Evaluation of the Performance Characteristics of a New POC Multiplex PCR Assay for the Diagnosis of Viral and Bacterial Neuromeningeal Infections
Abstract
1. Introduction
2. Materials and Methods
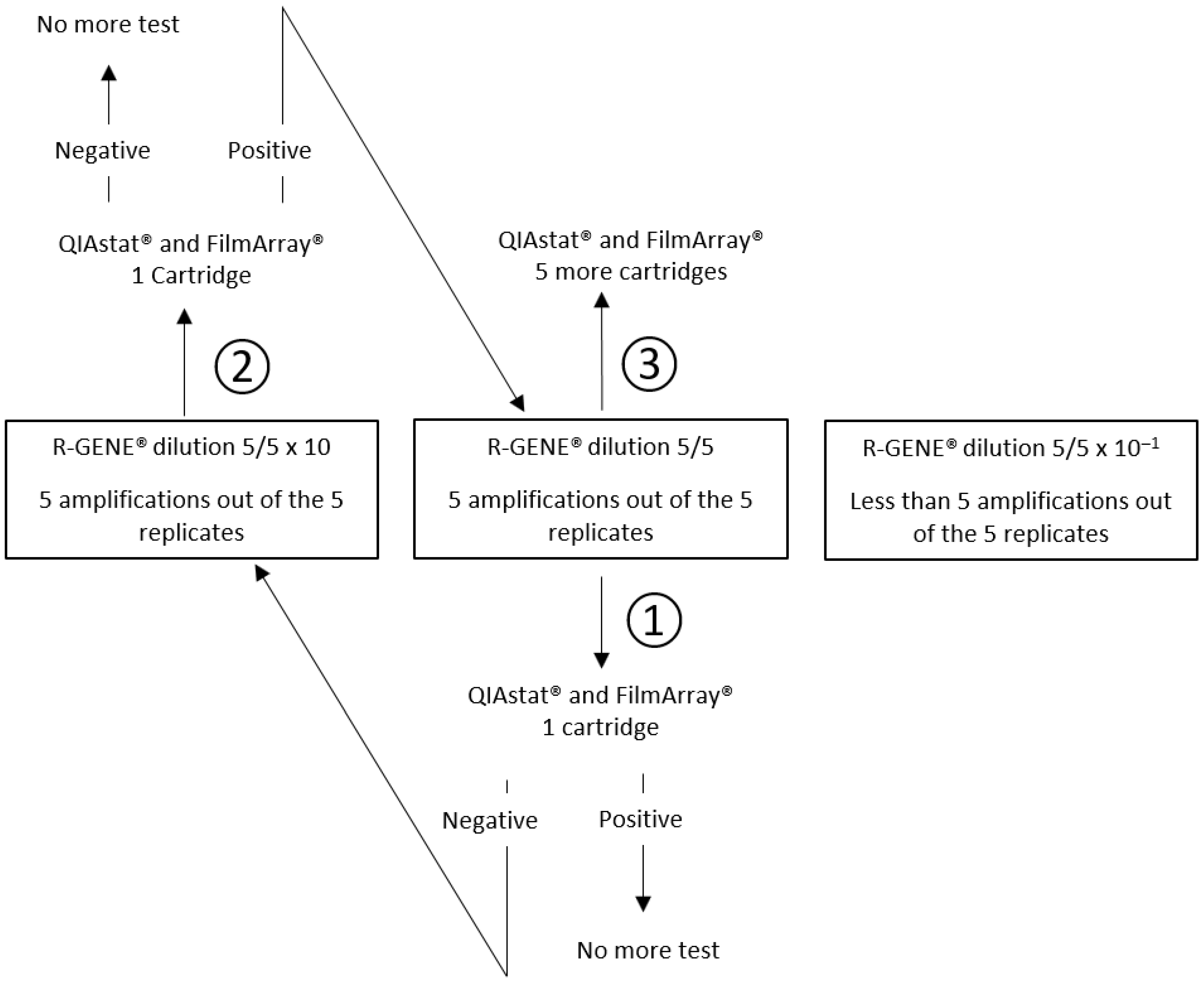
2.1. POC Syndromic PCR Meningitis/Encephalitis Panel
2.2. CSF Pool Preparation
2.3. Virus
2.4. Bacteria
2.5. Assessed Parameters
2.5.1. Detection of Low Viral and Mycoplasma pneumoniae Loads
2.5.2. Detection of Low Bacterial Concentrations
2.5.3. False Positive Results
2.5.4. Reproducibility and Carryover Contamination
2.5.5. Interferences
2.5.6. Clinical CSF Samples
3. Results
3.1. Detection of Low Viral and Mycoplasma pneumoniae Loads
3.2. Detection of Low Bacterial Concentrations
3.3. Analytical Specificity
3.4. Reproducibility and Carryover Contamination
3.5. Interferences
3.6. Clinical CSF Samples
3.7. Internal Control Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.J.; Westblade, L.F.; Gottesdiener, L.S.; Liang, K.; Li, H.A.; Wehmeyer, G.T.; Glesby, M.J.; Simon, M.S. Impact of a multiplex polymerase chain reaction panel on duration of empiric antibiotic therapy in suspected bacterial meningitis. Open Forum Infect. Dis. 2021, 8, ofab467. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.; Kim, C.; Zucker, J.; Green, D.A.; Sun, Y.; Whittier, S.; Thakur, K.T. Impact of implementing the cerebrospinal fluid FilmArray Meningitis/Encephalitis panel on duration of intravenous acyclovir treatment. Open Forum Infect. Dis. 2022, 9, ofac356. [Google Scholar] [CrossRef]
- Hoen, B.; Varon, E.; de Debroucker, T.; Fantin, B.; Grimprel, E.; Wolff, M.; Duval, X.; Expert and Reviewing Group. Management of acute community-acquired bacterial meningitis (excluding newborns). Long version with arguments. Med. Mal. Infect. 2019, 49, 405–441. [Google Scholar] [CrossRef] [PubMed]
- Menasalvas-Ruiz, A.I.; Salvador-Garcia, C.; Moreno-Docon, A.; Alfayate-Miguelez, S.; Perez Canovas, C.; Sanchez-Solis, M. Enterovirus reverse transcriptase polymerase chain reaction assay in cerebrospinal fluid: An essential tool in meningitis management in childhood. Enferm. Infecc. Microbiol. Clin. 2013, 31, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Gomez, J.; Tsokani, S.; Arango-Ferreira, C.; Atehortua-Munz, S.; Jimenez-Villegas, M.J.; Serrano-Tabares, C.; Veroniki, A.A.; Florez, I.D. Biofire Filmarray Meningitis/Encephalitis panel for the aetiological diagnosis of central nervous system infections: A systematic review and diagnostic test accuracy meta-analysis. EClinicalMedicine 2022, 44, 101275. [Google Scholar] [CrossRef] [PubMed]
