Motion-Corrected versus Conventional Diffusion-Weighted Magnetic Resonance Imaging of the Liver Using Non-Rigid Registration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Conventional DWI
2.3. Moco-DWI
2.4. Image Analysis
2.4.1. Qualitative Analysis
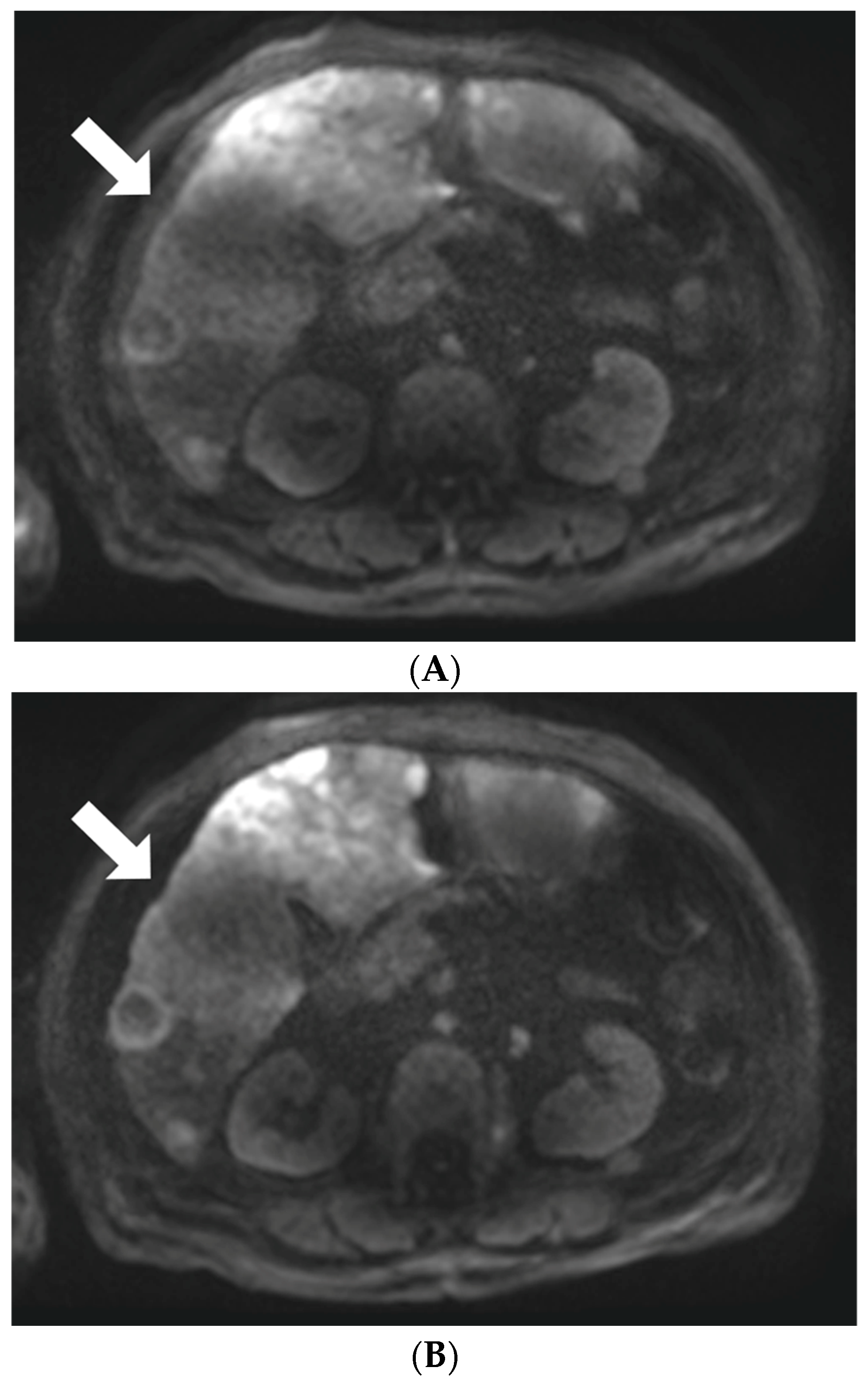
2.4.2. Lesion Detection and Characterization
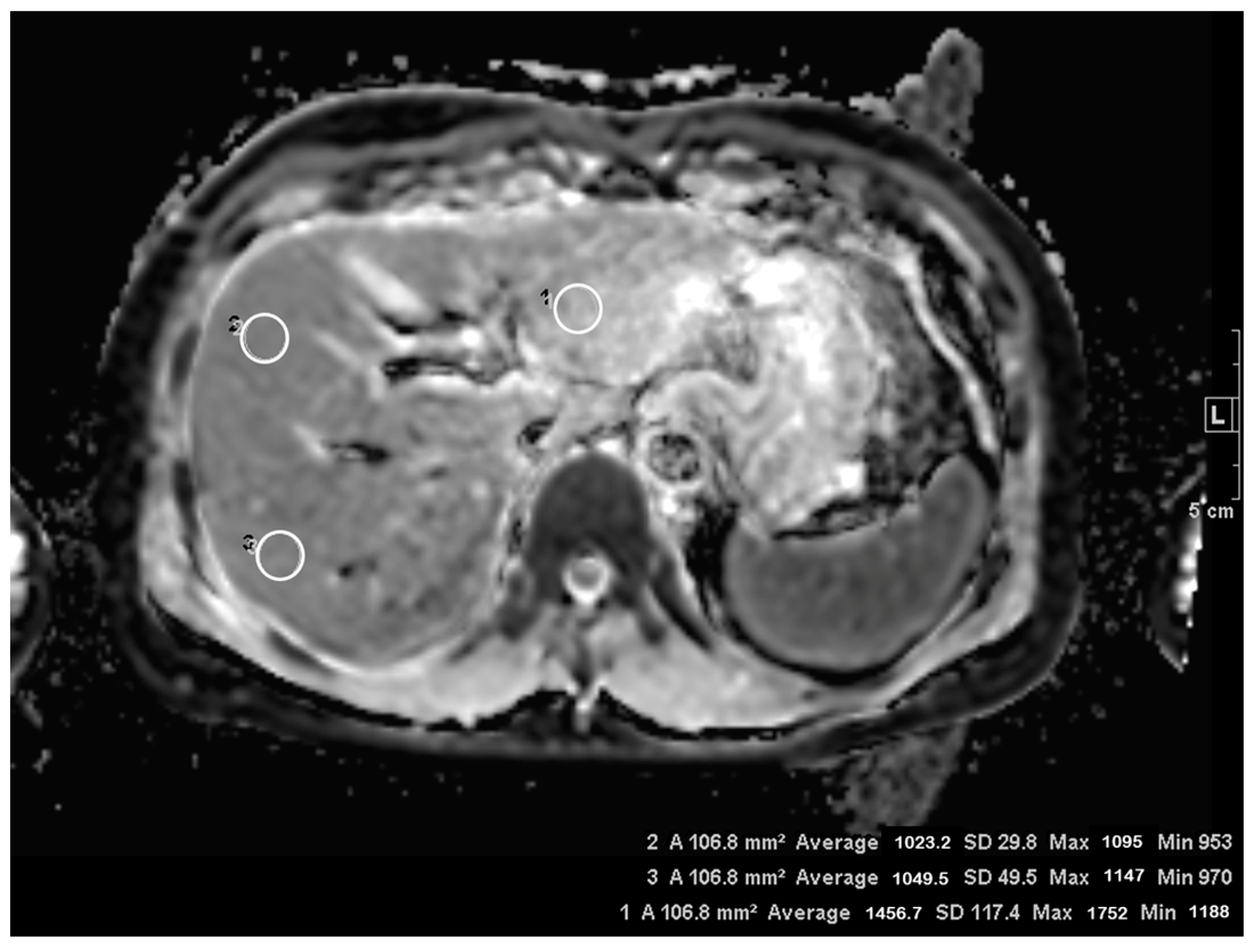
2.4.3. Quantitative Analysis
2.5. Statistical Analysis
3. Results
3.1. Subjective Image Quality
3.2. Lesion Visibility and Conspicuity
3.3. ADC Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koh, D.M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Taouli, B.; Koh, D.M. Diffusion-weighted MR imaging of the liver. Radiology 2010, 254, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cheng, Y.J.; Wang, S.T.; Wang, X.; Jin, Z.Y.; Qian, T.Y.; Zhu, J.X.; Nickel, M.D.; Xue, H.D. Simultaneous multi-slice accelerated diffusion-weighted imaging with higher spatial resolution for patients with liver metastases from neuroendocrine tumours. Clin. Radiol. 2021, 76, 81.e11–81.e19. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Hwang, J.; Hong, S.S.; Lee, E.J.; Jeong, J.; Benkert, T.; Sung, J.; Arberet, S. Clinical feasibility of accelerated diffusion weighted imaging of the abdomen with deep learning reconstruction: Comparison with conventional diffusion weighted imaging. Eur. J. Radiol. 2022, 154, 110428. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Do, R.K.; Shukla-Dave, A.; Deasy, J.O.; Lu, Y.; Akin, O. Motion correction of multi-b-value diffusion-weighted imaging in the liver. Acad. Radiol. 2012, 19, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, H.; Sharma, R.; Madhusudhan, K.S.; Kapoor, K.S. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: Comparison of image quality and apparent diffusion coefficient values. AJR Am. J. Roentgenol. 2009, 192, 915–922. [Google Scholar] [CrossRef]
- Obele, C.C.; Glielmi, C.; Ream, J.; Doshi, A.; Campbell, N.; Zhang, H.C.; Babb, J.; Bhat, H.; Chandarana, H. Simultaneous Multislice Accelerated Free-Breathing Diffusion-Weighted Imaging of the Liver at 3T. Abdom. Imaging 2015, 40, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Breuer, F.; Koopmans, P.J.; Norris, D.G.; Poser, B.A. Simultaneous multislice (SMS) imaging techniques. Magn. Reson. Med. 2016, 75, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.Z.; Tunnicliffe, E.M.; Frost, R.; Koopmans, P.J.; Tyler, D.J.; Robson, M.D. Accelerated human cardiac diffusion tensor imaging using simultaneous multislice imaging. Magn. Reson. Med. 2015, 73, 995–1004. [Google Scholar] [CrossRef]
