Pregnancy-Specific Glycoprotein 9 Enhances Store-Operated Calcium Entry and Nitric Oxide Release in Human Umbilical Vein Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Clinical Samples
2.2. Cell Culture
2.3. Western Blotting
2.4. NO Fluorescence Probe
2.5. [Ca2+]i Measurement
2.6. Statistical Analysis
3. Results
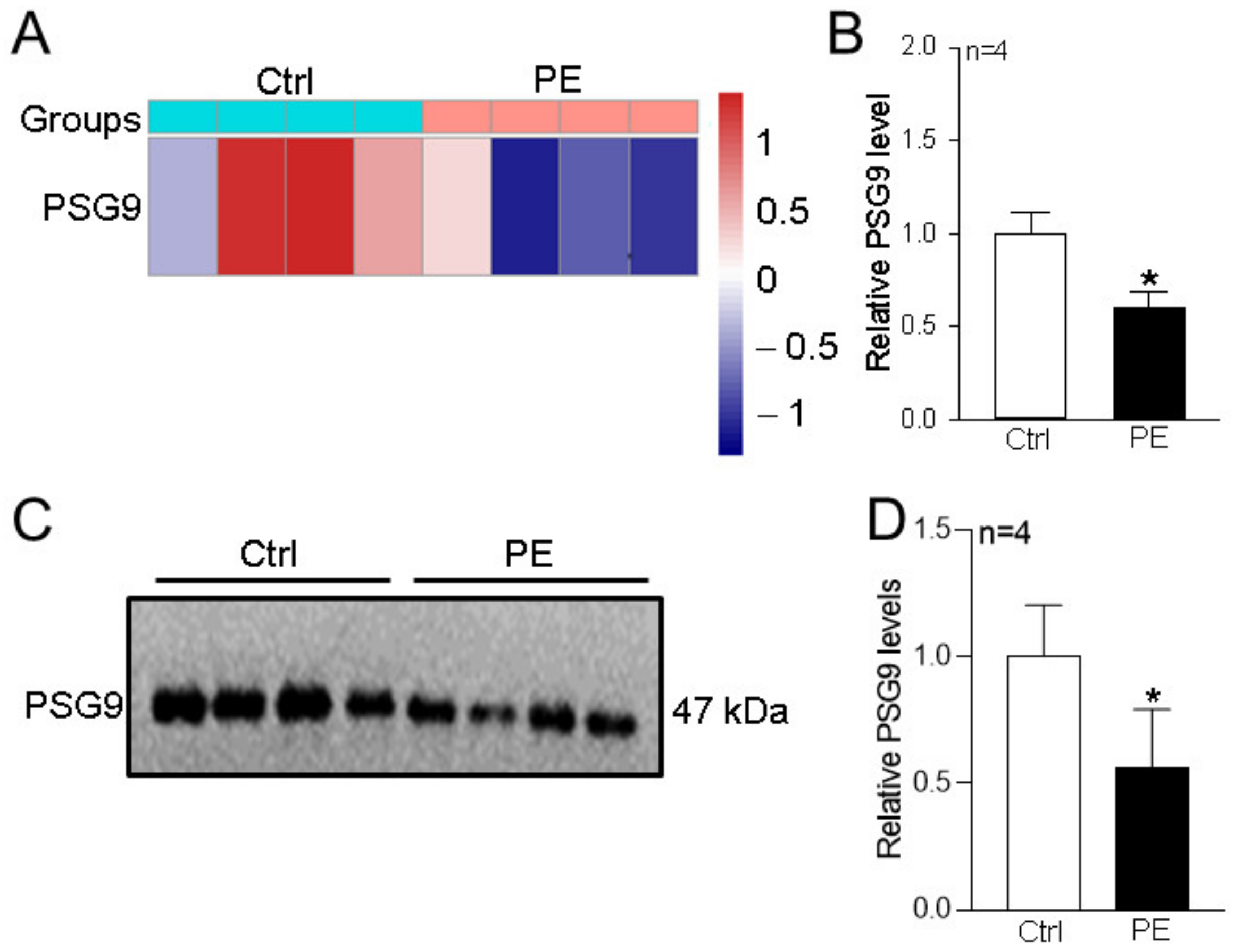
3.1. Changes in Serum PSG9 Levels in Patients with Preeclampsia
3.2. Effects of PSG9 on the Expression Levels of Orais and on SOCE in HUVECs
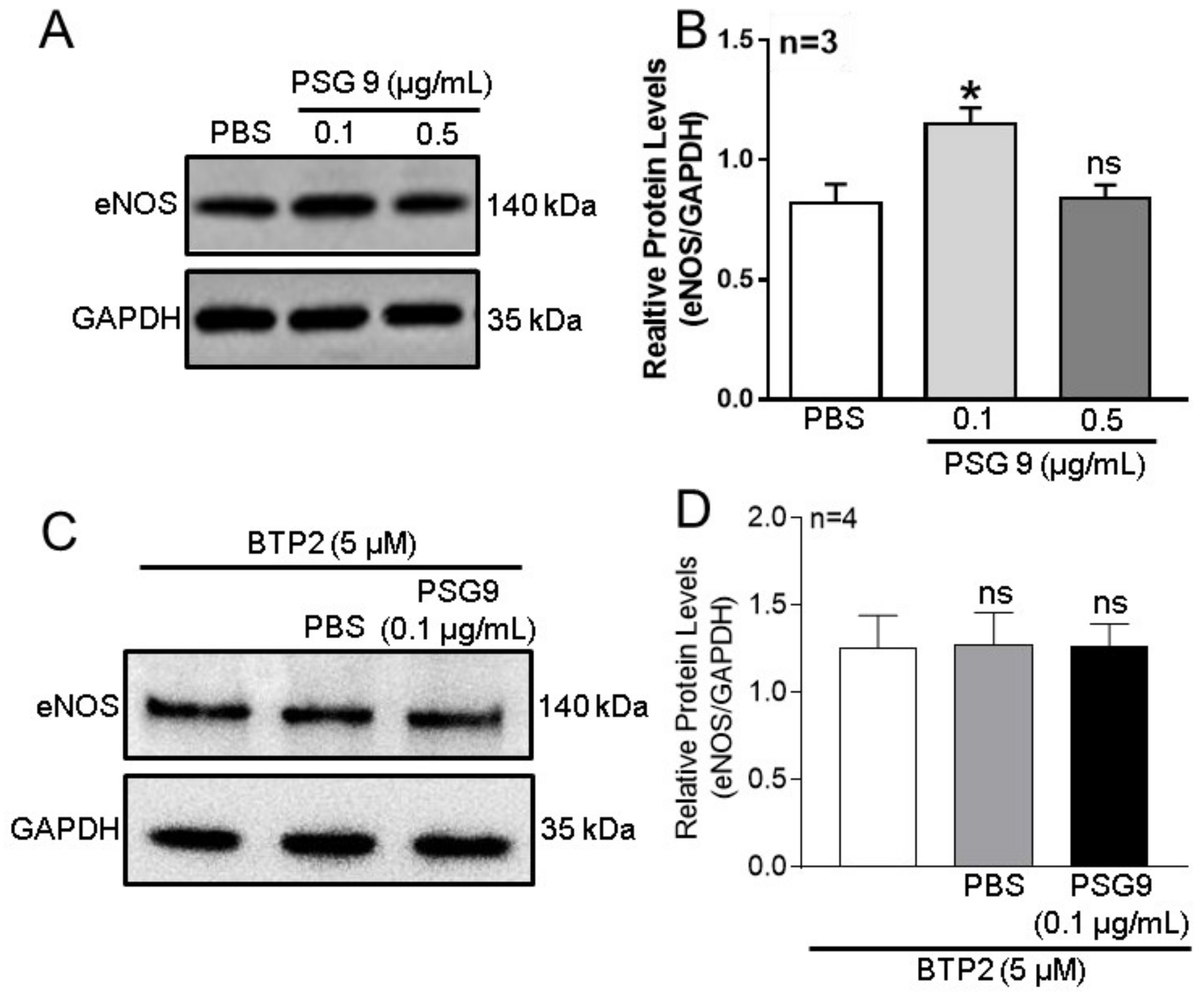
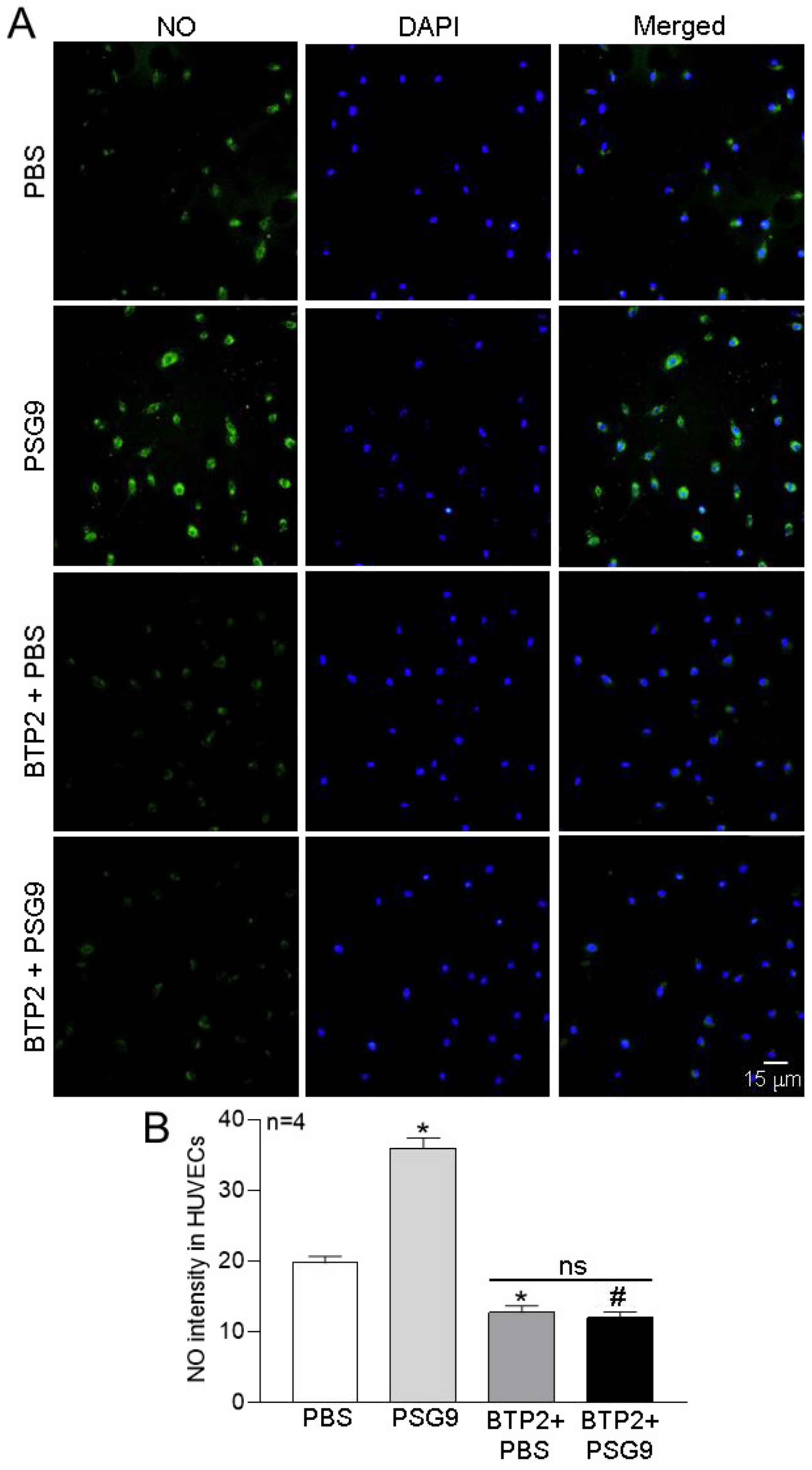
3.3. Role of Orais in PSG9-Enhanced eNOS Expression and NO Production in HUVECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Cheng, L.; Jin, J.; Liu, X.; Wang, F. Long non-coding RNA MALAT1 regulates trophoblast functions through VEGF/VEGFR1 signaling pathway. Arch. Gynecol. Obs. 2021, 304, 873–882. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: Gestational hypertension and preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar]
- Bokslag, A.; van Weissenbruch, M.; Mol, B.W.; de Groot, C.J. Preeclampsia; short and long-term consequences for mother and neonate. Early. Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.F. Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J. Obs. Gynecol. 2017, 56, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30 (Suppl. SA), S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.A.; Robertson, W.B.; Dixon, H.G. The role of the spiral arteries in the pathogenesis of preeclampsia. Obs. Gynecol. Annu. 1972, 1, 177–191. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Anthony, J.; Davey, D.A.; Rees, A.; Tiltman, A.; Vercruysse, L.; van Assche, A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br. J. Obs. Gynaecol. 1991, 98, 648–655. [Google Scholar] [CrossRef]
- Hecht, J.L.; Zsengeller, Z.K.; Spiel, M.; Karumanchi, S.A.; Rosen, S. Revisiting decidual vasculopathy. Placenta 2016, 42, 37–43. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tokunaga, N.; Isoda, H.; Kanayama, N.; Terao, T. Vasospasms are characteristic in cases with eclampsia/preeclampsia and HELLP syndrome: Proposal of an angiospastic syndrome of pregnancy. Semin. Thromb. Hemost. 2001, 27, 131–135. [Google Scholar] [CrossRef]