- Aberle, S.W.; Puchhammer-Stöckl, E. Diagnosis of herpesvirus infections of the central nervous system. J. Clin. Virol. 2002, 25 (Suppl. 1), S79–S85. [Google Scholar] [CrossRef]
- Binnicker, M.J.; Espy, M.J.; Irish, C.L. Rapid and direct detection of herpes simplex virus in cerebrospinal fluid by use of a commercial real-time PCR assay. J. Clin. Microbiol. 2004, 52, 4361–4362. [Google Scholar] [CrossRef]
- Ziyaeyan, M.; Alborzi, A.; Borhani Haghighi, A.; Jamalidoust, M.; Moeini, M.; Pourabbas, B. Diagnosis and quantitative detection of HSV DNA in samples from patients with suspected herpes simplex encephalitis. Braz. J. Infect. Dis. 2011, 15, 211–214. [Google Scholar] [CrossRef]
- Ramirez, K.A.; Choudhri, A.F.; Patel, A.; Lenny, N.T.; Thompson, R.E.; Berkelhammer Greenberg, L.; Clanton Watson, N.; Kocak, M.; DeVincenzo, J.P. Comparing molecular quantification of herpes simplex virus (HSV) in cerebrospinal fluid (CSF) with quantitative structural and functional disease severity in patients with HSV encephalitis (HSVE): Implications for improved therapeutic approaches. J. Clin. Virol. 2018, 107, 29–37. [Google Scholar] [CrossRef]
- Saraya, A.W.; Wacharapluesadee, S.; Petcharat, S.; Sittidetboripat, N.; Ghai, S.; Wilde, H.; Hemachudha, T. Normocellular CSF in herpes simplex encephalitis. BMC Res. Notes 2016, 9, 95. [Google Scholar] [CrossRef]
- Schloss, L.; van Loon, A.M.; Cinque, P.; Cleator, G.; Echevarria, J.M.; Falk, K.I.; Klapper, P.; Schirm, J.; Vestergaard, B.F.; Niesters, H.; et al. An international external quality assessment of nucleic acid amplification of herpes simplex virus. J. Clin. Virol. 2003, 28, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Schnuriger, A.; Vimont, S.; Godmer, A.; Gozlan, J.; Gallah, S.; Macé, M.; Lalande, V.; Saloum, K.; Perrier, M.; Veziris, N.; et al. Differential performance of the FilmArray Meningitis/Encephalitis assay to detect bacterial and viral pathogens in both pediatric and adult populations. Microbiol. Spectr. 2022, 10, e0277421. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, J.; Jerome, K.R. Applications of digital PCR for clinical microbiology. J. Clin. Microbiol. 2017, 55, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Tapparel, C.; Siegrist, F.; Petty, T.J.; Kaiser, L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013, 14, 282–293. [Google Scholar] [CrossRef]