- Tavakoli, A.; Attenberger, U.I.; Budjan, J.; Stemmer, A.; Nickel, D.; Kannengiesser, S.; Morelli, J.N.; Schoenberg, S.O.; Riffel, P. Improved Liver Diffusion-Weighted Imaging at 3 T Using Respiratory Triggering in Combination With Simultaneous Multislice Acceleration. Investig. Radiol. 2019, 54, 744–751. [Google Scholar] [CrossRef]
- Glutig, K.; Kruger, P.C.; Oberreuther, T.; Nickel, M.D.; Teichgraber, U.; Lorenz, M.; Mentzel, H.J.; Kramer, M. Preliminary results of abdominal simultaneous multi-slice accelerated diffusion-weighted imaging with motion-correction in patients with cystic fibrosis and impaired compliance. Abdom. Radiol. 2022, 47, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Park, H.S.; Kim, Y.J.; Yu, M.H.; Park, S.; Jung, S.I. Association of Hepatic Steatosis Index with Nonalcoholic Fatty Liver isease Diagnosed by Non-Enhanced CT in a Screening Population. Diagnostics 2021, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Takahara, T.; Niwa, T.; Ivancevic, M.K.; Herigault, G.; Van Cauteren, M.; Luijten, P.R. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. MAGMA 2009, 22, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, L.; Pan, D.; Huang, Y.; Yan, L.; Wang, G.; Liu, Y.; Liang, C.; Liu, Z. Liver diffusion-weighted MR imaging: Reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology 2014, 271, 113–125. [Google Scholar] [CrossRef]
- Filipe, J.P.; Curvo-Semedo, L.; Casalta-Lopes, J.; Marques, M.C.; Caseiro-Alves, F. Diffusion-weighted imaging of the liver: Usefulness of ADC values in the differential diagnosis of focal lesions and effect of ROI methods on ADC measurements. MAGMA 2013, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, S.S.; Byun, J.H.; Park, S.H.; Kim, J.K.; Park, B.; Kim, N.; Lee, M.G. Malignant hepatic tumors: Short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology 2010, 255, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Bruegel, M.; Holzapfel, K.; Gaa, J.; Woertler, K.; Waldt, S.; Kiefer, B.; Stemmer, A.; Ganter, C.; Rummeny, E.J. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur. Radiol. 2008, 18, 477–485. [Google Scholar] [CrossRef]