- Moore, T.; Dveksler, G. Pregnancy-specific glycoproteins: Complex gene families regulating maternal-fetal interactions. Int. J. Dev. Biol. 2014, 58, 273–280. [Google Scholar] [CrossRef]
- Salahshor, S.; Goncalves, J.; Chetty, R.; Gallinger, S.; Woodgett, J.R. Differential gene expression profile reveals deregulation of pregnancy specific beta1 glycoprotein 9 early during colorectal carcinogenesis. BMC Cancer 2005, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Rong, W.; Xiao, T.; Zhang, Y.; Xu, B.; Liu, Y.; Wang, L.; Wu, F.; Qi, J.; Zhao, X.; et al. Secretory/releasing proteome-based identification of plasma biomarkers in HBV-associated hepatocellular carcinoma. Sci. China Life Sci. 2013, 56, 638–646. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, S.; Yu, T.J.; Zhang, F.L.; Yang, F.; Huang, Y.N.; Ma, D.; Liu, G.Y.; Shao, Z.M.; Li, D.Q. Pregnancy-specific glycoprotein 9 acts as both a transcriptional target and a regulator of the canonical TGF-beta/Smad signaling to drive breast cancer progression. Clin. Transl. Med. 2020, 10, e245. [Google Scholar] [CrossRef] [PubMed]
- Rattila, S.; Kleefeldt, F.; Ballesteros, A.; Beltrame, J.S.; Ribeiro, M.L.; Ergun, S.; Dveksler, G. Pro-angiogenic effects of pregnancy-specific glycoproteins in endothelial and extravillous trophoblast cells. Reproduction 2020, 160, 737–750. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Yi, F.X.; Bird, I.M. eNOS activation and NO function: Pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium--new insights into eNOS regulation through adaptive cell signaling. J. Endocrinol. 2011, 210, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.J.; Khalil, R.A. Risk factors and mediators of the vascular dysfunction associated with hypertension in pregnancy. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 33–52. [Google Scholar] [CrossRef]