- Wang, M.; Ren, Q.; Zhang, Z.; Zhang, L.; Carr, M.J.; Li, J.; Zhou, H.; Shi, W. Rapid detection of hand, foot and mouth disease enterovirus genotypes by multiplex PCR. J. Virol. Methods 2018, 258, 7–12. [Google Scholar] [CrossRef]
- Volle, R.; Bailly, J.L.; Mirand, A.; Pereira, B.; Marque-Juillet, S.; Chambon, M.; Regagnon, C.; Brebion, A.; Henquell, C.; Peigue-Lafeuille, H.; et al. Variations in cerebrospinal fluid viral loads among enterovirus genotypes in patients hospitalized with laboratory-confirmed meningitis due to enterovirus. J. Infect. Dis. 2014, 210, 576–584. [Google Scholar] [CrossRef]
- Sooksawasdi Na Ayudhya, S.; Sips, G.J.; Bogers, S.; Leijten, L.M.E.; Laksono, B.M.; Smeets, L.C.; Bruning, A.; Benschop, K.; Wolthers, K.; van Riel, D.; et al. Detection of intrathecal antibodies to diagnose enterovirus infections of the central nervous system. J. Clin. Virol. 2022, 152, 105190. [Google Scholar] [CrossRef]
- Tansarli, G.S.; Chapin, K.C. Diagnostic test accuracy of the BioFire® FilmArray® meningitis/encephalitis panel: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 281–290. [Google Scholar] [CrossRef]
- La Scolea, L.J.; Dryja, D. Cerebrospinal fluid and blood of children with meningitis and its diagnostic significance. J. Clin. Microbiol. 1984, 19, 187–190. [Google Scholar] [CrossRef]
- Boudet, A.; Pantel, A.; Carles, M.J.; Boclé, H.; Charachon, S.; Enault, C.; Stéphan, R.; Cadot, L.; Lavigne, J.P.; Marchandin, H. A review of a 13-month period of FilmArray Meningitis/Encephalitis panel implementation as a first-line diagnosis tool at a university hospital. PLoS ONE 2019, 14, e0223887. [Google Scholar] [CrossRef]
- Bouam, A.; Vincent, J.J.; Drancourt, M.; Raoult, D.; Levy, P.Y. Preventing contamination of PCR-based multiplex assays including the use of a dedicated biosafety cabinet. Lett. Appl. Microbiol. 2021, 72, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Seme, K.; Mocilnik, T.; Fujs Komlos, K.; Doplihar, A.; Persing, D.H.; Poljak, M. GeneXpert enterovirus assay: One-year experience in a routine laboratory setting and evaluation on three proficiency panels. J. Clin. Microbiol. 2008, 46, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Baraldés, M.A.; Domingo, P.; Mauri, A.; Monmany, J.; Castellanos, M.; Pericas, R.; Vázquez, G. Group A streptococcal meningitis in the antibiotic era. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 572–578. [Google Scholar] [CrossRef] [PubMed]
