The Use of the Alberta Infant Motor Scale (AIMS) as a Diagnostic Scale for Infants with Autism
Abstract
1. Introduction
- Supine lying—a variety of supine positions, bringing the baby’s hands to the knees or feet, and rolling from the back to the stomach.
- Lying on the stomach—starting with lifting the head from the surface at different heights, reaching out with arm support, pivoting, belly crawling, four points kneeling, and reciprocal crawling.
- Sitting—from sitting the baby with a variety of supports to sitting without the support of the arms.
- Standing—starting with support standing, pulling to standing, standing and cruising with the support of objects, and ending with independent standing and walking.
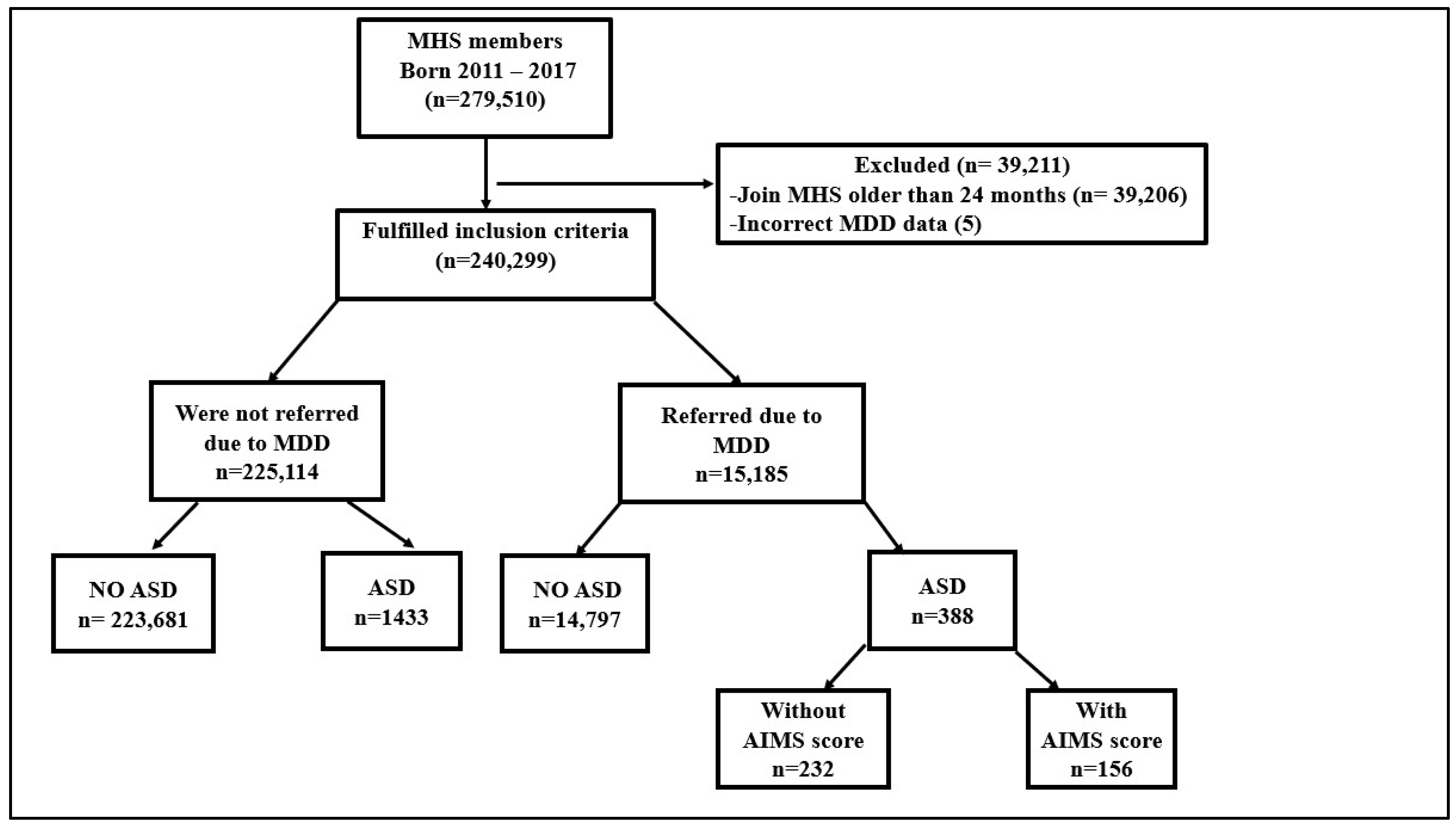
2. Method
3. Results
4. AIMS Score
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallahue, D.L.; Ozmun, J.C.; Goodway, J.D. Understanding motor Development: Infants, Children, Adolescents, Adults, 8th ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2019; ISBN 978-12-8417-494-6. [Google Scholar]
- Wilson, R.B.; Enticott, P.G.; Rinehart, N.J.; Piper, M.C.; Pinnell, L.E.; Darrah, J.; Maguire, T.; Paul, J.; Piper, M.C.; Pinnell, L.E.; et al. Construction and Validation of the Alberta Infant Motor Scale (AIMS). Can. J. Public Health 1992, 83, 46–50. [Google Scholar]
- Suir, I.; Boonzaaijer, M.; Nijmolen, P.; Westers, P.; Nuysink, J. Cross-Cultural Validity: Canadian Norm Values of the Alberta Infant Motor Scale Evaluated for Dutch Infants. Pediatr. Phys. Ther. 2019, 31, 354–358. [Google Scholar] [CrossRef]
- Saccani, R.; Valentini, N.C. Cross-cultural analysis of the motor development of Brazilian, Greek and Canadian infants assessed with the Alberta Infant Motor Scale. Rev. Paul. Pediatr. 2013, 31, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Zhang, D.; Robinson, C.C. Prevalence of developmental delays and participation in early intervention services for young children. Pediatrics 2008, 121, e1503–e1509. [Google Scholar] [CrossRef]