- Lopez, J.J.; Jardin, I.; Albarran, L.; Sanchez-Collado, J.; Cantonero, C.; Salido, G.M.; Smani, T.; Rosado, J.A. Molecular Basis and Regulation of Store-Operated Calcium Entry. Adv. Exp. Med. Biol. 2020, 1131, 445–469. [Google Scholar] [CrossRef]
- Tanwar, J.; Arora, S.; Motiani, R.K. Orai3: Oncochannel with therapeutic potential. Cell Calcium 2020, 90, 102247. [Google Scholar] [CrossRef]
- Kwon, J.; An, H.; Sa, M.; Won, J.; Shin, J.I.; Lee, C.J. Orai1 and Orai3 in Combination with Stim1 Mediate the Majority of Store-operated Calcium Entry in Astrocytes. Exp. Neurobiol. 2017, 26, 42–54. [Google Scholar] [CrossRef]
- Smyth, J.T.; Dehaven, W.I.; Jones, B.F.; Mercer, J.C.; Trebak, M.; Vazquez, G.; Putney, J.W., Jr. Emerging perspectives in store-operated Ca2+ entry: Roles of Orai, Stim and TRP. Biochim. Biophys. Acta 2006, 1763, 1147–1160. [Google Scholar] [CrossRef]
- Raman, C.S.; Li, H.; Martasek, P.; Kral, V.; Masters, B.S.; Poulos, T.L. Crystal structure of constitutive endothelial nitric oxide synthase: A paradigm for pterin function involving a novel metal center. Cell 1998, 95, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Tao, F.; Qin, Y.; Wu, M.; Cheng, J.; Xie, M.; Shen, B.; Ren, J.; Xu, X.; Huang, D.; et al. Serum proteins differentially expressed in early- and late-onset preeclampsia assessed using iTRAQ proteomics and bioinformatics analyses. PeerJ 2020, 8, e9753. [Google Scholar] [CrossRef] [PubMed]
- Lokki, A.I.; Teirila, L.; Triebwasser, M.; Daly, E.; Bhattacharjee, A.; Uotila, L.; Llort Asens, M.; Kurki, M.I.; Perola, M.; Auro, K.; et al. Dysfunction of complement receptors CR3 (CD11b/18) and CR4 (CD11c/18) in pre-eclampsia: A genetic and functional study. BJOG 2021, 128, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hernandez, I.; Alecsandru, D.; Garcia-Velasco, J.A.; Dominguez, F. Uterine natural killer cells: From foe to friend in reproduction. Hum. Reprod. Update 2021, 27, 720–746. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Lv, Y.; Chen, Y.; Rao, J. Crocin exhibits an antihypertensive effect in a rat model of gestational hypertension and activates the Nrf-2/HO-1 signaling pathway. Hypertens. Res. 2021, 44, 642–650. [Google Scholar] [CrossRef]