- van de Beek, D.; Drake, J.M.; Tunkel, A.R. Nosocomial bacterial meningitis. N. Engl. J. Med. 2010, 362, 146–154. [Google Scholar] [CrossRef] [PubMed]
| Species | Origin Description |
|---|---|
| Escherichia coli K1 | Clinical strain isolated from CSF |
| Haemophilus influenzae Type e | Clinical strain isolated from CSF |
| Listeria monocytogenes Type 4b | Clinical strain isolated from blood culture |
| Neisseria meningitidis Serotype B | Clinical strain isolated from CSF |
| Mycoplasma pneumoniae | M129 |
| Streptococcus agalactiae ST17 clone | Clinical strain isolated from CSF |
| Streptococcus mitis | DSM 12643 |
| Streptococcus oralis | DSM 20627 |
| Streptococcus pneumoniae | ATCC 49619 |
| Streptococcus pseudopneumoniae | DSM 18670 |
| Streptococcus pyogenes Serotype M77 | Clinical strain isolated from blood culture |
| Target | Dilution | Detected by R-GENE® | R-GENE® Median Ct Value | R-GENE® Median Titer (copies/mL) | Detected by FA | Detected by QS | QS Ct Value |
|---|---|---|---|---|---|---|---|
| EV-A71-C1 | 1 | 5/5 | 34.99 | ND | ND | 1/1 | 36.0 |
| 1/10 | 5/5 | 37.7 | ND | 1/1 | 3/6 | 38.5/38.7/38.5 | |
| 1/100 | 3/5 | 38.22 | ND | ND | ND | ND | |
| EV-D68-B3 | 1 | 5/5 | 32.71 | ND | 1/1 | 0/1 | ND |
| 1/10 | 5/5 | 35.57 | ND | 5/6 | 0/1 | ND | |
| 1/100 | 4/5 | 40 | ND | ND | ND | ND | |
| E-30 | 1 | 5/5 | 34.52 | ND | ND | 1/1 | 37.5 |
| 1/10 | 5/5 | 37.49 | ND | 1/1 | 0/6 | ND | |
| 1/100 | 3/5 | 40 | ND | ND | ND | ND | |
| E-6 | 1/10 | 5/5 | 38.58 | ND | 1/1 | 1/1 | 38.5 |
| 1/100 | 3/5 | 40 | ND | ND | ND | ND | |
| PeV-1 | 1/10 | 5/5 | 38.41 | ND | 1/1 | 1/1 | 33.4 |
| 1/100 | 3/5 | 40 | ND | ND | ND | ND | |
| PeV-3 | 1/10 | 5/5 | 37.52 | ND | 1/1 | 1/1 | 35.0 |
| 1/100 | 3/5 | 40 | ND | ND | ND | ND | |
| HSV-1 | 1/10 | 5/5 | 36.41 | 1280 | 1/1 | 1/1 | 35.4 |
| 1/100 | 2/5 | 39.03 | <250 | ND | ND | ND | |
| HSV-2 | 1/10 | 5/5 | 35.39 | 277 | 1/1 | 1/1 | 36.5 |
| 1/100 | 3/5 | 37.61 | <100 | ND | ND | ND | |
| HHV-6 | 1/10 | 5/5 | 35.83 | 534 | 1/1 | 1/1 | 37.2 |
| 1/100 | 1/5 | 39.28 | <200 | ND | ND | ND | |
| VZV | 1/10 | 5/5 | 37.25 | <300 | 1/1 | 1/1 | 35.6 |
| 1/100 | 3/5 | 40 | <300 | ND | ND | ND | |