- Noritz, G.H.; Murphy, N.A. Motor delays: Early identification and evaluation. Pediatrics 2013, 131, e2016–e2027. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 1; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Brugha, T.S.; Erskine, H.E.; Scheurer, R.W.; Vos, T.; Scott, J.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015, 45, 601–613. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Weisskopf, M.G.; Levine, H.; Pinto, O.; Raz, R. Incidence time trends and socioeconomic factors in the observed incidence of autism spectrum disorder in israel: A nationwide nested case–control study. Autism Res. 2019, 12, 1870–1879. [Google Scholar] [CrossRef]
- Wong, V.C.N.; Hui, S.L.H. Epidemiological study of autism spectrum disorder in China. J. Child Neurol. 2008, 23, 67–72. [Google Scholar] [CrossRef]
- Taylor, B.; Jick, H.; MacLaughlin, D. Prevalence, and incidence rates of autism in the UK: Time trend from 2004-2010 in children aged 8 years. BMJ Open 2013, 3, e003219. [Google Scholar] [CrossRef] [PubMed]
- van Bakel, M.M.E.; Delobel-Ayoub, M.; Cans, C.; Assouline, B.; Jouk, P.S.; Raynaud, J.P.; Arnaud, C. Low but Increasing Prevalence of Autism Spectrum Disorders in a French Area from Register-Based Data. J. Autism Dev. Disord. 2015, 45, 3255–3261. [Google Scholar] [CrossRef] [PubMed]
- Ouellette-Kuntz, H.; Coo, H.; Lloyd, J.E.V.; Kasmara, L.; Holden, J.J.A.; Lewis, M.E.S. Trends in special education code assignment for autism: Implications for prevalence estimates. J. Autism Dev. Disord. 2007, 37, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Fairthorne, J.; de Klerk, N.; Leonard, H.M.; Schieve, L.A.; Yeargin-Allsopp, M. Maternal Race–Ethnicity, Immigrant Status, Country of Birth, and the Odds of a Child with Autism. Child Neurol. Open 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Idring, S.; Rai, D.; Dal, H.; Dalman, C.; Sturm, H.; Zander, E.; Lee, B.K.; Serlachius, E.; Magnusson, C. Autism spectrum disorders in the Stockholm youth cohort: Design, prevalence and validity. PLoS ONE 2012, 7, e41280. [Google Scholar] [CrossRef]
- Buescher, A.V.S.; Cidav, Z.; Knapp, M.; Mandell, D.S. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014, 168, 721–728. [Google Scholar] [CrossRef]