- Horne, C.H.; Towler, C.M.; Pugh-Humphreys, R.G.; Thomson, A.W.; Bohn, H. Pregnancy specific beta1-glycoprotein--a product of the syncytiotrophoblast. Experientia 1976, 32, 1197. [Google Scholar] [CrossRef]
- Zhou, G.Q.; Baranov, V.; Zimmermann, W.; Grunert, F.; Erhard, B.; Mincheva-Nilsson, L.; Hammarstrom, S.; Thompson, J. Highly specific monoclonal antibody demonstrates that pregnancy-specific glycoprotein (PSG) is limited to syncytiotrophoblast in human early and term placenta. Placenta 1997, 18, 491–501. [Google Scholar] [CrossRef]
- Ha, C.T.; Wu, J.A.; Irmak, S.; Lisboa, F.A.; Dizon, A.M.; Warren, J.W.; Ergun, S.; Dveksler, G.S. Human pregnancy specific beta-1-glycoprotein 1 (PSG1) has a potential role in placental vascular morphogenesis. Biol. Reprod. 2010, 83, 27–35. [Google Scholar] [CrossRef]
- Martinez, F.F.; Cervi, L.; Knubel, C.P.; Panzetta-Dutari, G.M.; Motran, C.C. The role of pregnancy-specific glycoprotein 1a (PSG1a) in regulating the innate and adaptive immune response. Am. J. Reprod. Immunol. 2013, 69, 383–394. [Google Scholar] [CrossRef]
- Hendrix, P.; Tang, Z.; Silasi, M.; Racicot, K.E.; Mor, G.; Abrahams, V.M.; Guller, S. Herpesvirus-infected Hofbauer cells activate endothelial cells through an IL-1beta-dependent mechanism. Placenta 2020, 91, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Milovanov, A.P.; Voloshchuk, I.N. Deported syncytiotrophoblast and placental microparticles in the mother’s body during normal pregnancy and preeclampsia (28 years later). Arkh Patol. 2017, 79, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rakner, J.J.; Silva, G.B.; Mundal, S.B.; Thaning, A.J.; Elschot, M.; Ostrop, J.; Thomsen, L.C.V.; Bjorge, L.; Gierman, L.M.; Iversen, A.C. Decidual and placental NOD1 is associated with inflammation in normal and preeclamptic pregnancies. Placenta 2021, 105, 23–31. [Google Scholar] [CrossRef]
