvas, T.; Fazli, L.; et al. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 385–389. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. (68)Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Greifenstein, L.; Kramer, C.S.; Moon, E.S.; Rösch, F.; Klega, A.; Landvogt, C.; Müller, C.; Baum, R.P. From Automated Synthesis to In Vivo Application in Multiple Types of Cancer-Clinical Results with [(68)Ga]Ga-DATA(5m).SA.FAPi. Pharmaceuticals 2022, 15, 1000. [Google Scholar] [CrossRef]
- Liu, F.; Qi, L.; Liu, B.; Liu, J.; Zhang, H.; Che, D.; Cao, J.; Shen, J.; Geng, J.; Bi, Y.; et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: A meta-analysis. PLoS ONE 2015, 10, e0116683. [Google Scholar] [CrossRef] [PubMed]
- Mona, C.E.; Benz, M.R.; Hikmat, F.; Grogan, T.R.; Lueckerath, K.; Razmaria, A.; Riahi, R.; Slavik, R.; Girgis, M.D.; Carlucci, G.; et al. Correlation of (68)Ga-FAPi-46 PET Biodistribution with FAP Expression by Immunohistochemistry in Patients with Solid Cancers: Interim Analysis of a Prospective Translational Exploratory Study. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1021–1026. [Google Scholar] [CrossRef]
- Hintz, H.M.; Gallant, J.P.; Vander Griend, D.J.; Coleman, I.M.; Nelson, P.S.; LeBeau, A.M. Imaging Fibroblast Activation Protein Alpha Improves Diagnosis of Metastatic Prostate Cancer with Positron Emission Tomography. Clin. Cancer Res. 2020, 26, 4882–4891. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Karathanasis, A.; Tzortzis, V. Expression of Fibroblast Activation Protein Is Enriched in Neuroendocrine Prostate Cancer and Predicts Worse Survival. Genes 2022, 13, 135. [Google Scholar] [CrossRef]
- Kessel, K.; Seifert, R.; Weckesser, M.; Boegemann, M.; Huss, S.; Kratochwil, C.; Haberkorn, U.; Giesel, F.; Rahbar, K. Prostate-specific membrane antigen and fibroblast activation protein distribution in prostate cancer: Preliminary data on immunohistochemistry and PET imaging. Ann. Nucl. Med. 2022, 36, 293–301. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Lan, L.; Liu, H.; Wang, Y.; Deng, J.; Peng, D.; Feng, Y.; Wang, L.; Chen, Y.; Qiu, L. The potential utility of [(68) Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [(18)F]FDG. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Liao, T.; Rao, Z.; Gong, W.; Ou, L.; Chen, Y.; Zhang, C. Comparison of the Relative Diagnostic Performance of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F]FDG PET/CT for the Detection of Bone Metastasis in Patients with Different Cancers. Front. Oncol. 2021, 11, 737827. [Google Scholar] [CrossRef]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients with Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef]
- Fendler, W.P.; Pabst, K.M.; Kessler, L.; Costa, P.F.; Ferdinandus, J.; Weber, M.; Lippert, M.; Lueckerath, K.; Umutlu, L.; Kostbade, K.; et al. Safety and Efficacy of 90Y-FAPI-46 Radioligand Therapy in Patients with Advanced Sarcoma and Other Cancer Entities. Clin. Cancer Res. 2022, 28, 4346–4353. [Google Scholar] [CrossRef]
- Khreish, F.; Rosar, F.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U.; Ezziddin, S. Positive FAPI-PET/CT in a metastatic castration-resistant prostate cancer patient with PSMA-negative/FDG-positive disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. A Peer Rev. Indep. Open Access J. 2009, 3, e123–e130. [Google Scholar]