- Raz, R.; Lerner-Geva, L.; Leon, O.; Chodick, G.; Gabis, L.V. A survey of out-of-pocket expenditures for children with autism spectrum disorder in Israel. J. Autism Dev. Disord. 2013, 43, 2295–2302. [Google Scholar] [CrossRef]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Dobkins, K.; et al. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics 2011, 128, e488–e495. [Google Scholar] [CrossRef]
- Ozonoff, S.; Young, G.S.; Goldring, S.; Greiss-Hess, L.; Herrera, A.M.; Steele, J.; Macari, S.; Hepburn, S.; Rogers, S.J. Gross motor development, movement abnormalities, and early identification of autism. J. Autism Dev. Disord. 2008, 38, 644–656. [Google Scholar] [CrossRef]
- Provost, B.; Lopez, B.R.; Heimerl, S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. J. Autism Dev. Disord. 2007, 32, 321–328. [Google Scholar] [CrossRef]
- Licari, M.K.; Alvares, G.A.; Varcin, K.; Evans, K.L.; Cleary, D.; Reid, S.L.; Glasson, E.J.; Bebbington, K.; Reynolds, J.E.; Wray, J.; et al. Prevalence of Motor Difficulties in Autism Spectrum Disorder: Analysis of a Population-Based Cohort. Autism Res. 2020, 13, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, B.; Sargent, B.; Fetters, L. Cross-cultural validity of standardized motor development screening and assessment tools: A systematic review. Dev. Med. Child Neurol. 2016, 58, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Warren, Z.; McPheeters, M.L.; Sathe, N.; Foss-Feig, J.H.; Glasser, A.; Veenstra-VanderWeele, J. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics 2011, 127, e1303–e1311. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.R. Early motor delays as diagnostic clues in autism spectrum disorder. Eur. J. Pediatr. 2017, 176, 1259–1262. [Google Scholar] [CrossRef]
- Holloway, J.M.; Long, T.; Biasini, F. Concurrent Validity of Two Standardized Measures of Gross Motor Function in Young Children with Autism Spectrum Disorder. Phys. Occup. Ther. Pediatr. 2019, 39, 193–203. [Google Scholar] [CrossRef]
- Liu, T.; Breslin, C.M. Fine and gross motor performance of the MABC-2 by children with autism spectrum disorder and typically developing children. Res. Autism Spectr. Disord. 2013, 7, 1244–1249. [Google Scholar] [CrossRef]