- Tuerxun, D.; Aierken, R.; Zhang, Y.M.; Huang, Y.; Sui, S.; Li, X.Y.; Abulikemu, Z.; Dilixiati, N. Astragaloside IV alleviates lipopolysaccharide-induced preeclampsia-like phenotypes via suppressing the inflammatory responses. Kaohsiung J. Med. Sci. 2021, 37, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Kandel, M.; MacDonald, T.M.; Walker, S.P.; Cluver, C.; Bergman, L.; Myers, J.; Hastie, R.; Keenan, E.; Hannan, N.J.; Cannon, P.; et al. PSG7 and 9 (Pregnancy-Specific beta-1 Glycoproteins 7 and 9): Novel Biomarkers for Preeclampsia. J. Am. Heart Assoc. 2022, 11, e024536. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.C.; Pudwell, J.; Smith, G.N. Postpartum microvascular functional alterations following severe preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1393–H1402. [Google Scholar] [CrossRef]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef]
- Lillo, M.A.; Gaete, P.S.; Puebla, M.; Ardiles, N.M.; Poblete, I.; Becerra, A.; Simon, F.; Figueroa, X.F. Critical contribution of Na(+)-Ca(2+) exchanger to the Ca(2+)-mediated vasodilation activated in endothelial cells of resistance arteries. FASEB J. 2018, 32, 2137–2147. [Google Scholar] [CrossRef]
- Bkaily, G.; d’Orleans-Juste, P.; Naik, R.; Perodin, J.; Stankova, J.; Abdulnour, E.; Rola-Pleszczynski, M. PAF activation of a voltage-gated R-type Ca2+ channel in human and canine aortic endothelial cells. Br. J. Pharm. 1993, 110, 519–520. [Google Scholar] [CrossRef]
- Koo, B.H.; Hwang, H.M.; Yi, B.G.; Lim, H.K.; Jeon, B.H.; Hoe, K.L.; Kwon, Y.G.; Won, M.H.; Kim, Y.M.; Berkowitz, D.E.; et al. Arginase II Contributes to the Ca(2+)/CaMKII/eNOS Axis by Regulating Ca(2+) Concentration Between the Cytosol and Mitochondria in a p32-Dependent Manner. J. Am. Heart Assoc. 2018, 7, e009579. [Google Scholar] [CrossRef]