- Murtz, P.; Flacke, S.; Traber, F.; van den Brink, J.S.; Gieseke, J.; Schild, H.H. Abdomen: Diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology 2002, 224, 258–264. [Google Scholar] [CrossRef]
- Nasu, K.; Kuroki, Y.; Sekiguchi, R.; Kazama, T.; Nakajima, H. Measurement of the apparent diffusion coefficient in the liver: Is it a reliable index for hepatic disease diagnosis? Radiat. Med. 2006, 24, 438–444. [Google Scholar] [CrossRef]
- Szklaruk, J.; Son, J.B.; Wei, W.; Bhosale, P.; Javadi, S.; Ma, J. Comparison of free breathing and respiratory triggered diffusion-weighted imaging sequences for liver imaging. World J. Radiol. 2019, 11, 134–143. [Google Scholar] [CrossRef]
- Taouli, B.; Sandberg, A.; Stemmer, A.; Parikh, T.; Wong, S.; Xu, J.; Lee, V.S. Diffusion-weighted imaging of the liver: Comparison of navigator triggered and breathhold acquisitions. J. Magn. Reson. Imaging 2009, 30, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Metens, T.; Ferraresi, K.F.; Farchione, A.; Moreno, C.; Bali, M.A.; Matos, C. Normal hepatic parenchyma visibility and ADC quantification on diffusion-weighted MRI at 3 T: Influence of age, gender, and iron content. Eur. Radiol. 2014, 24, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Takahara, T.; Koh, D.M.; Nievelstein, R.A.; Luijten, P.R. Comparison and reproducibility of ADC measurements in breathhold, respiratory triggered, and free-breathing diffusion-weighted MR imaging of the liver. J. Magn. Reson. Imaging 2008, 28, 1141–1148. [Google Scholar] [CrossRef] [PubMed]


| Protocol Parameter | |
|---|---|
| b-values (s/mm2) | 50, 800 |
| TR/TE (ms) | 5200/51 |
| Field-of-view (mm2) | 379 × 260 |
| Matrix | 140 × 96 |
| In-plane resolution (mm2) | 1.36 × 1.36 (interpolated) |
| Slice thickness (mm) | 5 |
| Number of slices | 35 |
| Flip angle (degree) | 90 |
| Parallel imaging factor | 2 |
| Number of excitations | 2 (b = 50 s/mm2), 4 (b = 800 s/mm2) |
| Fat suppression | SPAIR |
| Acquisition time (m:s) | 03:36 (free breathing) |
| Subjective Image Analysis | c-DWI | moco-DWI | p-Value |
|---|---|---|---|
| Overall image quality | 2.68 ± 0.544 | 2.82 ± 0.573 | 0.0018 |
| Liver margin sharpness | 2.84 ± 0.56 | 2.9 ± 0.58 | 0.0244 |
| Intrahepatic vessel delineation | 2.68 ± 0.668 | 2.73 ± 0.633 | 0.0448 |
| Pancreas margin sharpness | 2.75 ± 0.643 | 2.78 ± 0.652 | 0.1586 |