- Piper, M.C.; Darrah, J. Motor Assessment of the Developing Infant Alberta Infant Motor Scale (AIMS), 1st ed.; WB Saunders: Philadelphia, PA, USA, 1994. [Google Scholar]
- Lackovic, M.; Nikolic, D.; Filimonovic, D.; Petronic, I.; Mihajlovic, S.; Golubovic, Z.; Pavicevic, P.; Cirovic, D. Reliability, consistency, and temporal stability of Alberta infant motor scale in Serbian infants. Children 2020, 7, 16. [Google Scholar] [CrossRef]
- de Albuquerque, P.L.; de Farias Guerra, M.Q.; de Carvalho Lima, M.; Eickmann, S.H. Concurrent validity of the Alberta Infant Motor Scale to detect delayed gross motor development in preterm infants: A comparative study with the Bayley III. Dev. Neurorehabilit. 2018, 21, 408–414. [Google Scholar] [CrossRef]
- Darrah, J.; Piper, M.; Watt, M.J. Assessment of gross motor skills of at-risk infants: Predictive validity of the Alberta Infant Motor Scale. Dev. Med. Child Neurol. 1998, 40, 485–491. [Google Scholar] [CrossRef]
- Bhat, A.N.; Galloway, J.C.; Landa, R.J. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev. 2012, 35, 838–846. [Google Scholar] [CrossRef]
- Baranek, G.T. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J. Autism Dev. Disord. 1999, 29, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Iverson, J.M.; Shic, F.; Wall, C.A.; Chawarska, K.; Curtin, S.; Estes, A.; Gardner, J.M.; Hutman, T.; Landa, R.J.; Levin, A.R.; et al. Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. J. Abnorm. Psychol. 2019, 128, 69–80. [Google Scholar] [CrossRef]
- Hatakenaka, Y.; Kotani, H.; Yasumitsu-Lovell, K.; Suzuki, K.; Fernell, E.; Gillberg, C. Infant Motor Delay and Early Symptomatic Syndromes Eliciting Neurodevelopmental Clinical Examinations in Japan. Pediatr. Neurol. 2016, 54, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pagnozzi, A.M.; Conti, E.; Calderoni, S.; Fripp, J.; Rose, S.E. A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. Int. J. Dev. Neurosci. 2018, 71, 68–82. [Google Scholar] [CrossRef]