- Pang, Y.; Meng, T.; Xu, W.; Shang, Q.; Chen, H. 68 Ga-FAPI PET/CT Detected Non-PSMA/FDG-Avid Primary Tumor in De Novo Metastatic Prostate Cancer. Clin. Nucl. Med. 2022, 47, 1108–1111. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, Y.; Ding, H.; Cai, L.; Zhou, Z.; Song, Z.; Chen, Y. [(68)Ga]Ga-DOTA-FAPI-04 PET/CT imaging in a case of prostate cancer with shoulder arthritis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1254–1255. [Google Scholar] [CrossRef]
- Isik, E.G.; Has-Simsek, D.; Sanli, O.; Sanli, Y.; Kuyumcu, S. Fibroblast Activation Protein-Targeted PET Imaging of Metastatic Castration-Resistant Prostate Cancer Compared With 68Ga-PSMA and 18F-FDG PET/CT. Clin. Nucl. Med. 2022, 47, e54–e55. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.; Manafi-Farid, R.; Amini, H.; Divband, G.; Moghadam, S.Z. 68 Ga-FAPI-46 PET/CT in a Metastatic Castration-Resistant Prostate Cancer Patient With Low PSMA Expression. Clin. Nucl. Med. 2022, 47, 972–973. [Google Scholar] [CrossRef]
- Baratto, L.; Duan, H.; Laudicella, R.; Toriihara, A.; Hatami, N.; Ferri, V.; Iagaru, A. Physiological (68)Ga-RM2 uptake in patients with biochemically recurrent prostate cancer: An atlas of semi-quantitative measurements. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 115–122. [Google Scholar] [CrossRef] [PubMed]
| Authors [ref.] | Year | FAPi Tracer | No. of Patients (PCa) | Scenario | ISUP | PSA (ng/mL) | Main Findings |
|---|---|---|---|---|---|---|---|
| Kesch et al. [11] | 2021 | [68Ga]Ga-DOTA-FAPi-04 | 94 (94 *) | PCa, mCRPCa, NEPCa | 5 *** | NA | In the microarray analysis, the authors observed significantly higher H-index values for more advanced PCa (mCPRCa and NEPCa) than those of benign tissues and early PCa, as confirmed by imaging of [68Ga]Ga-DOTA-FAPi-04. |
| Greifenstein et al. [13] | 2022 | [68Ga]Ga-DATA5m.SA.FAPi | 6 (1) | mPCa | 3 | NA | [68Ga]Ga-DATA5m.SA.FAPi had excellent diagnostic properties, detecting soft tissue and bone metastases, with high TBR and a remarkably high tumour SUVmax in mPCa (ISUP 3) with extensive disease. |
| Kratochwil et al. [19] | 2019 | [68Ga]Ga-DOTA-FAPi-04 | 80 (4 **) | PSMA-negative PCa | NA | NA | Intermediate-to-high [68Ga]Ga-DOTA-FAPi-04 uptake (SUVmax 6-12) was observed in a patient with PSMA-negative PCa. Low uptake (SUVmax < 6) was observed in 3 patients with NEPCa. |
| Authors [ref.] | Year | FAPi Tracer | No. of Patients (PCa) | Scenario | ISUP | PSA (ng/mL) | Main Findings |
|---|---|---|---|---|---|---|---|
| Kessel et al. [18] | 2021 | [68Ga]Ga-DOTA-FAPi-46 | 14 (11 *) | PCa diagnosis and mCRPCa | 5 (3) NA (3) ** | 23.8 [2.4–106] ** | The role of FAPi in PCa diagnosis remains subject to further investigations, as it is also overexpressed in inflammatory diseases. Measurement of FAPi expression levels might be recommended as a complementary diagnostic tool for later PCa stages together with the use of PSMA and/or FDG or DOTATATE-PET. |
| Pang et al. [27] | 2022 | [68Ga]Ga-DOTA-FAPi-04 | 1 (1) | mPCa diagnosis | 5 | >60 | The technique of [68Ga]Ga-DOTA-FAPi-04 PET/CT may be a useful imaging modality for the detection and localisation of non-PSMA avid primary PCa. |
| Authors [ref.] | Year | FAPi Tracer | No. of Patients (PCa) | Scenario | ISUP | PSA (ng/mL) | Main Findings |
|---|---|---|---|---|---|---|---|
| Lan et al. [21] | 2021 | [68Ga]Ga-DOTA-FAPi-04 | 123 (1) | NA | NA | NA | In 1 patient with PCa, the authors observed lower SUVmax values for [68Ga]Ga-DOTA-FAPi-04 than those for [18F]FDG PET/CT. |