- Horinouchi, T.; Mazaki, Y.; Terada, K.; Miwa, S. Extracellular Ca(2+) promotes nitric oxide production via Ca(2+)-sensing receptor-Gq/11 protein-endothelial nitric oxide synthase signaling in human vascular endothelial cells. J. Pharm. Sci. 2020, 143, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Su, X.H.; Jin, J.Y.; Zhou, Z.Q.; Sun, S.S.; Wen, J.F.; Kang, D.G.; Lee, H.S.; Cho, K.W.; Jin, S.N. Rumex acetosa L. induces vasorelaxation in rat aorta via activation of PI3-kinase/Akt- AND Ca(2+)-eNOS-NO signaling in endothelial cells. J. Physiol. Pharm. 2015, 66, 907–915. [Google Scholar]


| Feature | Control Mean (SD) (n = 4) | Preeclampsia Mean (SD) (n = 4) | t Value |
|---|---|---|---|
| Age (years) | 26.00 ± 1.83 | 24.75 ± 1.71 | 0.951 |
| Gestational weeks (days) | 272.25 ± 13.25 | 268.75 ± 7.63 | 0.458 |
| SP (mmHg) | 120.00 ± 8.04 | 167.50 ± 14.52 * | −5.722 |
| DP (mmHg) | 74.5 ± 9.47 | 105.00 ± 11.28 * | −4.141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Meng, Q.; Wang, Q.; Wu, M.; Fang, Y.; Tu, C.; Hu, X.; Shen, B.; Chen, H.; Xu, X. Pregnancy-Specific Glycoprotein 9 Enhances Store-Operated Calcium Entry and Nitric Oxide Release in Human Umbilical Vein Endothelial Cells. Diagnostics 2023, 13, 1134. https://doi.org/10.3390/diagnostics13061134
Qin Y, Meng Q, Wang Q, Wu M, Fang Y, Tu C, Hu X, Shen B, Chen H, Xu X. Pregnancy-Specific Glycoprotein 9 Enhances Store-Operated Calcium Entry and Nitric Oxide Release in Human Umbilical Vein Endothelial Cells. Diagnostics. 2023; 13(6):1134. https://doi.org/10.3390/diagnostics13061134
Chicago/Turabian StyleQin, Ying, Qinggang Meng, Qunhua Wang, Mingzhu Wu, Yan Fang, Chengcheng Tu, Xinyang Hu, Bing Shen, Hongbo Chen, and Xiaohong Xu. 2023. "Pregnancy-Specific Glycoprotein 9 Enhances Store-Operated Calcium Entry and Nitric Oxide Release in Human Umbilical Vein Endothelial Cells" Diagnostics 13, no. 6: 1134. https://doi.org/10.3390/diagnostics13061134
APA StyleQin, Y., Meng, Q., Wang, Q., Wu, M., Fang, Y., Tu, C., Hu, X., Shen, B., Chen, H., & Xu, X. (2023). Pregnancy-Specific Glycoprotein 9 Enhances Store-Operated Calcium Entry and Nitric Oxide Release in Human Umbilical Vein Endothelial Cells. Diagnostics, 13(6), 1134. https://doi.org/10.3390/diagnostics13061134