| Susceptibility artifact | 2.37 ± 0.661 | 2.39 ± 0.646 | 0.1586 |
| Cardiac motion artifact | 1.98 ± 0.547 | 2.01 ± 0.581 | 0.0832 |
| Motion artifact | 2.35 ± 0.674 | 2.38 ± 0.681 | 0.0832 |
| Noise | 2.43 ± 0.651 | 2.49 ± 0.654 | 0.0244 |
| Post Hoc Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Left | Right Anterior | Right Posterior | p-Value | Left vs. Right Anterior | Left vs. Right Posterior | Right Anterior vs. Right Posterior | |
| c-DWI | 1.194 ± 0.028 | 1.030 ± 0.149 | 1.023 ± 0.02 | <0.001 | <0.001 | <0.001 | 1 |
| moco-DWI | 1.183 ± 0.027 | 1.027 ± 0.014 | 1.013 ± 0.02 | <0.001 | <0.001 | <0.001 | 1 |
| p-Value | 0.0853 | 0.4468 | 0.0584 | ||||
| Post Hoc Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Left | Right Anterior | Right Posterior | p-Value | Left vs. Right Anterior | Left vs. Right Posterior | Right Anterior vs. Right Posterior | |
| c-DWI | 0.096 ± 0.005 | 0.078 ± 0.005 | 0.072 ± 0.004 | <0.001 | 0.0031 | <0.0001 | 0.3096 |
| moco-DWI | 0.092 ± 0.005 | 0.076 ± 0.004 | 0.07 ± 0.004 | <0.001 | 0.0281 | 0.0001 | 0.1535 |
| p-Value | 0.2866 | 0.3328 | 0.4789 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.S.; Park, H.S.; Park, S.; Kim, Y.J.; Yu, M.H.; Jung, S.I.; Paek, M.; Nickel, M.D. Motion-Corrected versus Conventional Diffusion-Weighted Magnetic Resonance Imaging of the Liver Using Non-Rigid Registration. Diagnostics 2023, 13, 1008. https://doi.org/10.3390/diagnostics13061008
Son JS, Park HS, Park S, Kim YJ, Yu MH, Jung SI, Paek M, Nickel MD. Motion-Corrected versus Conventional Diffusion-Weighted Magnetic Resonance Imaging of the Liver Using Non-Rigid Registration. Diagnostics. 2023; 13(6):1008. https://doi.org/10.3390/diagnostics13061008
Chicago/Turabian StyleSon, Je Seung, Hee Sun Park, Sungeun Park, Young Jun Kim, Mi Hye Yu, Sung Il Jung, Munyoung Paek, and Marcel Dominik Nickel. 2023. "Motion-Corrected versus Conventional Diffusion-Weighted Magnetic Resonance Imaging of the Liver Using Non-Rigid Registration" Diagnostics 13, no. 6: 1008. https://doi.org/10.3390/diagnostics13061008
APA StyleSon, J. S., Park, H. S., Park, S., Kim, Y. J., Yu, M. H., Jung, S. I., Paek, M., & Nickel, M. D. (2023). Motion-Corrected versus Conventional Diffusion-Weighted Magnetic Resonance Imaging of the Liver Using Non-Rigid Registration. Diagnostics, 13(6), 1008. https://doi.org/10.3390/diagnostics13061008