| Xu et al. [28] | 2020 | [68Ga]Ga-DOTA-FAPi-04 | 1 (1) | PCa diagnosis | 2 | 4.6 | In a patient with PCa (cT1c, ISUP 2), the [68Ga]Ga-DOTA-FAPi-04 PET/CT findings were similar to the [18F]FDG PET/CT findings, which indicates that [68Ga]Ga-DOTA-FAPi-04 may not be specific to PCa. |
| Authors [ref.] | Year | FAPi Tracer | No. of Patients (PCa) | Scenario | ISUP | PSA (ng/mL) | Main Findings |
|---|---|---|---|---|---|---|---|
| Assadi et al. [23] | 2021 | [68Ga]Ga-DOTA-FAPi-46 and [177Lu]Lu-DOTA-FAPi-46 | 21 (2 *) | mPCa | NA | NA | RLT with [177Lu]Lu-DOTA-FAPi-46 is feasible, with dosimetry and toxicity values that are similar to those of [177Lu]Lu-DOTATATE and [177Lu]Lu-PSMA and an acceptable tumour retention time (up to 10 days after administration). In 1 patients with mPCa, the authors reported SD after 1 RLT cycle (1.85 GBq). |
| Fendler et al. [24] | 2022 | [68Ga]Ga-DOTA-FAPi-46 and [90Y]FAPi-46 | 21 (1) | mCRPCa | NA | NA | In spite of the short retention time, [90Y]FAPi-46 RLT is safe, with organ radiation doses below the critical range, high response rates, and prolonged survival in patients with mCRPCa. |
| Khreish et al. [25] | 2019 | [68Ga]Ga-DOTA-FAPi-04 | 1 (1) | mCRPCa | NA | NA | The technique of [68Ga]Ga-DOTA-FAPi-04 PET/CT revealed a higher detection rate than that of [68Ga]Ga-PSMA PET/CT and [18F]FDG PET/CT, opening new RLT opportunities for FAPi molecules in patients with highly dedifferentiated PCa, overcoming the limitation of PSMA expression heterogeneity. |
| Isik et al. [29] | 2022 | [68Ga]Ga-DOTA-FAPi-04 | 2 (2) | mCRPCa | NA | NA | FAPi molecules are promising novel tracers with theranostics applications in patients with mCRPCa, particularly those with heterogeneous tumour phenotypes. |
| Aryana et al. [30] | 2022 | [68Ga]Ga-DOTA-FAPi-46 | 1 (1) | mCRPCa | NA | 1603 | [68Ga]Ga-FAPi-46 theranostics may have a potential application in the treatment of patients with mCRPCa who have negative or low PSMA expression levels or failed [177Lu]Lu-PSMA therapy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudicella, R.; Spataro, A.; Crocè, L.; Giacoppo, G.; Romano, D.; Davì, V.; Lopes, M.; Librando, M.; Nicocia, A.; Rappazzo, A.; et al. Preliminary Findings of the Role of FAPi in Prostate Cancer Theranostics. Diagnostics 2023, 13, 1175. https://doi.org/10.3390/diagnostics13061175
Laudicella R, Spataro A, Crocè L, Giacoppo G, Romano D, Davì V, Lopes M, Librando M, Nicocia A, Rappazzo A, et al. Preliminary Findings of the Role of FAPi in Prostate Cancer Theranostics. Diagnostics. 2023; 13(6):1175. https://doi.org/10.3390/diagnostics13061175
Chicago/Turabian StyleLaudicella, Riccardo, Alessandro Spataro, Ludovica Crocè, Giulia Giacoppo, Davide Romano, Valerio Davì, Maria Lopes, Maria Librando, Antonio Nicocia, Andrea Rappazzo, and et al. 2023. "Preliminary Findings of the Role of FAPi in Prostate Cancer Theranostics" Diagnostics 13, no. 6: 1175. https://doi.org/10.3390/diagnostics13061175
APA StyleLaudicella, R., Spataro, A., Crocè, L., Giacoppo, G., Romano, D., Davì, V., Lopes, M., Librando, M., Nicocia, A., Rappazzo, A., Celesti, G., Torre, F. L., Pagano, B., Garraffa, G., Bauckneht, M., Burger, I. A., Minutoli, F., & Baldari, S. (2023). Preliminary Findings of the Role of FAPi in Prostate Cancer Theranostics. Diagnostics, 13(6), 1175. https://doi.org/10.3390/diagnostics13061175