- Casanova, M.F.; El-Baz, A.S.; Kamat, S.S.; Dombroski, B.A.; Khalifa, F.; Elnakib, A.; Soliman, A.; Allison-McNutt, A.; Switala, A.E. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol. Commun. 2013, 1, 67. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Patra, J.; Tiwari, N.; Salankar, N.; Ansari, M.N.; Ahmad, W. Dysregulation of multiple signaling neurodevelopmental pathways during embryogenesis: A possible cause of autism spectrum disorder. Cells 2021, 10, 958. [Google Scholar] [CrossRef]
- de Kegel, A.; Peersman, W.; Onderbeke, K.; Baetens, T.; Dhooge, I.; van Waelvelde, H. New reference values must be established for the Alberta Infant Motor Scales for accurate identification of infants at risk for motor developmental delay in Flanders. Child Care Health Dev. 2013, 39, 260–267. [Google Scholar] [CrossRef]
- van Iersel, P.A.M.; la Bastide-van Gemert, S.; Wu, Y.C.; Hadders-Algra, M. Alberta Infant Motor Scale: Cross-cultural analysis of gross motor development in Dutch and Canadian infants and introduction of Dutch norms. Early Hum. Dev. 2020, 151, 105239. [Google Scholar] [CrossRef]
- Syrengelas, D.; Siahanidou, T.; Kourlaba, G.; Kleisiouni, P.; Bakoula, C.; Chrousos, G.P. Standardization of the Alberta infant motor scale in full-term Greek infants: Preliminary results. Early Hum. Dev. 2010, 86, 245–249. [Google Scholar] [CrossRef]
- Kepenek-Varol, B.; Hoşbay, Z.; Varol, S.; Torun, E. Assessment of motor development using the Alberta infant motor scale in full-term infants. Turk. J. Pediatr. 2020, 62, 94–102. [Google Scholar] [CrossRef]
- Almeida, K.M.; Dutra, M.V.P.; de Mello, R.R.; Reis, A.B.R.; Martins, P.S. Validade concorrente e confiabilidade da Alberta Infant Motor Scale em lactentes nascidos prematuros. J. Pediatr. 2008, 84, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, C.H.; Massetti, T.; Crocetta, T.B.; da Silva, T.D.; Mustacchi, Z.; Guarnieri, R.; de Abreu, L.C.; de Araújo, A.V.L.; de Menezes, L.D.C.; Monteiro, C.B.D.M.; et al. Systematic Review of the Main Motor Scales for Clinical Assessment of Individuals with down Syndrome. Dev. Neurorehabilit. 2020, 23, 39–49. [Google Scholar] [CrossRef]
- Kaplan, S.L.; Coulter, C.; Sargent, B. Physical Therapy Management of Congenital Muscular Torticollis: A 2018 Evidence-Based Clinical Practice Guideline from the APTA Academy of Pediatric Physical Therapy. Pediatr. Phys. Ther. 2018, 30, 240–290. [Google Scholar] [CrossRef] [PubMed]