| Mp | 1/10 | 5/5 | 36.21 | ND | ND | 1/1 | 34.8 |
| 1/100 | 2/5 | 39.32 | ND | ND | ND | ND |
| Bacterial Target | Concentration (CFU/mL) | Detected by QS | QS Ct Value | Detected by FA |
|---|---|---|---|---|
| E. coli (Ec) | 1000 | 1/1 | 34.7 | 1/1 |
| L. monocytogenes (Lm) | 1000 | 1/1 | 36 | 1/1 |
| H. influenzae (Hi) | 1000 | 1/1 | 30 | 1/1 |
| N. meningitidis (Nm) | 100 | 1/1 | 34.1 | 1/1 |
| S. pneumoniae (Sp) | 100 | 1/1 | 35.6 | 1/1 |
| 200 | 1/1 | 34.7 | 1/1 | |
| S. agalactiae (GBS) | 1000 | 0/1 | ND | 1/1 |
| 1750 | 0/1 | ND | ND | |
| 3000 | 1/1 | 35.9 | ND | |
| S. pyogenes (GAS) | 1000 | 1/1 | 38.2 | ND |
| Assays (n) | False Positive Assays (n) | |
|---|---|---|
| QS | 48 | 3 |
| FA | 37 | 1 |
| Routinely-Used PCR Ct Value | Detected by QS | QS Ct Value | Detected by FA | |
|---|---|---|---|---|
| CSF + N. meningitidis | ND | 1/1 | 31.3 | 1/1 |
| CSF + S. pneumoniae | ND | 1/1 | 19.8 | 1/1 |
| CSF + HSV-2 | 37.2 | 1/1 | 36.5 | 1/1 |
| CSF + VZV | 33.7 | 1/1 | 33.0 | 1/1 |
| CSF + PeV | 34.3 | 1/1 | 31.9 | 1/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Bars, H.; Madany, N.; Lamoureux, C.; Beauruelle, C.; Vallet, S.; Payan, C.; Pilorgé, L. Evaluation of the Performance Characteristics of a New POC Multiplex PCR Assay for the Diagnosis of Viral and Bacterial Neuromeningeal Infections. Diagnostics 2023, 13, 1110. https://doi.org/10.3390/diagnostics13061110
Le Bars H, Madany N, Lamoureux C, Beauruelle C, Vallet S, Payan C, Pilorgé L. Evaluation of the Performance Characteristics of a New POC Multiplex PCR Assay for the Diagnosis of Viral and Bacterial Neuromeningeal Infections. Diagnostics. 2023; 13(6):1110. https://doi.org/10.3390/diagnostics13061110
Chicago/Turabian StyleLe Bars, Hervé, Neil Madany, Claudie Lamoureux, Clémence Beauruelle, Sophie Vallet, Christopher Payan, and Léa Pilorgé. 2023. "Evaluation of the Performance Characteristics of a New POC Multiplex PCR Assay for the Diagnosis of Viral and Bacterial Neuromeningeal Infections" Diagnostics 13, no. 6: 1110. https://doi.org/10.3390/diagnostics13061110
APA StyleLe Bars, H., Madany, N., Lamoureux, C., Beauruelle, C., Vallet, S., Payan, C., & Pilorgé, L. (2023). Evaluation of the Performance Characteristics of a New POC Multiplex PCR Assay for the Diagnosis of Viral and Bacterial Neuromeningeal Infections. Diagnostics, 13(6), 1110. https://doi.org/10.3390/diagnostics13061110