- Schertz, M.; Zuk, L.; Zin, S.; Nadam, L.; Schwartz, D.; Bienkowski, R.S. Motor and cognitive development at one-year follow-up in infants with torticollis. Early Hum. Dev. 2008, 84, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Boonzaaijer, M.; van Dam, E.; van Haastert, I.C.; Nuysink, J. Concurrent validity between live and home video observations using the Alberta infant motor scale. Pediatr. Phys. Ther. 2017, 29, 146–151. [Google Scholar] [CrossRef]
- Olivier, O. A Comparsion of Treatment Protocols for Infants with Motor Delay. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2012. [Google Scholar]
- Atun-Einy, O.; Ashkenazi-Shahar, T. En route to Change Professional: Expanding the Physical Therapy Toolbox for Early Screening of Autistic Spectrum Disorder During Infancy. J. Isr. Physiother. Soc.–JIPTS 2020, 1, 6–21. [Google Scholar]
- Atun-Einy, O.; Lotan, M.; Harel, Y.; Shavit, E.; Burstein, S.; Kempner, G. Physical therapy for young children diagnosed with Autism Spectrum Disorders-clinical frameworks model in an Israeli setting. Front. Pediatr. 2013, 1, 19. [Google Scholar] [CrossRef]
- Branson, D.; Vigil, D.C.; Bingham, A. Community childcare providers’ role in the early detection of autism spectrum disorders. Early Child. Educ. J. 2008, 35, 523–530. [Google Scholar] [CrossRef]
- Atun-Einy, O.; Ben-Sasson, A. Pediatric allied healthcare professionals’ knowledge and self-efficacy regarding ASD. Res. Autism Spectr. Disord. 2018, 47, 1–13. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Atun-Einy, O.; Yahav-Jonas, G.; Lev-On, S.; Gev, T. Training Physical Therapists in Early ASD Screening. J. Autism Dev. Disord. 2018, 48, 3929–3938. [Google Scholar] [CrossRef]
- Vivanti, G.; Manzi, B.; Benvenuto, A. An Italian Prospective Study on Autism Treatment: The Earlier, the Better? Autism-Open Access 2011, 1, 3. [Google Scholar] [CrossRef]
- Hyman, S.L.; Levy, S.E.; Myers, S.M.; Children, O.N.; Disabilities, W. Identification, Evaluation, and Management of Children with Autism Spectrum Disorder. Pediatrics 2020, 145, e20193447. [Google Scholar] [CrossRef] [PubMed]
| Gender | |
|---|---|
| Boys | 298 (76.8%) |
| Father’s age | |
| Mean ± SD (years) | 31.6 ± 12 |
| Mother’s age | |
| Mean ± SD (years) | 31.2 ± 6.9 |
| Sector | |
| General Population | 271 (69.8%) |
| Ultra-Orthodox Jews | 83 (21.3%) |
| Israeli Arabs | 34 (11%) |
| Pregnancy Description | |
| Risk | 66 (17%) |
| Age of Gestational (Weeks) | |
| 37> | 66 (17%) |
| Birth Weight (Grams) | |
| 2500> | 70 (18.1%) |
| Apgar Score (n = 226) | |
| 1 Min | 8.4 ± 1.7 |
| 1 Min < 8 | 27 (6.9%) |
| 5 Min | 9.3 ± 1.1 |
| 5 Min < 9 | 21 (5.4%) |
| Typical Motor Development >25th | Suspicion MDD 6th–25th | MDD 5th≥ | |
|---|---|---|---|
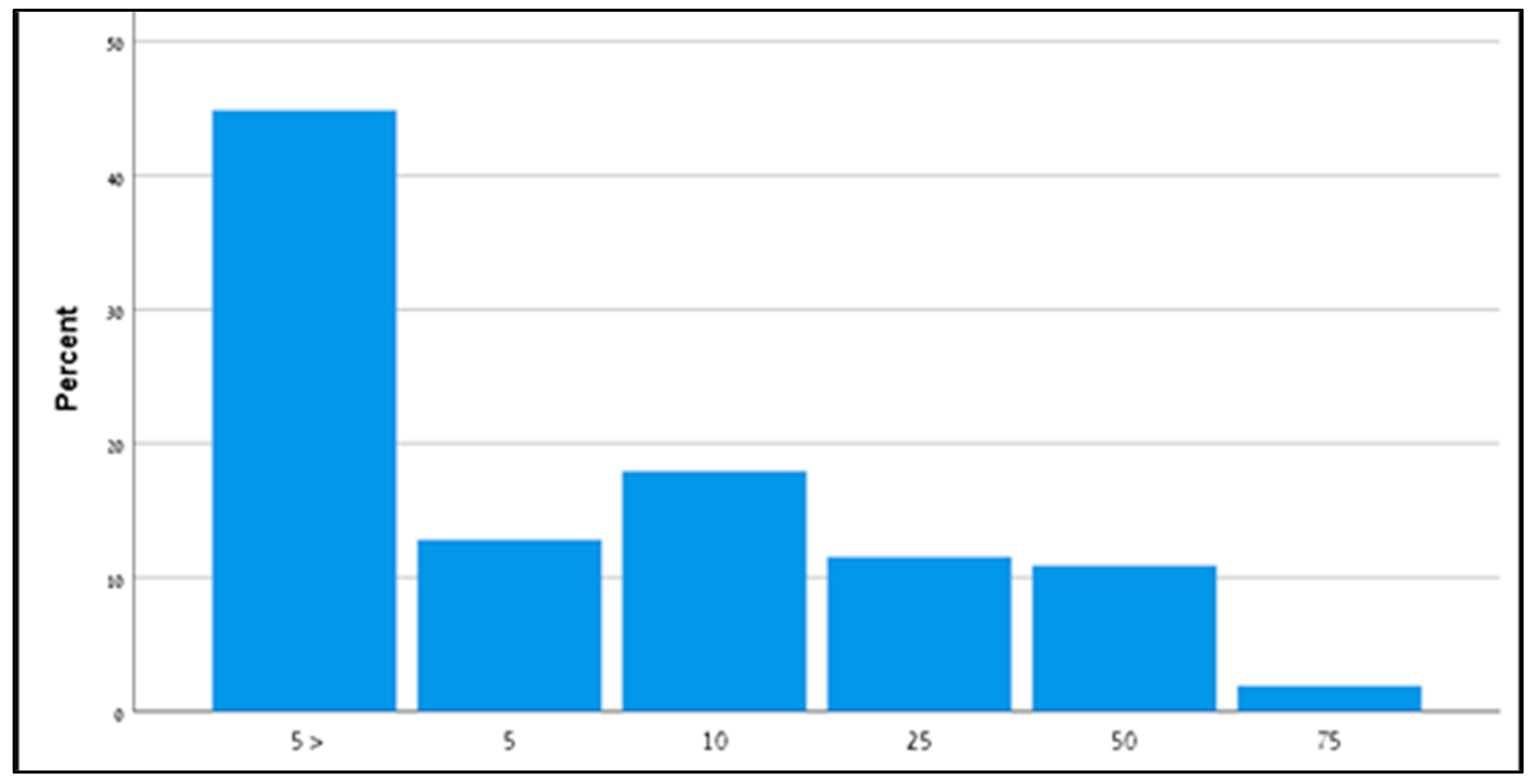
| General results (n = 156) | 20 (13%) | 46 (29%) | 90 (58%) |
| Reason for referral | |||
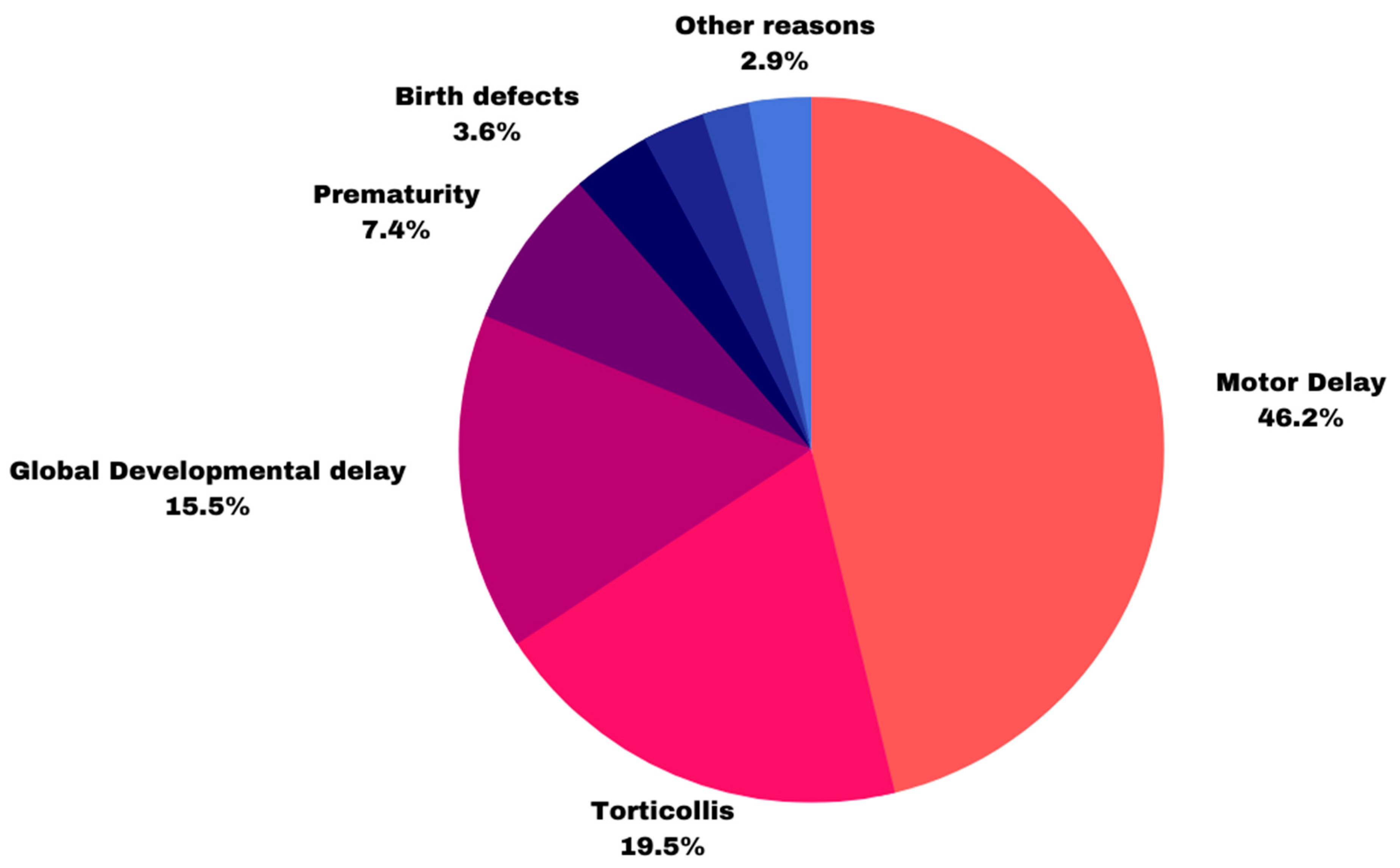
| Motor delay (n = 91) | 2(2%) | 20 (22%) | 69 (76%) |
| Torticollis (n = 42) | 9 (21%) | 24 (57%) | 9 (21%) |
| Global developmental delay (n = 15) | 4 (27%) | 1 (6%) | 10 (66%) |
| Age of first visit DPT | |||
| 0–6 Months (n = 52) | 8 (15%) | 24 (46%) | 20 (39%) |
| 7–12 Months (n = 57) | 5 (8%) | 13 (22%) | 39 (68%) |
| 13–24 Months (n = 47) | 7 (14%) | 9 (19%) | 31 (66%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochav-Lev, M.; Bennett-Back, O.; Lotan, M.; Stein-Zamir, C. The Use of the Alberta Infant Motor Scale (AIMS) as a Diagnostic Scale for Infants with Autism. Diagnostics 2023, 13, 1045. https://doi.org/10.3390/diagnostics13061045
Kochav-Lev M, Bennett-Back O, Lotan M, Stein-Zamir C. The Use of the Alberta Infant Motor Scale (AIMS) as a Diagnostic Scale for Infants with Autism. Diagnostics. 2023; 13(6):1045. https://doi.org/10.3390/diagnostics13061045
Chicago/Turabian StyleKochav-Lev, Mooly, Odeya Bennett-Back, Meir Lotan, and Chen Stein-Zamir. 2023. "The Use of the Alberta Infant Motor Scale (AIMS) as a Diagnostic Scale for Infants with Autism" Diagnostics 13, no. 6: 1045. https://doi.org/10.3390/diagnostics13061045
APA StyleKochav-Lev, M., Bennett-Back, O., Lotan, M., & Stein-Zamir, C. (2023). The Use of the Alberta Infant Motor Scale (AIMS) as a Diagnostic Scale for Infants with Autism. Diagnostics, 13(6), 1045. https://doi.org/10.3390/diagnostics13061045






